Gastrointestinal Tract Stabilized Protein Delivery Using Disulfide Thermostable Exoshell System
Abstract
:1. Introduction
2. Results
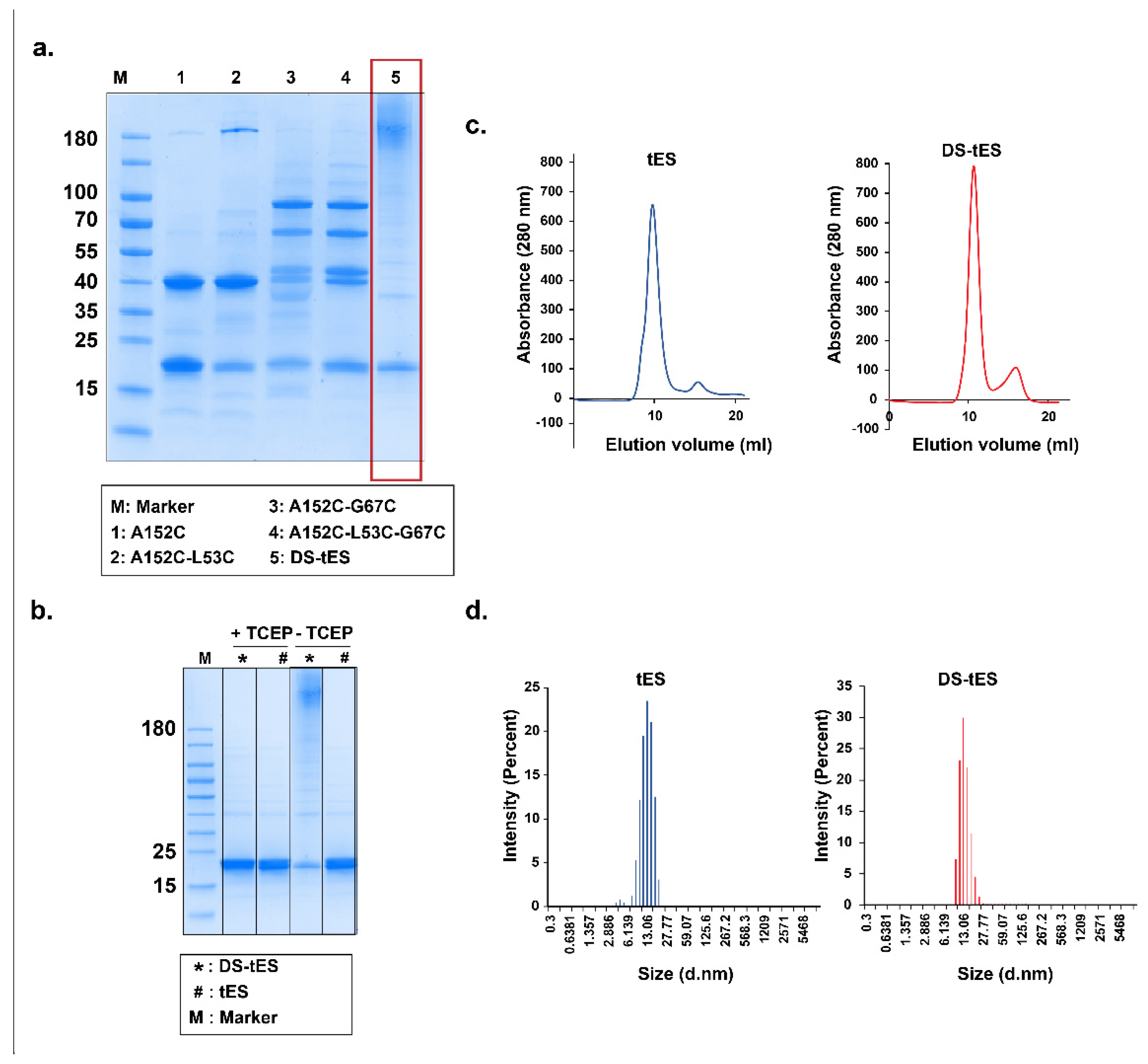
2.1. Design and Screening for Disulfide tES
2.2. Purification and Characterization of DS-tES
2.3. DS-tES Stabilized HRP Activity in Simulated GI Conditions
2.4. DS-tES Permeabilizes through Intestinal Cell Layers
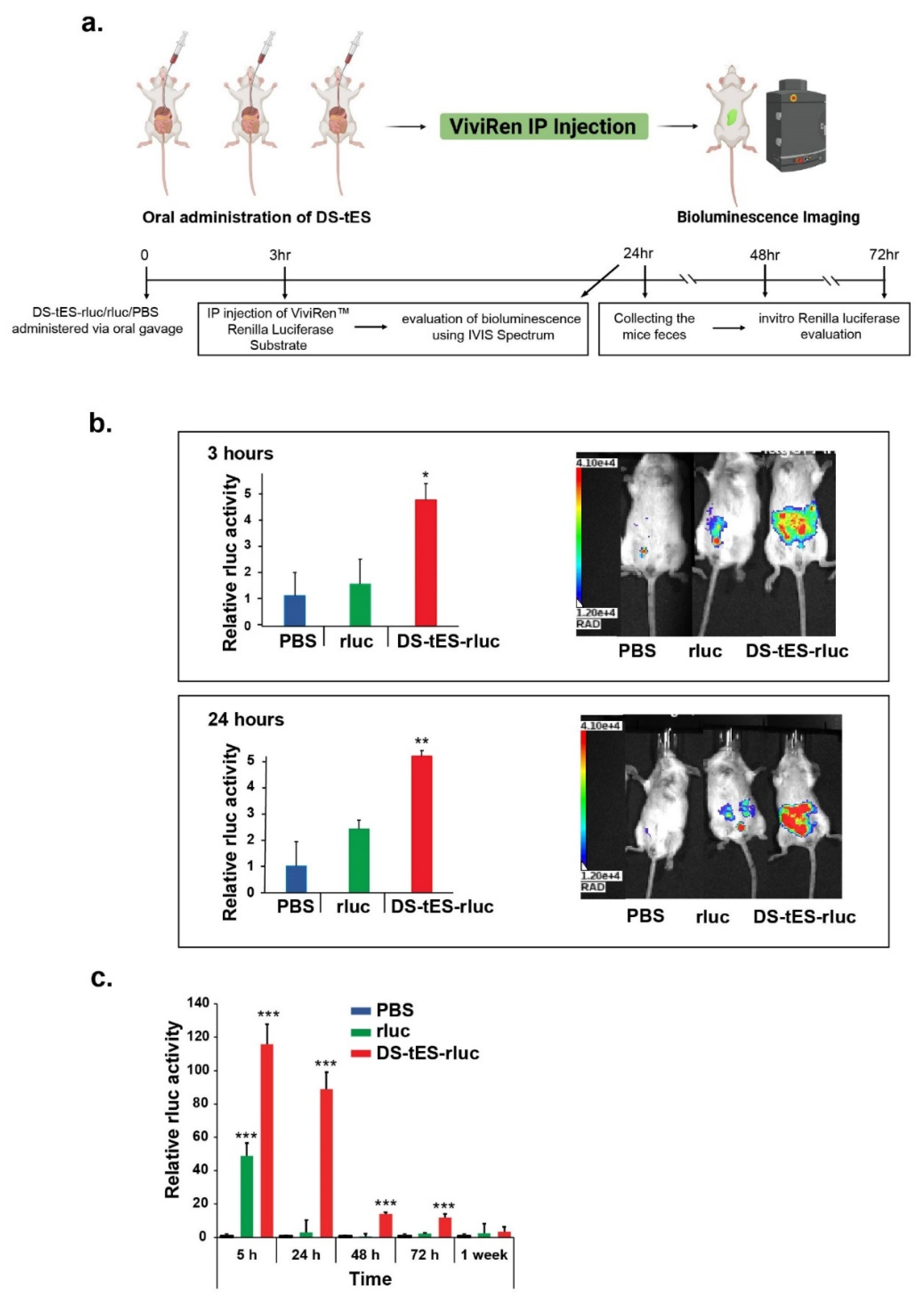
2.5. Oral Delivery of DS-tES and Its In Vivo Evaluation
3. Discussion
4. Materials and Methods
4.1. Preparation of DS-tES
4.2. Characterization of DS-tES
4.3. Preparation of DS-tES-HRP/rluc
4.4. In Vitro Experiments
4.4.1. Pepsin Assay
4.4.2. Trypsin Assay
4.4.3. Cell Permeability
4.5. In Vivo Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral administration of protein nanoparticles: An emerging route to disease treatment. Pharmacol. Res. 2020, 158, 104685. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 62, 618411. [Google Scholar] [CrossRef] [PubMed]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract–revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Leng, P.; Liu, Y. Oral drug delivery with nanoparticles into the gastrointestinal mucosa. Fundam. Clin. Pharmacol. 2021, 35, 86–96. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Usmani, S.S.; Bedi, G.; Samuel, J.S.; Singh, S.; Kalra, S.; Kumar, P.; Ahuja, A.A.; Sharma, M.; Gautam, A.; Raghava, G.P. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE 2017, 12, e0181748. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin. Drug Deliv. 2015, 12, 1459–1473. [Google Scholar] [CrossRef]
- Liao, R.; Pon, J.; Chungyoun, M.; Nance, E. Enzymatic protection and biocompatibility screening of enzyme-loaded polymeric nanoparticles for neurotherapeutic applications. Biomaterials 2020, 257, 120238. [Google Scholar] [CrossRef]
- Fu, A.; Tang, R.; Hardie, J.; Farkas, M.E.; Rotello, V.M. Promises and pitfalls of intracellular delivery of proteins. Bioconjugate Chem. 2014, 25, 1602–1608. [Google Scholar] [CrossRef]
- Estrada, L.H.; Champion, J. Protein nanoparticles for therapeutic protein delivery. Biomater. Sci. 2015, 3, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnology 2021, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J. Control. Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Perfecto, A.; Rodriguez-Ramiro, I.; Rodriguez-Celma, J.; Sharp, P.; Balk, J.; Fairweather-Tait, S. Pea ferritin stability under gastric pH conditions determines the mechanism of iron uptake in Caco-2 cells. J. Nutr. 2018, 148, 1229–1235. [Google Scholar] [CrossRef]
