Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. X-ray Powder Diffraction
2.3. Transmission Fe Mössbauer Spectroscopy
2.4. Electron Microscopy
2.5. Adsorption Measurements
2.6. Magnetometry
3. Results
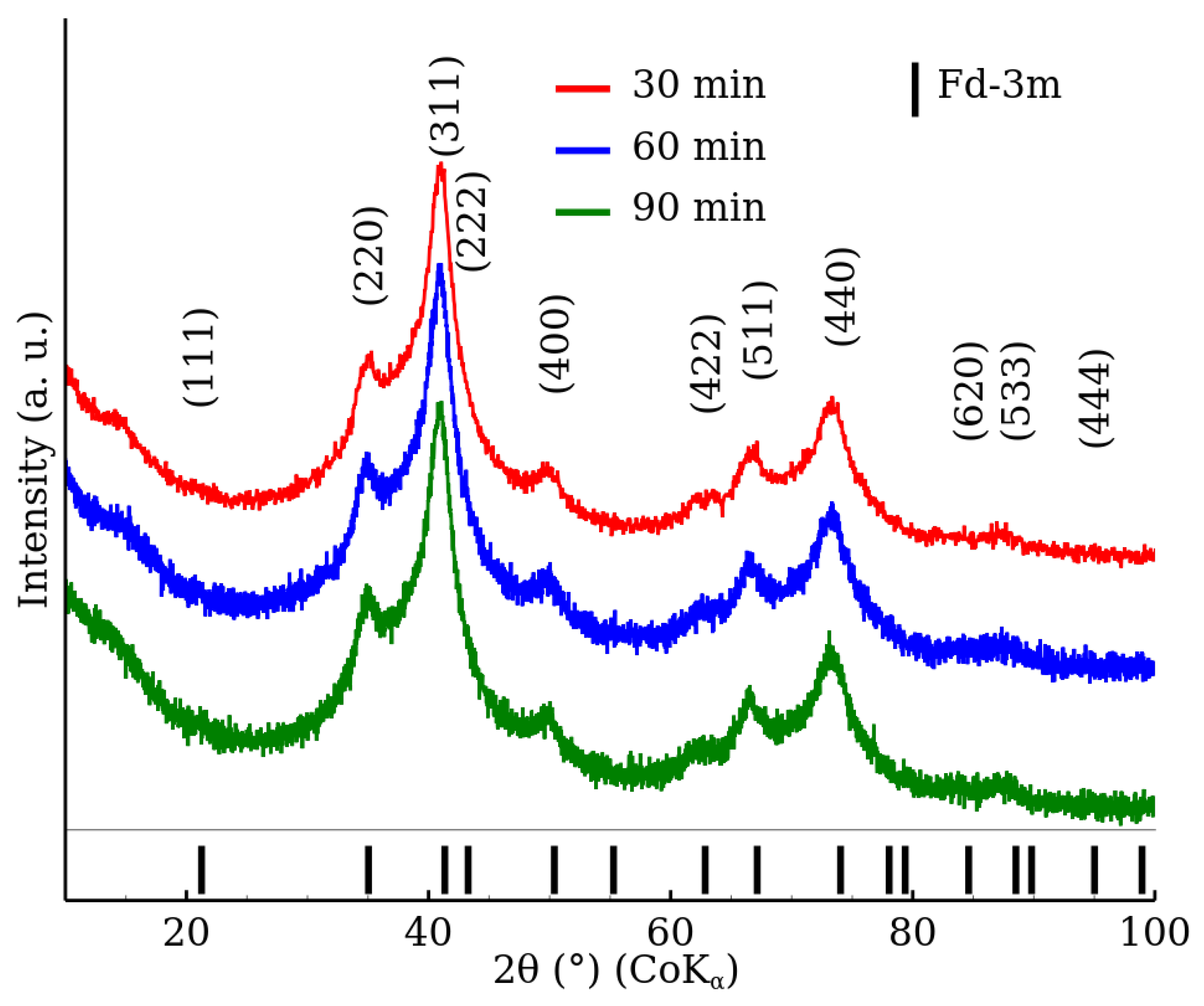
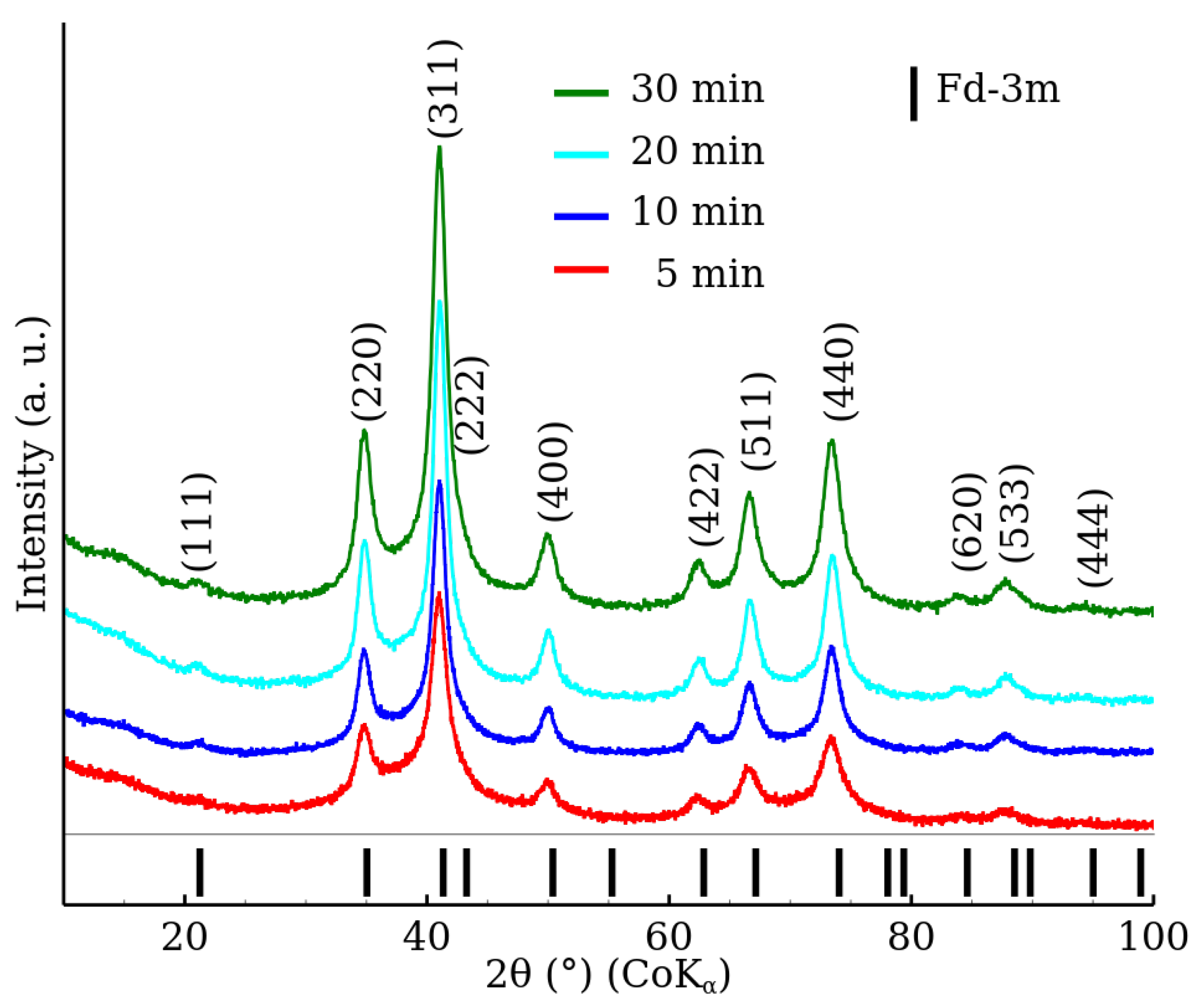
3.1. X-ray Powder Diffraction
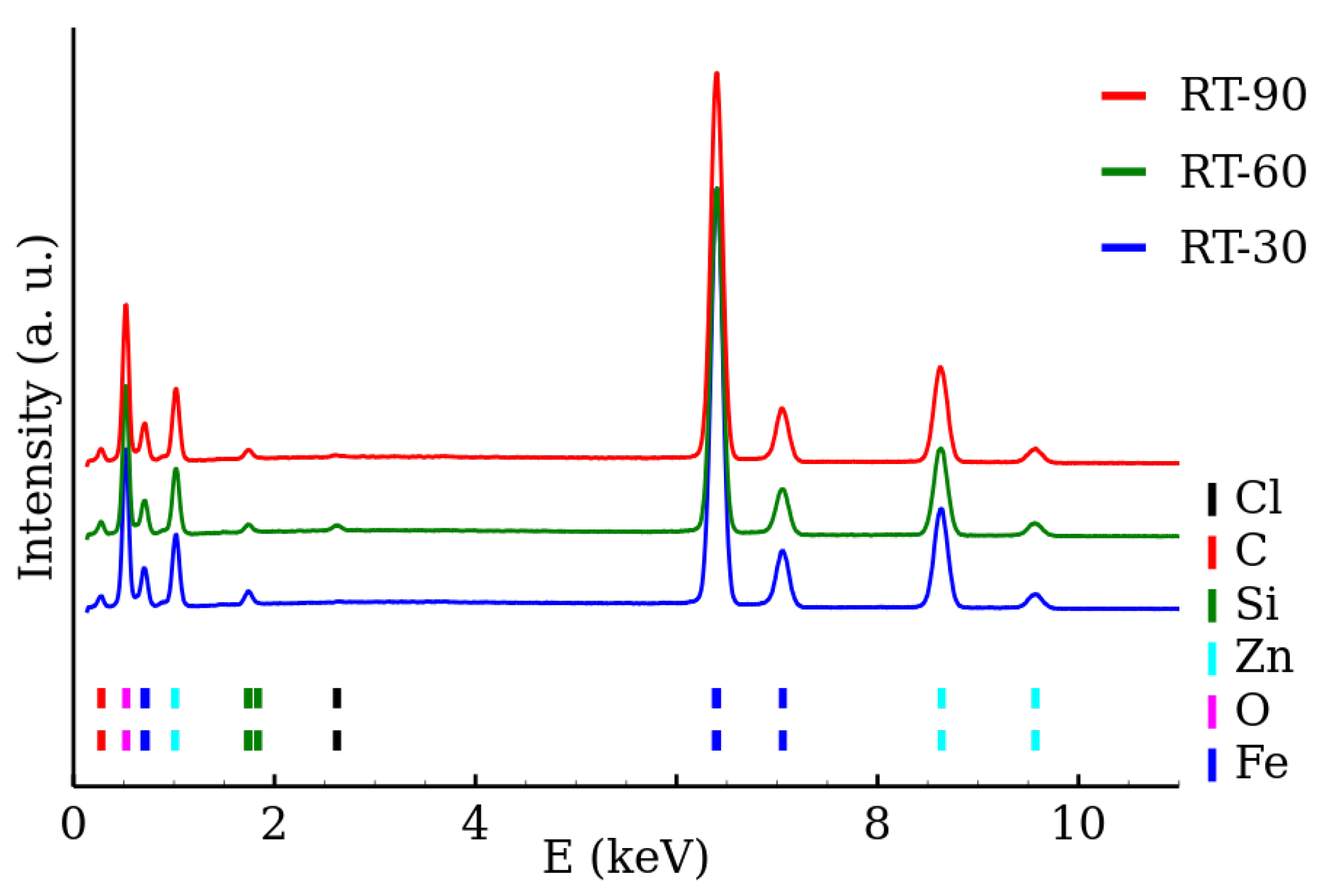
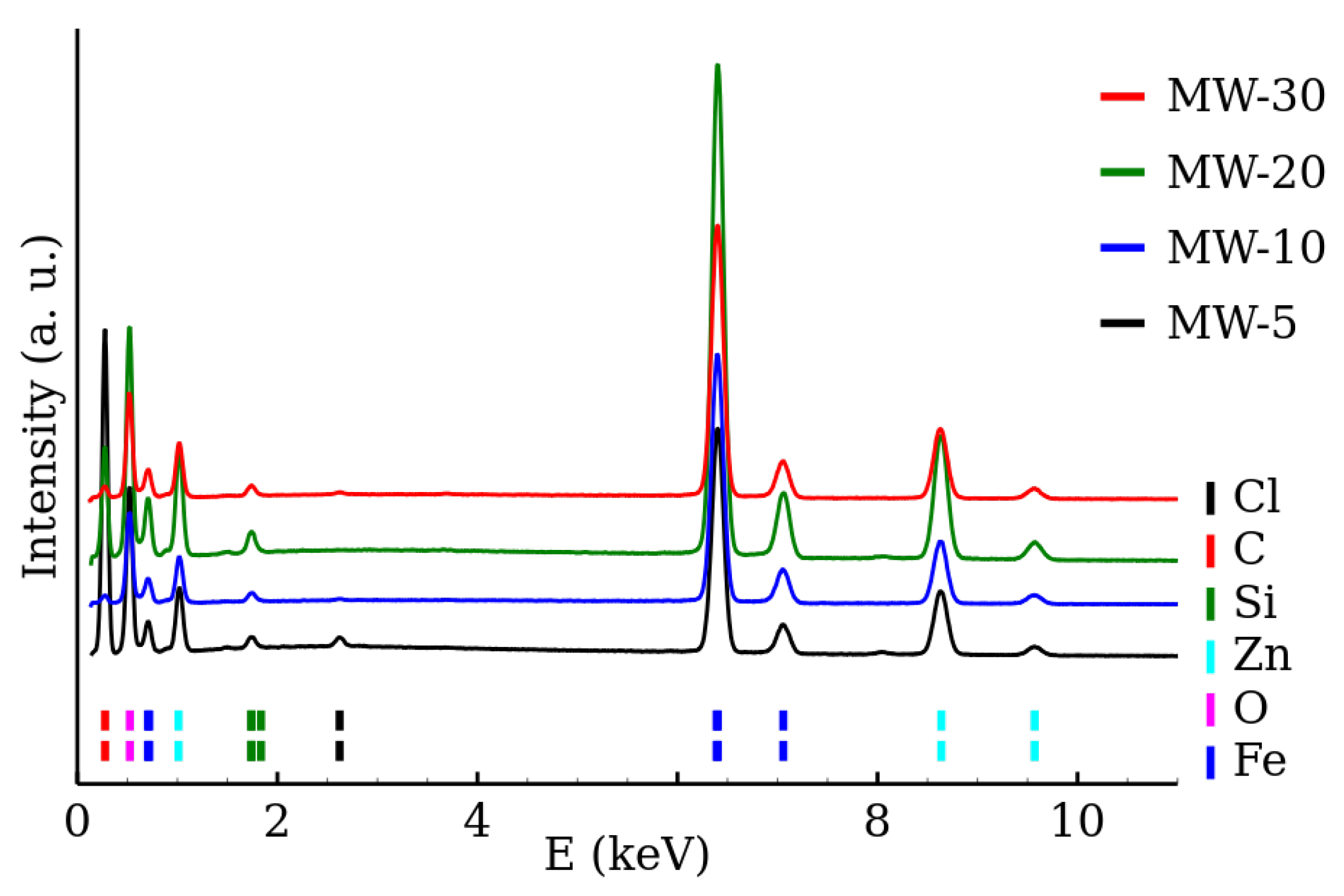
3.2. Transmission Electron Microscopy and Elemental Analysis
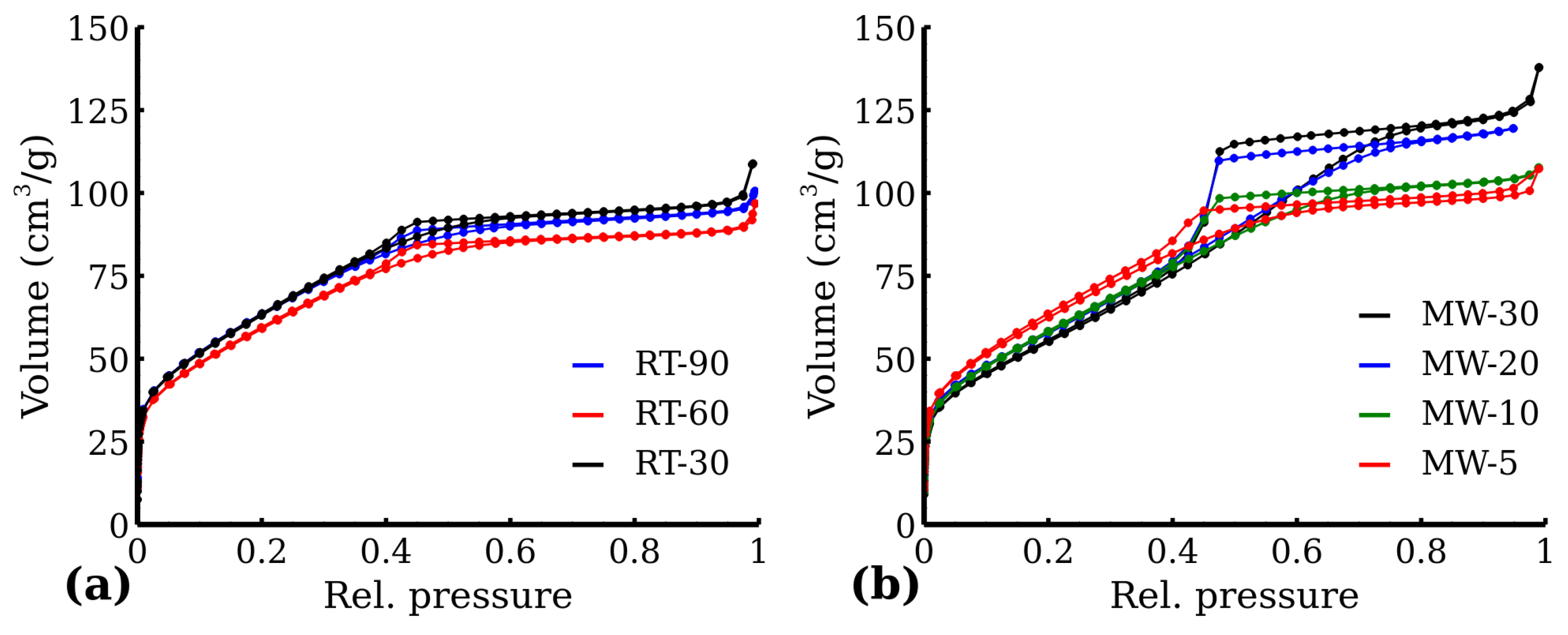
3.3. Specific Surface Area
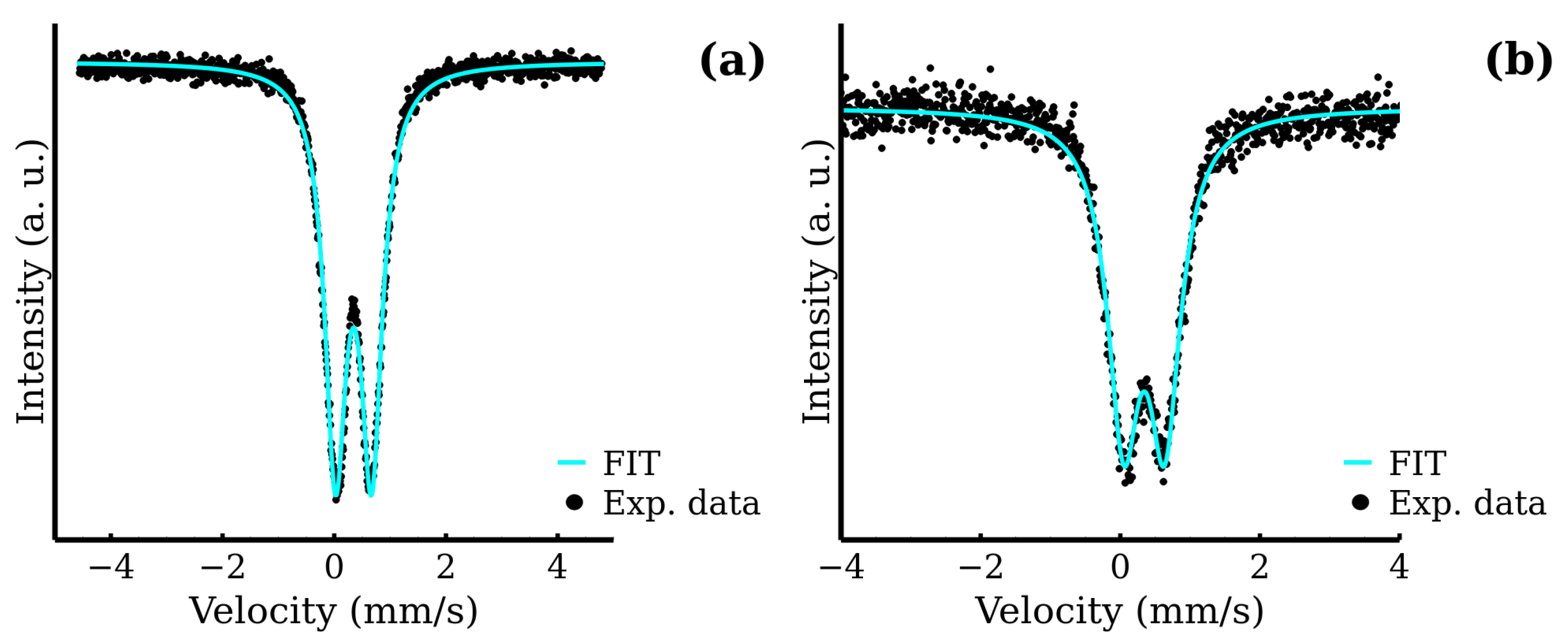
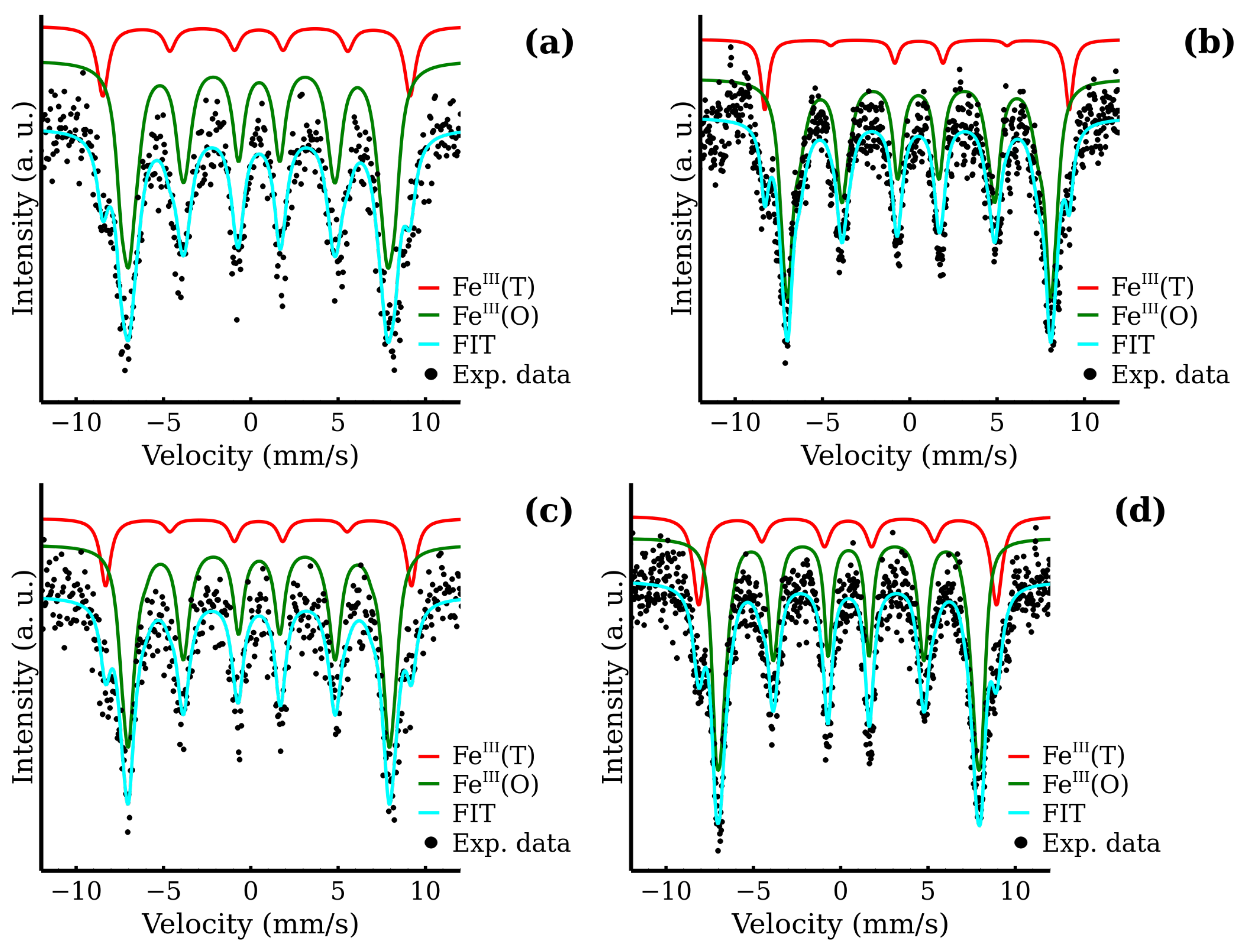
3.4. Mössbauer Spectroscopy as a Tool for Determination of Inversion Factor
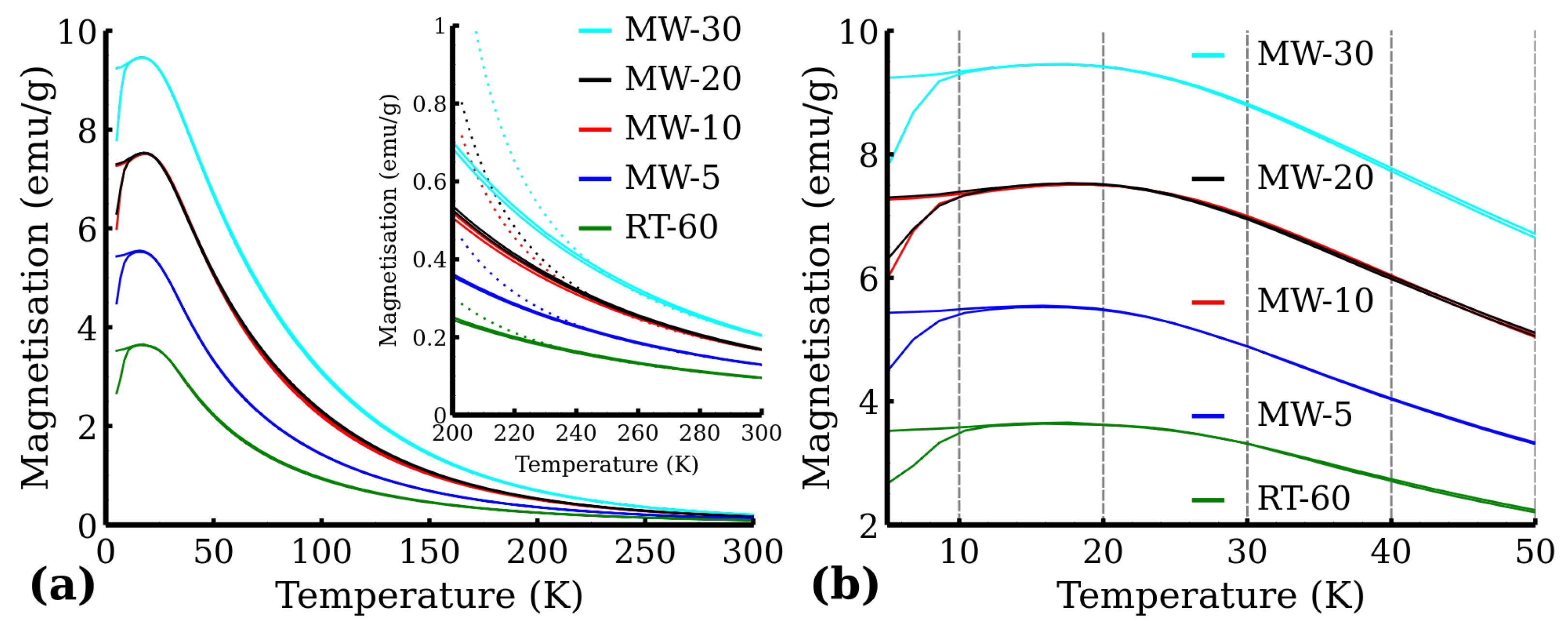
3.5. Magnetometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imran Din, M.; Rafique, F.; Hussain, M.S.; Arslan Mehmood, H.; Waseem, S. Recent Developments in the Synthesis and Stability of Metal Ferrite Nanoparticles. Sci. Prog. 2019, 102, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, V.; Shanmugavani, A.; Sanjeeviraja, C.; Kalai Selvan, R. Microwave Assisted Combustion Synthesis of CdFe2O4: Magnetic and Electrical Properties. J. Magn. Magn. Mater. 2012, 324, 2100–2107. [Google Scholar] [CrossRef]
