Ecofriendly Composite as a Promising Material for Highly-Performance Uranium Recovery from Different Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
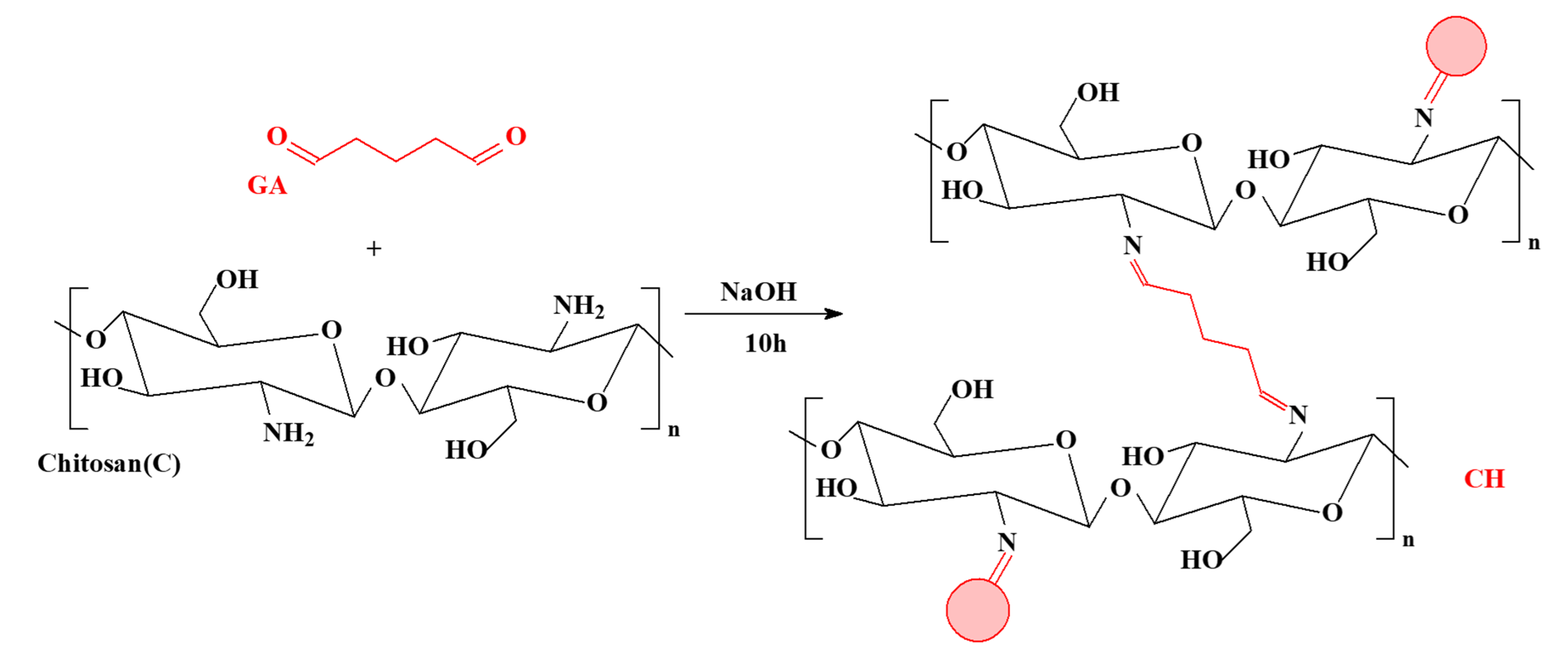
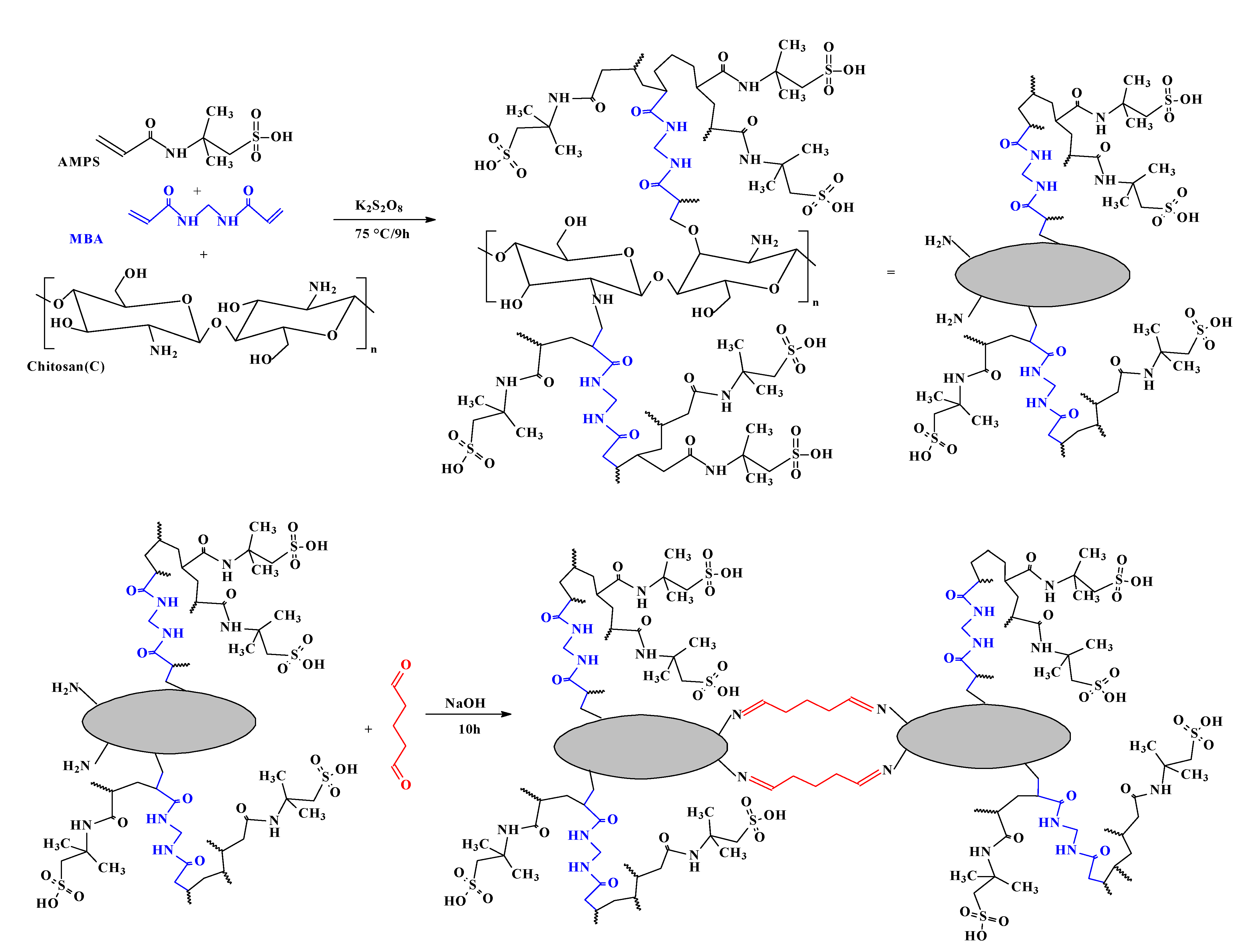
2.2. Synthesis of Sorbent
2.2.1. Synthesis of the Reference Material (Chitosan Crosslinked)