- Kalgaonkar, S.; Lönnerdal, B. Effects of dietary factors on iron uptake from ferritin by Caco-2 cells. J. Nutr. Biochem. 2008, 19, 33–39. [Google Scholar] [CrossRef]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral delivery for protein and peptide drugs. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Deshpande, S.; Masurkar, N.D.; Girish, V.M.; Desai, M.; Chakraborty, G.; Chan, J.M.; Drum, C.L. Thermostable exoshells fold and stabilize recombinant proteins. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Deshpande, S.; Vallerinteavide Mavelli, G.; Aksoyoglu, A.; Bafna, J.; Winterhalter, M.; Kini, R.M.; Lane, D.P.; Drum, C.L. A general approach to protein folding using thermostable exoshells. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vallerinteavide Mavelli, G.; Sadeghi, S.; Vaidya, S.S.; Kong, S.N.; Drum, C.L. Nanoencapsulation as a General Solution for Lyophilization of Labile Substrates. Pharmaceutics 2021, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Masurkar, N.D.; Vallerinteavide Mavelli, G.; Deshpande, S.; Kok Yong Tan, W.; Yee, S.; Kang, S.-A.; Lim, Y.-P.; Kai-Hua Chow, E.; Drum, C.L. Bioorthogonal Catalysis for Treatment of Solid Tumors Using Thermostable, Self-Assembling, Single Enzyme Nanoparticles and Natural Product Conversion with Indole-3-acetic Acid. ACS Nano 2022, 16, 10292–10301. [Google Scholar] [CrossRef] [PubMed]
- Reinwarth, M.; Glotzbach, B.; Tomaszowski, M.; Fabritz, S.; Avrutina, O.; Kolmar, H. Oxidative Folding of Peptides with Cystine-Knot Architectures: Kinetic Studies and Optimization of Folding Conditions. Chembiochem 2013, 14, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006, 7, 271–275. [Google Scholar] [CrossRef]
- Okumura, M.; Saiki, M.; Yamaguchi, H.; Hidaka, Y. Acceleration of disulfide-coupled protein folding using glutathione derivatives. FEBS J. 2011, 278, 1137–1144. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Kalimuthu, P.; Abraham John, S. Solvent Induced Disulfide Bond Formation in 2, 5-dimercapto-1, 3, 4-thiadiazole. Nat. Preced. 2007. [Google Scholar] [CrossRef]
- Du, C.; Huang, Y.; Borwankar, A.; Tan, Z.; Cura, A.; Yee, J.C.; Singh, N.; Ludwig, R.; Borys, M.; Ghose, S. Using hydrogen peroxide to prevent antibody disulfide bond reduction during manufacturing process. In Proceedings of the MAbs, Stockholm, Sweden, 14–15 July 2018; pp. 500–510. [Google Scholar]
- Okumura, M.; Shimamoto, S.; Hidaka, Y. A chemical method for investigating disulfide-coupled peptide and protein folding. FEBS J. 2012, 279, 2283–2295. [Google Scholar] [CrossRef]