- Naz, K.; Khan, J.K.; Khalid, M.; Akhtar, M.S.; Gilani, Z.A.; Noor ul Huda Khan Asghar, H.M.; Mersal, G.A.M.; Ibrahim, M.M.; Muhammad, A.; Ashiq, M.G.B. Structural, Dielectric, Impedance and Electric Modulus Analysis of Ni Substituted Copper Spinel Ferrites Nanoparticles for Microwave Device Applications. Mater. Chem. Phys. 2022, 285, 126091. [Google Scholar] [CrossRef]
- Faraz, A.; Ahmad, N.M. Electrical and Dielectric Characteristics of Magnetic Ceramics of Mn1-xNixFe2O4 Spinel Nanoferrites. Adv. Appl. Ceram. 2012, 111, 381–388. [Google Scholar] [CrossRef]
- Martinson, K.D.; Popkov, V.I. Two-step combustion synthesis of nanocrystalline Zn1-xMnxFe2O4 (0<=x<=1) spinel ferrites with linear tuning of magnetic parameters. Nanosyst. Phys. Chem. Math. 2021, 12, 634–640. [Google Scholar]
- Yadav, R.S.; Anju; Jamatia, T.; Kuřitka, I.; Vilčáková, J.; Škoda, D.; Urbánek, P.; Machovský, M.; Masař, M.; Urbánek, M.; et al. Superparamagnetic ZnFe2O4 Nanoparticles-Reduced Graphene Oxide-Polyurethane Resin Based Nanocomposites for Electromagnetic Interference Shielding Application. Nanomaterials 2021, 11, 1112. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; de la Peña O’Shea, V.A.; Keller, N. Ferrite Materials for Photoassisted Environmental and Solar Fuels Applications. Top. Curr. Chem. 2019, 378, 6. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Manikandan, A.; Ragu, R.; Dinesh, A.; Paulraj, P.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Madhavan, J.; Xavier, B.; et al. Exploring the Influence of Varying Ph on Structural, Electro-Optical, Magnetic and Photo-Fenton Properties of Mesoporous ZnFe2O4 Nanocrystals. Environ. Pollut. 2021, 272, 115983. [Google Scholar] [CrossRef] [PubMed]
- Borade, R.M.; Somvanshi, S.B.; Kale, S.B.; Pawar, R.P.; Jadhav, K.M. Spinel Zinc Ferrite Nanoparticles: An Active Nanocatalyst for Microwave Irradiated Solvent Free Synthesis of Chalcones. Mater. Res. Express 2020, 7, 016116. [Google Scholar] [CrossRef]
- Joe Sherin, J.F.; Bessy, T.C.; Asha, S.; Kumar, C.V.; Huessien, D.; Bindhu, M.R.; Rasheed, R.A.; Alarjani, K.M. Microwave Assisted Hydrothermally Synthesized Cobalt Doped Zinc Ferrites Nanoparticles for the Degradation of Organic Dyes and Antimicrobial Applications. Environ. Res. 2022, 208, 112687. [Google Scholar] [CrossRef]