2.2.2. Synthesis of the Functionalized Chitosan Composite
2.3. Characterization of Materials
2.4. Sorption Procedures
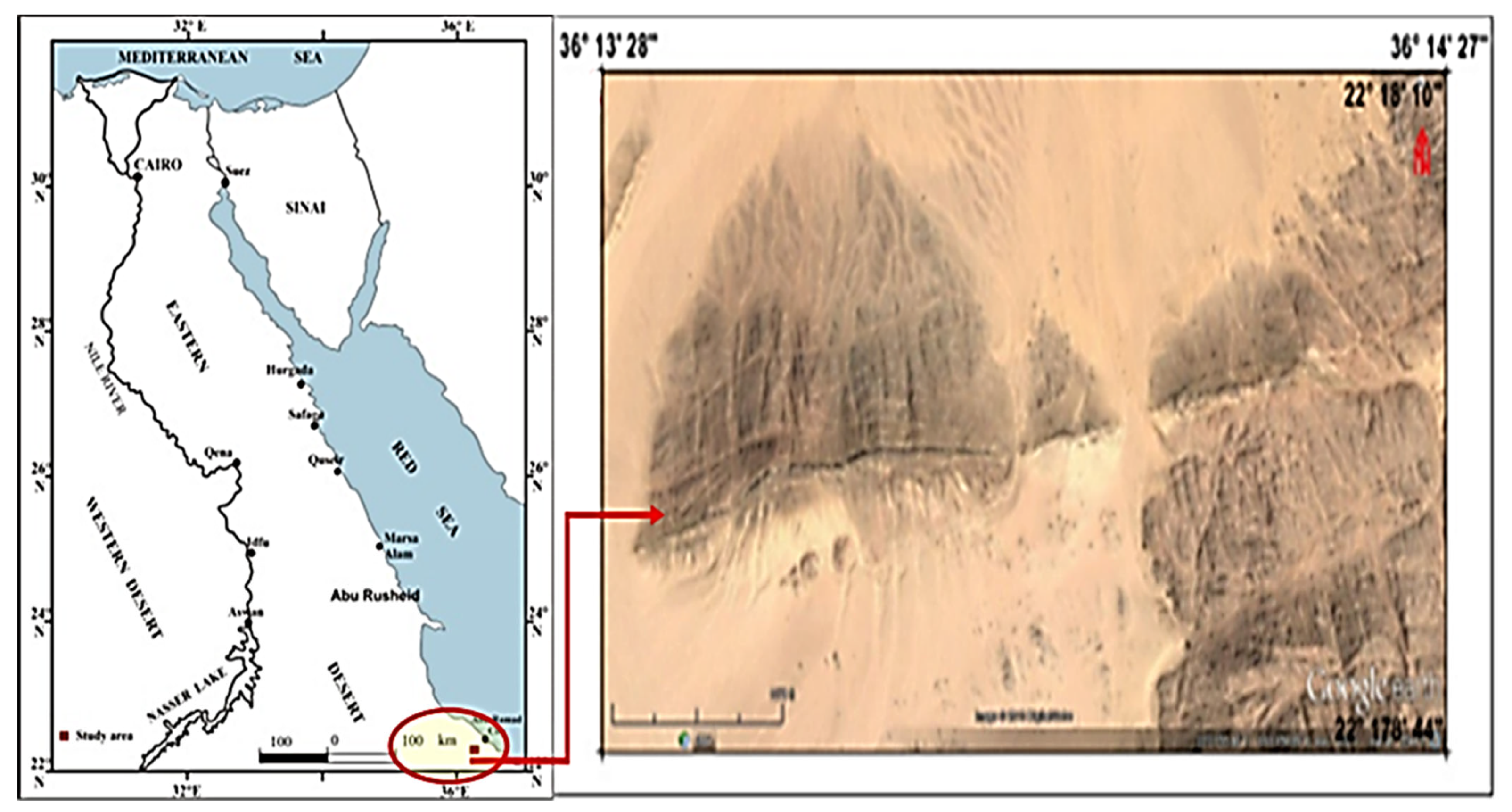
2.5. Ore Specification
2.6. Mineralogical Characteristics of the Studied Sample
2.7. Results of H2SO4 Agitation Leaching Process
3. Results and Discussion