- Weismiller, H.A.; Holub, T.J.; Krzesinski, B.J.; Margittai, M. A thiol-based intramolecular redox switch in four-repeat tau controls fibril assembly and disassembly. J. Biol. Chem. 2021, 297, 101021. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties: A review. Polymers 2020, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Fürst, A.; Akkus-Dagdeviren, Z.B.; Arshad, S.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Less Reactive Thiol Ligands: Key towards Highly Mucoadhesive Drug Delivery Systems. Polymers 2020, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Schmittner, M.; Duschl, A.; Horejs-Hoeck, J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS ONE 2014, 9, e113840. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Fukui, C.; Morishita, Y.; Haishima, Y. A biological study establishing the endotoxin limit for in vitro proliferation of human mesenchymal stem cells. Regen. Ther. 2017, 7, 45–51. [Google Scholar] [CrossRef]
- Ferruzza, S.; Rossi, C.; Scarino, M.L.; Sambuy, Y. A protocol for differentiation of human intestinal Caco-2 cells in asymmetric serum-containing medium. Toxicol. Vitr. 2012, 26, 1252–1255. [Google Scholar] [CrossRef]
- Joshi, G.; Kumar, A.; Sawant, K. Bioavailability enhancement, Caco-2 cells uptake and intestinal transport of orally administered lopinavir-loaded PLGA nanoparticles. Drug Deliv. 2016, 23, 3492–3504. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The enhancing effect of N-acetylcysteine modified hyaluronic acid-octadecylamine micelles on the oral absorption of paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]
- Martien, R.; Loretz, B.; Sandbichler, A.M.; Schnuerch, A.B. Thiolated chitosan nanoparticles: Transfection study in the Caco-2 differentiated cell culture. Nanotechnology 2008, 19, 045101. [Google Scholar] [CrossRef]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y. Improving the stability of wheat gliadin nanoparticles–Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar] [CrossRef]
- Feng, G.; Han, K.; Li, Y.; Yang, Q.; Feng, W.; Wang, J.; Yang, X. Undigestible gliadin peptide nanoparticles penetrate mucus and reduce mucus production driven by intestinal epithelial cell damage. J. Agric. Food Chem. 2021, 69, 7979–7989. [Google Scholar] [CrossRef]
- Minko, T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv. Drug Deliv. Rev. 2004, 56, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Souris, J.S.; Cheng, S.-H.; Chu, C.-H.; Wang, Y.-C.; Konda, V.; Dougherty, U.; Bissonnette, M.; Mou, C.-Y.; Chen, C.-T. Lectin-functionalized mesoporous silica nanoparticles for endoscopic detection of premalignant colonic lesions. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Agüeros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Casein nanoparticles as carriers for the oral delivery of folic acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef]