- Rana, G.; Dhiman, P.; Kumar, A.; Vo, D.-V.N.; Sharma, G.; Sharma, S.; Naushad, M. Recent Advances on Nickel Nano-Ferrite: A Review on Processing Techniques, Properties and Diverse Applications. Chem. Eng. Res. Des. 2021, 175, 182–208. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Judith Vijaya, J.; John Kennedy, L. Spinel Ferrite Nanoparticles: Synthesis, Crystal Structure, Properties, and Perspective Applications. Springer Proc. Phys. 2017, 195, 305–325. [Google Scholar]
- Sreeja, V.; Vijayanand, S.; Deka, S.; Joy, P.A. Magnetic and Mössbauer Spectroscopic Studies of Nizn Ferrite Nanoparticles Synthesized by a Combustion Method. Hyperfine Interact. 2008, 183, 99–107. [Google Scholar] [CrossRef]
- Siddique, M.; Butt, N.M. Effect of Particle Size on Degree of Inversion in Ferrites Investigated by Mössbauer Spectroscopy. Phys. B Condens. Matter 2010, 405, 4211–4215. [Google Scholar] [CrossRef]
- Ashok, A.; Ratnaji, T.; John Kennedy, L.; Judith Vijaya, J.; Gnana Pragash, R. Magnetically Recoverable Mg Substituted Zinc Ferrite Nanocatalyst for Biodiesel Production: Process Optimization, Kinetic and Thermodynamic Analysis. Renew. Energy 2021, 163, 480–494. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.-N.; Choi, S.H.; Jang, J.-H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Diodati, S.; Walton, R.I.; Mascotto, S.; Gross, S. Low-Temperature Wet Chemistry Synthetic Approaches towards Ferrites. Inorg. Chem. Front. 2020, 7, 3282–3314. [Google Scholar] [CrossRef]
- Ochmann, M.; Linderhof, F.M.; Machala, L. Spinel Ferrites Nanoparticles for Alloy Steel Protective Layers. In Proceedings of the 12th International Conference on Nanomaterials—Research & Application (NANOCON), Brno, Czech Republic, 21–23 October 2020. [Google Scholar]
- Zhao, J.; Yang, L.; Chen, T.; Li, F. Magnetic Co1-xZnxFe2O4 Granular Films Fabricated via Layered Double Hydroxide Precursors. J. Phys. Chem. Solids 2012, 73, 1500–1504. [Google Scholar] [CrossRef]
- Koleva, K.; Velinov, N.; Tsoncheva, T.; Mitov, I. Mössbauer Study of Cu1-xZnxFe2O4 Catalytic Materials. Hyperfine Interact. 2013, 226, 89–97. [Google Scholar] [CrossRef]
- Sorescu, M.; Diamandescu, L.; Ramesh, P.D.; Roy, R.; Daly, A.; Bruno, Z. Evidence for Microwave-Induced Recrystallization in Nizn Ferrites. Mater. Chem. Phys. 2007, 101, 410–414. [Google Scholar] [CrossRef]