3.1. Sorbent Characterization
3.1.1. Textural Properties
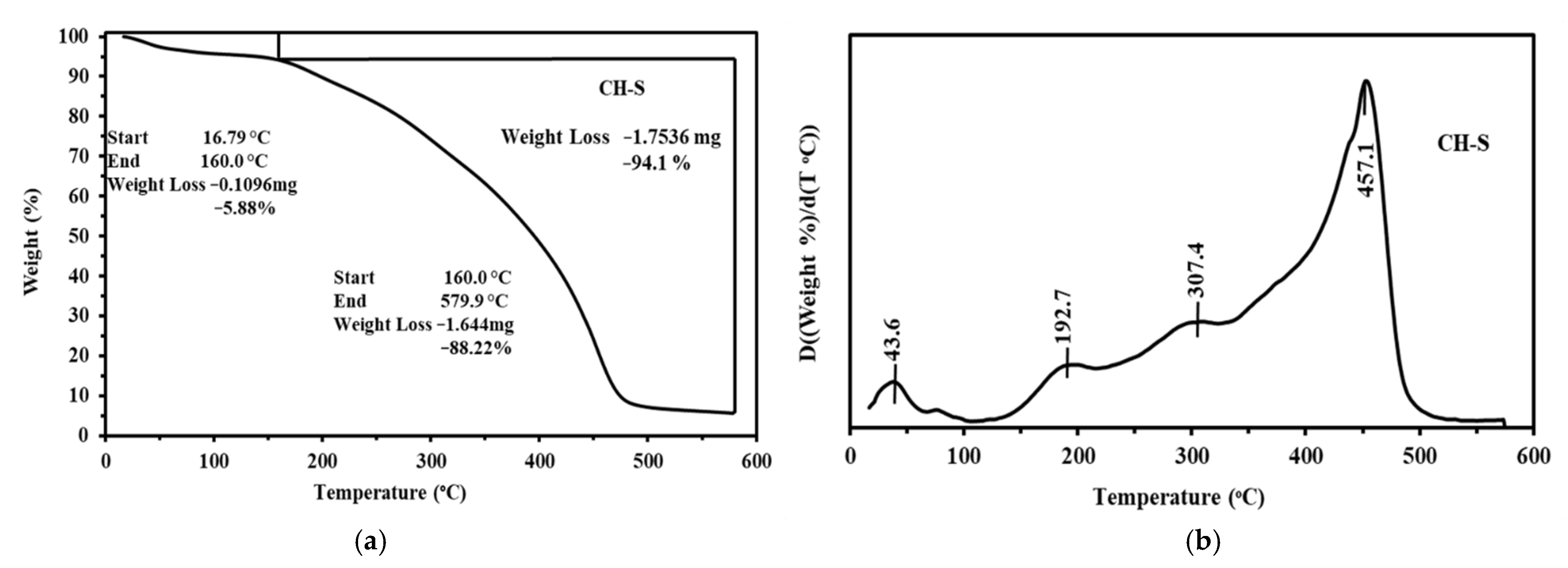
3.1.2. Thermogravimetric Analysis
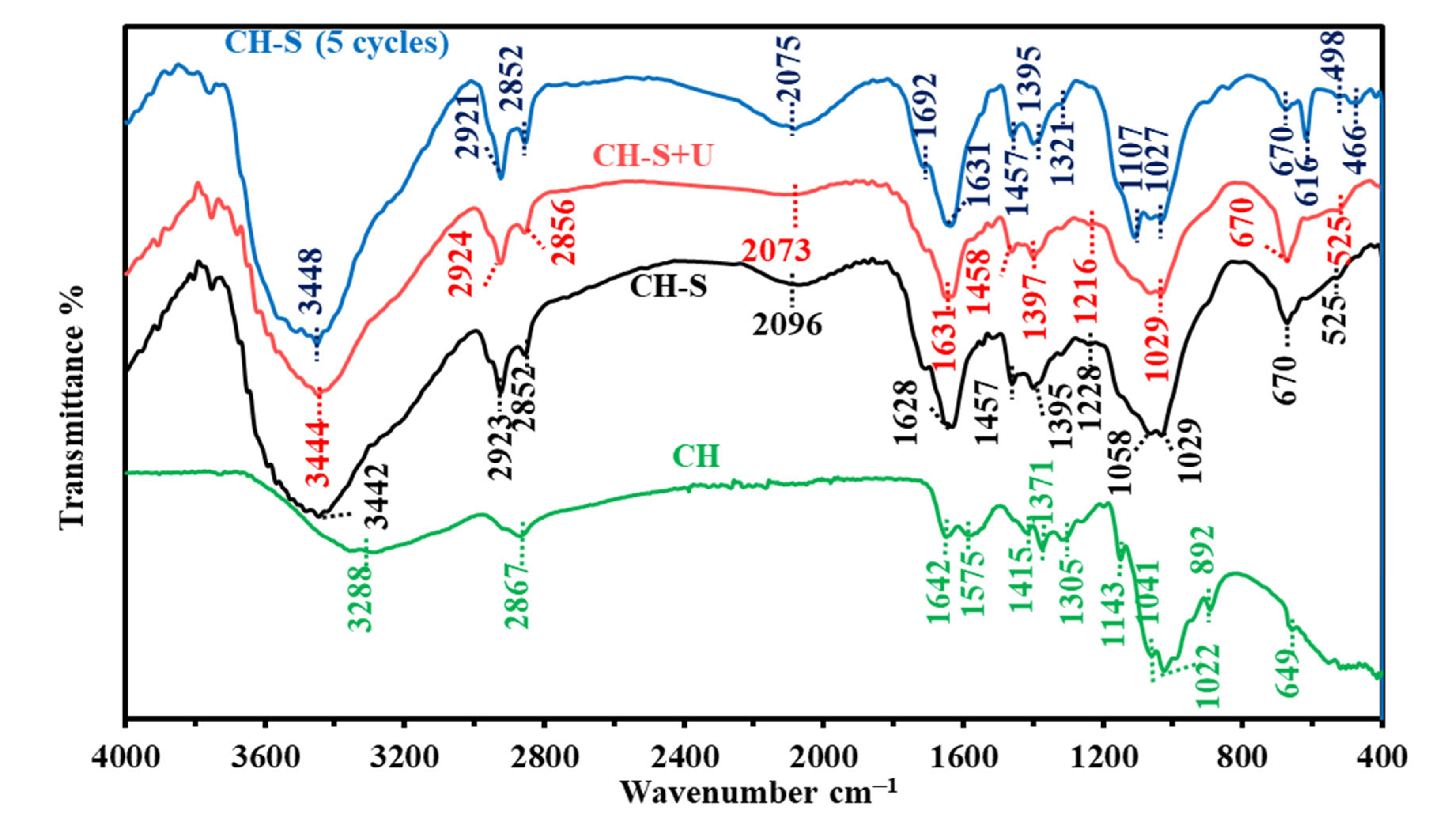
3.1.3. FTIR Spectroscopy
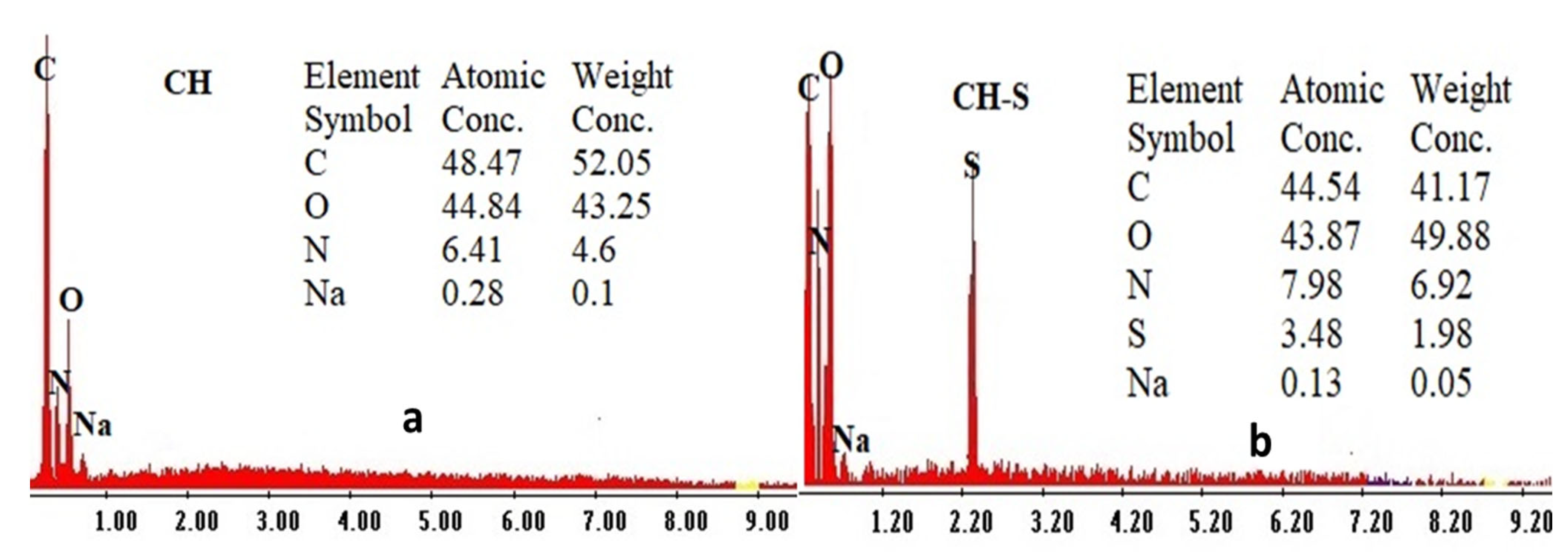
3.1.4. Elemental Analysis
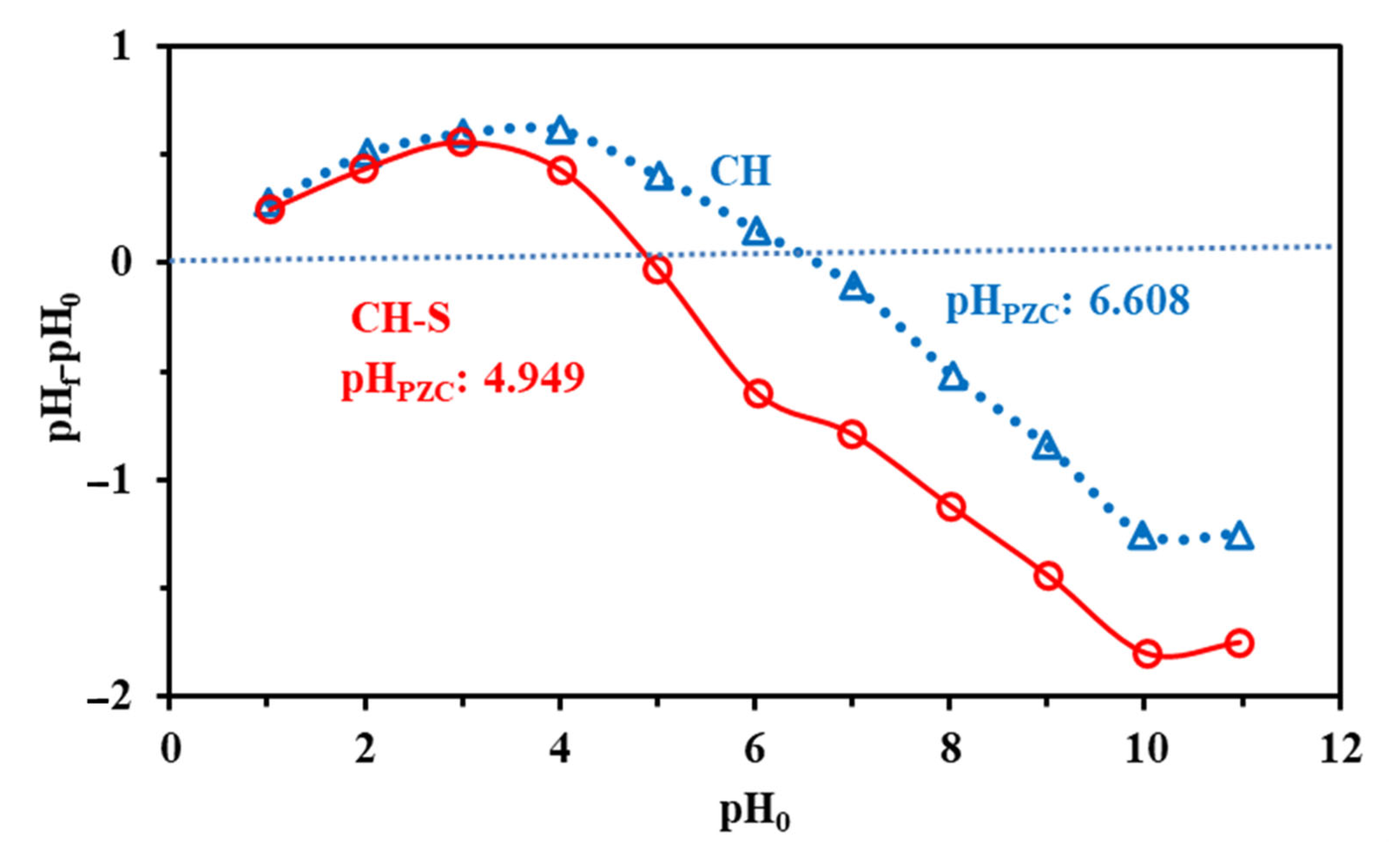
3.1.5. Surface Charge—pHPZC