- Shapira, A.; Assaraf, Y.G.; Livney, Y.D. Beta-casein nanovehicles for oral delivery of chemotherapeutic drugs. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef]
- Umamaheshwari, R.; Ramteke, S.; Jain, N.K. Anti-Helicobacter pylori effect of mucoadhesive nanoparticles bearing amoxicillin in experimental gerbils model. Aaps Pharmscitech 2004, 5, 60–68. [Google Scholar] [CrossRef]
- Irache, J.M.; González-Navarro, C.J. Zein nanoparticles as vehicles for oral delivery purposes. Nanomedicine 2017, 12, 1209–1211. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef]
- He, J.; Fan, K.; Yan, X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release 2019, 311, 288–300. [Google Scholar] [CrossRef]
- Yin, S.; Davey, K.; Dai, S.; Liu, Y.; Bi, J. A critical review of ferritin as a drug nanocarrier: Structure, properties, comparative advantages and challenges. Particuology 2022, 64, 65–84. [Google Scholar] [CrossRef]
- Landreh, M.; Sahin, C.; Gault, J.; Sadeghi, S.; Drum, C.L.; Uzdavinys, P.; Drew, D.; Allison, T.M.; Degiacomi, M.T.; Marklund, E.G. Predicting the shapes of protein complexes through collision cross section measurements and database searches. Anal. Chem. 2020, 92, 12297–12303. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.M.; Schellekens, R.C.; Van Rieke, H.M.; Wanke, C.; Iordanov, V.; Stellaard, F.; Wutzke, K.D.; Dijkstra, G.; van der Zee, M.; Woerdenbag, H.J. Gastrointestinal pH and transit time profiling in healthy volunteers using the IntelliCap system confirms ileo-colonic release of ColoPulse tablets. PLoS ONE 2015, 10, e0129076. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Koneru, T.; McCord, E.; Pawar, S.; Tatiparti, K.; Sau, S.; Iyer, A.K. Transferrin: Biology and use in receptor-targeted nanotherapy of gliomas. ACS Omega 2021, 6, 8727–8733. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front. Immunol. 2021, 12, 583. [Google Scholar] [CrossRef]
- Tortorella, S.; Karagiannis, T.C. The significance of transferrin receptors in oncology: The development of functional nano-based drug delivery systems. Curr. Drug Deliv. 2014, 11, 427–443. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Mahdinloo, S.; Farshbaf, M.; Sarfraz, M.; Fatahi, Y.; Atyabi, F.; Valizadeh, H. Transferrin receptor-mediated liposomal drug delivery: Recent trends in targeted therapy of cancer. Expert Opin. Drug Deliv. 2022, 19, 685–705. [Google Scholar] [CrossRef]