- Rahim, D.A.; Fang, W.; Zhu, G.; Wibowo, H.; Hantoko, D.; Hu, Q.; Susanto, H.; Gao, Z.; Yan, M. Microwave-Assisted Synthesis of Zn-Fe Adsorbent Supported on Alumina: Effect of Zn to Fe Ratio on Syngas Desulfurization Performance. Chem. Eng. Process. Process Intensif. 2021, 168, 108565. [Google Scholar] [CrossRef]
- Alcalá, O.; Briceño, S.; Brämer-Escamilla, W.; Silva, P. Toroidal Cores of Mnx Co1−xFe2 O4/PAA Nanocomposites with Potential Applications in Antennas. Mater. Chem. Phys. 2017, 192, 17–21. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Optical, Magnetic and Structural Properties of ZnFe2O4 Nanoparticles Synthesized by Conventional and Microwave Assisted Combustion Method: A Comparative Investigation. Optik 2017, 129, 57–68. [Google Scholar] [CrossRef]
- Manikandan, A.; Vijaya, J.J.; Mary, J.A.; Kennedy, L.J.; Dinesh, A. Structural, Optical and Magnetic Properties of Fe3O4 Nanoparticles Prepared by a Facile Microwave Combustion Method. J. Ind. Eng. Chem. 2014, 20, 2077–2085. [Google Scholar] [CrossRef]
- Sutka, A.; Mezinskis, G. Sol-Gel Auto-Combustion Synthesis of Spinel-Type Ferrite Nanomaterials. Front. Mater. Sci. 2012, 6, 128–141. [Google Scholar] [CrossRef]