3.2. Sorption Studies
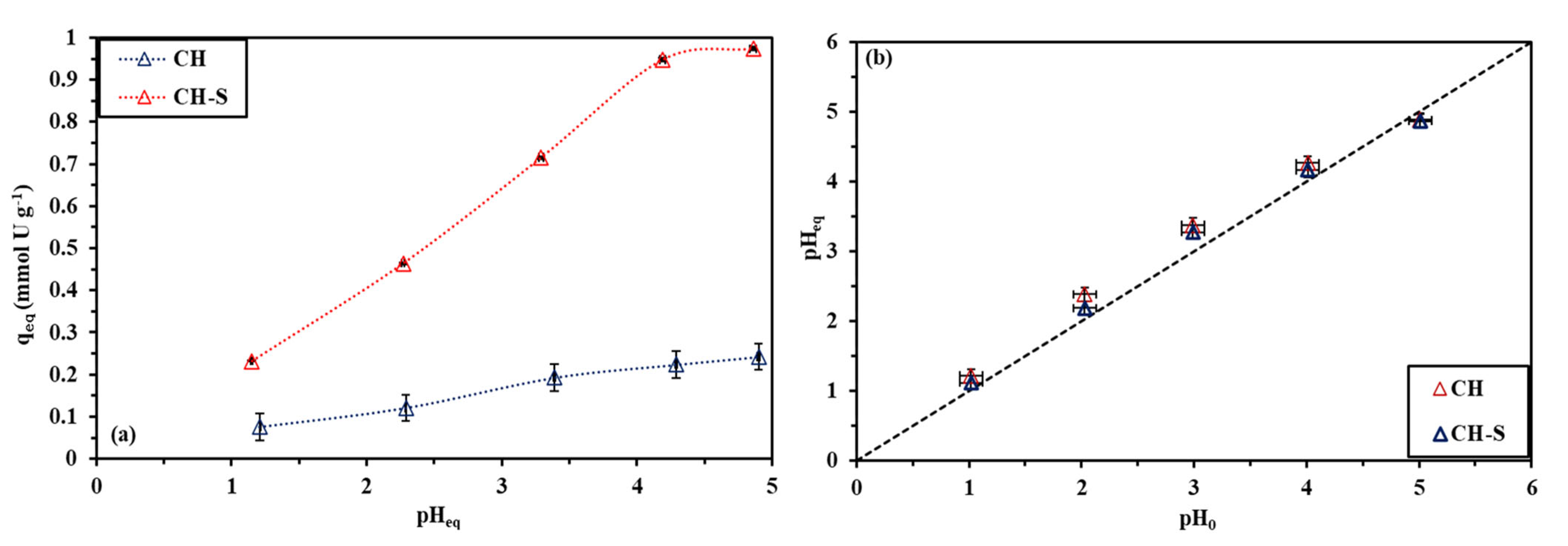
3.2.1. pH Effect
3.2.2. Uptake Kinetics
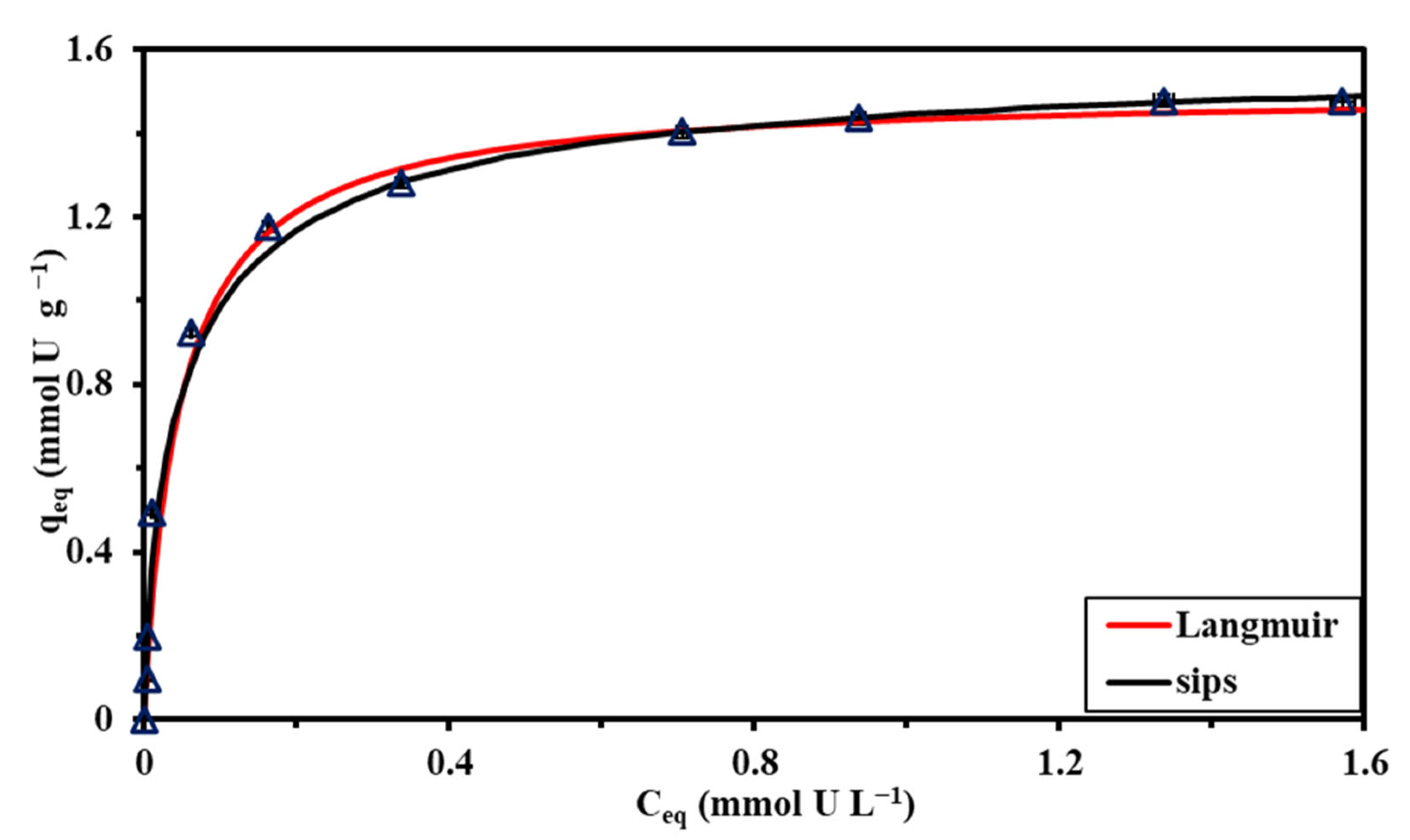
3.2.3. Sorption Isotherms
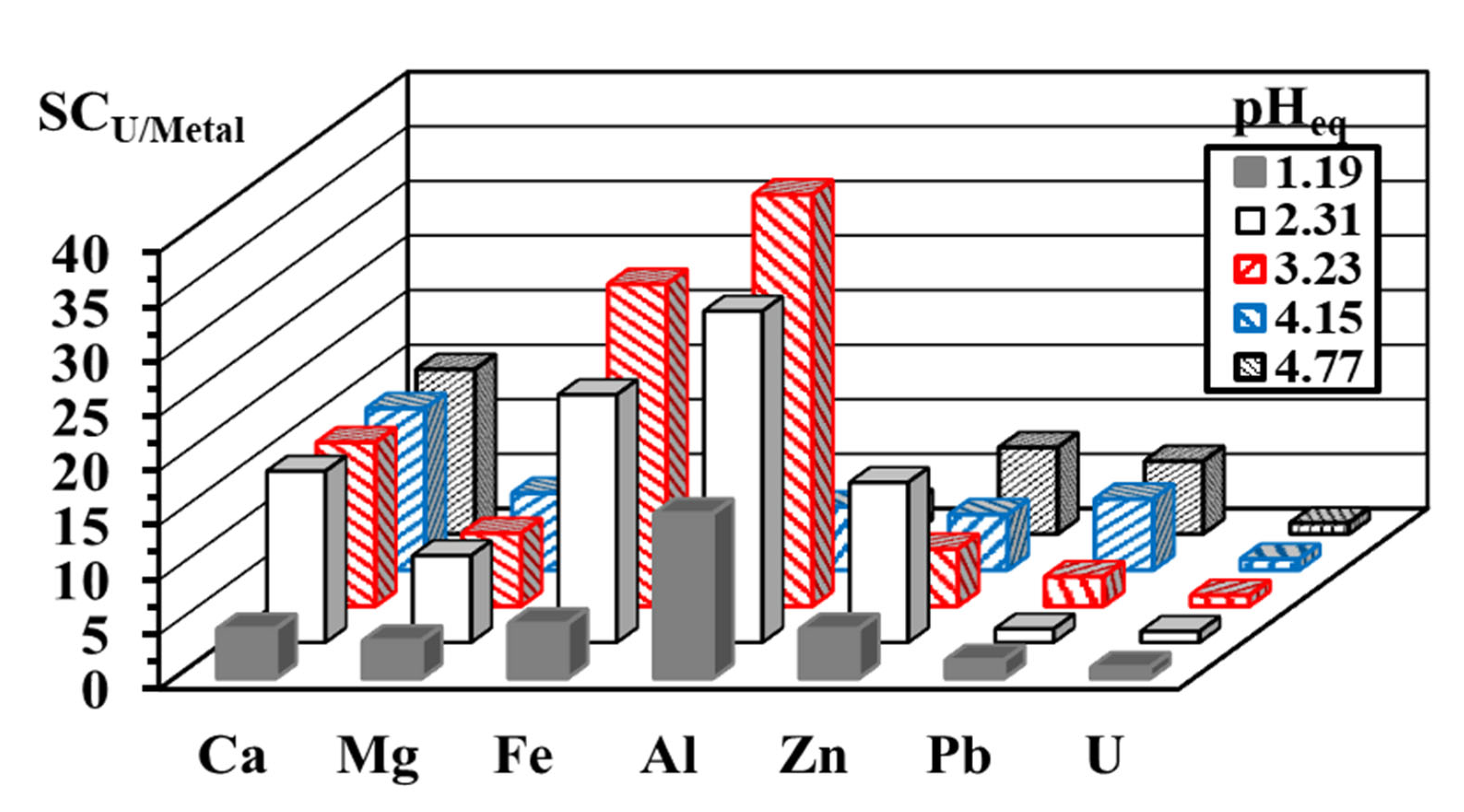
3.2.4. Sorption Mechanism
3.2.5. Metal Desorption and Recycling Properties