- Montemiglio, L.C.; Testi, C.; Ceci, P.; Falvo, E.; Pitea, M.; Savino, C.; Arcovito, A.; Peruzzi, G.; Baiocco, P.; Mancia, F. Cryo-EM structure of the human ferritin–transferrin receptor 1 complex. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916. [Google Scholar]
- Lodhi, M.S.; Khan, M.T.; Bukhari, S.M.H.; Sabir, S.H.; Samra, Z.Q.; Butt, H.; Akram, M.S. Probing Transferrin Receptor Overexpression in Gastric Cancer Mice Models. ACS Omega 2021, 6, 29893–29904. [Google Scholar] [CrossRef]
- Widera, A.; Bai, Y.; Shen, W.-C. The transepithelial transport of a G-CSF-transferrin conjugate in Caco-2 cells and its myelopoietic effect in BDF1 mice. Pharm. Res. 2004, 21, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.M.; Mantaj, J.; Cheng, Y.; Vllasaliu, D. Delivery of nanoparticles across the intestinal epithelium via the transferrin transport pathway. Pharmaceutics 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Fan, Y.; Zheng, N.; He, B.; Yuan, L.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials 2013, 34, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin–nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef]
- Calisti, L.; Trabuco, M.C.; Boffi, A.; Testi, C.; Montemiglio, L.C.; Des Georges, A.; Benni, I.; Ilari, A.; Taciak, B.; Białasek, M. Engineered ferritin for lanthanide binding. PLoS ONE 2018, 13, e0201859. [Google Scholar] [CrossRef]
- Laurent, Q.; Martinent, R.; Lim, B.; Pham, A.-T.; Kato, T.; López-Andarias, J.; Sakai, N.; Matile, S. Thiol-Mediated Uptake. Jacs Au 2021, 1, 710–728. [Google Scholar] [CrossRef]
- Cheng, Y.; Pham, A.-T.; Kato, T.; Lim, B.; Moreau, D.; López-Andarias, J.; Zong, L.; Sakai, N.; Matile, S. Inhibitors of thiol-mediated uptake. Chem. Sci. 2021, 12, 626–631. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi, S.; Vallerinteavide Mavelli, G.; Vaidya, S.S.; Drum, C.L. Gastrointestinal Tract Stabilized Protein Delivery Using Disulfide Thermostable Exoshell System. Int. J. Mol. Sci. 2022, 23, 9856. https://doi.org/10.3390/ijms23179856
Sadeghi S, Vallerinteavide Mavelli G, Vaidya SS, Drum CL. Gastrointestinal Tract Stabilized Protein Delivery Using Disulfide Thermostable Exoshell System. International Journal of Molecular Sciences. 2022; 23(17):9856. https://doi.org/10.3390/ijms23179856
Chicago/Turabian StyleSadeghi, Samira, Girish Vallerinteavide Mavelli, Siddhesh Sujit Vaidya, and Chester Lee Drum. 2022. "Gastrointestinal Tract Stabilized Protein Delivery Using Disulfide Thermostable Exoshell System" International Journal of Molecular Sciences 23, no. 17: 9856. https://doi.org/10.3390/ijms23179856