- Wani, T.A.; Suresh, G. Plant-Mediated Green Synthesis of Magnetic Spinel Ferrite Nanoparticles: A Sustainable Trend in Nanotechnology. Adv. Sustain. Syst. 2022, 6, 2200035. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Yun, H.-S.; Kim, S.-Y.; Shim, J.-H.; Park, K.-J.; You, C.-Y.; Cho, N.-H. Effects of Microwave Irradiation on the Synthesis of Znfe2O4 Nanopowders with Hydrated Compounds. Sci. Adv. Mater. 2021, 13, 1125–1135. [Google Scholar] [CrossRef]
- Kore, E.K.; Shahane, G.S.; Mulik, R.N. Effect of ‘Zn’ Substitution on Structural, Morphological, Magnetic and Optical Properties of Co–Zn Ferrite Nanoparticles for Ferrofluid Application. J. Mater. Sci. Mater. Electron. 2022, 33, 9815–9829. [Google Scholar] [CrossRef]
- Ochmann, M.; Machala, L.; Kašlík, J. Microwave Synthesis of Magnetite Nanoparticles and Mg-Doped Magnetite Nanoparticles by Precipitation of Fe2+ Ions. J. Nanosci. Nanotechnol. 2021, 21, 5165–5173. [Google Scholar] [CrossRef]
- MAUD: Material Analysis Using Diffraction. Available online: http://maud.radiographema.eu/ (accessed on 24 June 2022).
- Palacky University Mössbauer. Available online: https://mossbauer.vtpup.cz/ (accessed on 24 June 2022).
- Klencsár, Z. Mössbauer Spectrum Analysis by Evolution Algorithm. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1997, 129, 527–533. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789048166336. [Google Scholar]
- Krishna, K.R.; Ravinder, D.; Kumar, K.V.; Lincon, C.A. Synthesis, XRD and SEM Studies of Zinc Substitution in Nickel Ferrites by Citrate Gel Technique. World J. Condens. Matter Phys. 2012, 2, 153–159. [Google Scholar] [CrossRef]
- Grasset, F.; Labhsetwar, N.; Li, D.; Park, D.C.; Saito, N.; Haneda, H.; Cador, O.; Roisnel, T.; Mornet, S.; Duguet, E.; et al. Synthesis and Magnetic Characterization of Zinc Ferrite Nanoparticles with Different Environments: Powder, Colloidal Solution, and Zinc Ferrite-Silica Core-Shell Nanoparticles. Langmuir 2002, 18, 8209–8216. [Google Scholar] [CrossRef]
- Mokhosi, S.R.; Mdlalose, W.; Nhlapo, A.; Singh, M. Advances in the Synthesis and Application of Magnetic Ferrite Nanoparticles for Cancer Therapy. Pharmaceutics 2022, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Kmječ, T.; Kohout, J.; Dopita, M.; Veverka, M.; Kuriplach, J. Mössbauer Spectroscopy of Triphylite (LiFePO4) at Low Temperatures. Condens. Matter 2019, 4, 86. [Google Scholar] [CrossRef] [Green Version]




| Sample | t | ||||
|---|---|---|---|---|---|
| (g) | (g) | (mL) | (min) | (W) | |
| RT-30 | 0.541 | 0.136 | 3 | 30 | 0 |
| RT-60 | 0.541 | 0.136 | 3 | 60 | 0 |
| RT-90 | 0.541 | 0.136 | 3 | 90 | 0 |
| MW-5 | 0.541 | 0.136 | 3 | 5 | 800 |
| MW-10 | 0.541 | 0.136 | 3 | 10 | 800 |
| MW-20 | 0.541 | 0.136 | 3 | 20 | 800 |
| MW-30 | 0.541 | 0.136 | 3 | 30 | 800 |
| Sample | Lattice Parameter (Å) | Mean Coherent Length (Å) |
|---|---|---|
| RT-30 | 8.49 | 28 |
| RT-60 | 8.49 | 29 |
| RT-90 | 8.49 | 29 |
| MW-5 | 8.47 | 62 |
| MW-10 | 8.47 | 78 |
| MW-20 | 8.46 | 86 |
| MW-30 | 8.47 | 77 |
| Fe | Zn | O | Si | Balance | |
|---|---|---|---|---|---|
| Sample | |||||
| (%) | (%) | (%) | (%) | (%) | |
| RT-30 | 27.3 | 13.5 | 57.7 | 1.5 | 0.0 |
| RT-60 | 25.8 | 12.5 | 60.5 | 1.0 | 0.2 |
| RT-90 | 25.2 | 12.7 | 60.9 | 1.0 | 0.2 |
| MW-5 | 19.7 | 10.1 | 68.3 | 1.0 | 0.9 |
| MW-10 | 26.6 | 13.0 | 58.5 | 1.6 | 0.2 |
| MW-20 | 25.1 | 12.9 | 60.1 | 1.7 | 0.2 |
| MW-30 | 25.9 | 13.0 | 59.1 | 1.6 | 0.3 |
| Sample | BET Area | Type of Isotherm |
|---|---|---|
| % | IUPAC | |
| (m g) | Classification | |
| RT-30 | 225 | I |
| RT-60 | 220 | I |
| RT-90 | 234 | I |
| MW-5 | 231 | I |
| MW-10 | 217 | I/IV |
| MW-20 | 214 | IV |
| MW-30 | 207 | IV |
| Sample | FWHM | RA | Identification | |||
|---|---|---|---|---|---|---|
| (mm s) | (mm s) | (mm s) | (mm s) | (%) | ||
| RT-30 | 0.34 | 0.65 | — | 0.45 | 100 | FeIII |
| RT-60 | 0.35 | 0.66 | — | 0.46 | 100 | FeIII |
| RT-90 | 0.35 | 0.67 | — | 0.48 | 100 | FeIII |
| MW-5 | 0.35 | 0.65 | — | 0.47 | 100 | FeIII |
| MW-5 | 0.39 | −0.16 | 52.6 | 0.91 | 33 | FeIII(T) |
| (5 , 5 ) | 0.45 | −0.10 | 46.0 | 0.62 | 67 | FeIII(O) |
| MW-10 | 0.35 | 0.64 | — | 0.47 | 100 | FeIII |
| MW-10 | 0.40 | −0.24 | 53.0 | 0.84 | 27 | FeIII(T) |
| (5 , 5 ) | 0.46 | −0.03 | 46.3 | 0.56 | 73 | FeIII(O) |
| MW-20 | 0.34 | 0.62 | — | 0.47 | 100 | FeIII |
| MW-20 | 0.41 | −0.12 | 52.8 | 0.76 | 30 | FeIII(T) |
| (5 , 5 ) | 0.46 | −0.04 | 46.3 | 0.52 | 70 | FeIII(O) |
| MW-30 | 0.34 | 0.59 | — | 0.55 | 100 | FeIII |
| MW-30 | 0.41 | −0.03 | 53.0 | 0.77 | 26 | FeIII(T) |
| (5 , 5 ) | 0.46 | 0.00 | 45.9 | 0.51 | 74 | FeIII(O) |
| Sample | Curie Constant | Weiss Constant | |
|---|---|---|---|
| (emu K Oe g) | (K) | () | |
| RT-60 | 10.5 | ||
| MW-5 | 11.9 | ||
| MW-10 | 13.1 | ||
| MW-20 | 12.9 | ||
| MW-30 | 13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochmann, M.; Vrba, V.; Kopp, J.; Ingr, T.; Malina, O.; Machala, L. Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles. Nanomaterials 2022, 12, 2987. https://doi.org/10.3390/nano12172987
Ochmann M, Vrba V, Kopp J, Ingr T, Malina O, Machala L. Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles. Nanomaterials. 2022; 12(17):2987. https://doi.org/10.3390/nano12172987
Chicago/Turabian StyleOchmann, Martin, Vlastimil Vrba, Josef Kopp, Tomáš Ingr, Ondřej Malina, and Libor Machala. 2022. "Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles" Nanomaterials 12, no. 17: 2987. https://doi.org/10.3390/nano12172987
APA StyleOchmann, M., Vrba, V., Kopp, J., Ingr, T., Malina, O., & Machala, L. (2022). Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles. Nanomaterials, 12(17), 2987. https://doi.org/10.3390/nano12172987