3.2.6. Results of the Uranium Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalafalla, M.S. Biotechnological recovery of uranium (VI) from Abu Zeneima spent ore residue using green lixiviant. J. Radioanal. Nucl. Chem. 2022, 331, 2503–2513. [Google Scholar] [CrossRef]
- Hamza, M.F. Uranium recovery from concentrated chloride solution produced from direct acid leaching of calcareous shale, Allouga ore materials, southwestern Sinai, Egypt. J. Radioanal. Nucl. Chem. 2018, 315, 613–626. [Google Scholar] [CrossRef]
- Hamza, M.F. Grafting of quaternary ammonium groups for uranium (VI) recovery: Application on natural acidic leaching liquor. J. Radioanal. Nucl. Chem. 2019, 322, 519–532. [Google Scholar] [CrossRef]
- Hamza, M.F.; Sallam, O.R.; Khalafalla, M.S.; Abbas, A.E.A.; Wei, Y. Geological and radioactivity studies accompanied by uranium recovery: Um Bogma Formation, southwestern Sinai, Egypt. J. Radioanal. Nucl. Chem. 2020, 324, 1039–1051. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd (II) and Pb (II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef] [PubMed]
- Dousti, Z.; Dolatyari, L.; Yaftian, M.R.; Rostamnia, S. Adsorption of Eu(III), Th(IV), and U(VI) by mesoporous solid materials bearing sulfonic acid and sulfamic acid functionalities. Sep. Sci. Technol. 2019, 54, 2609–2624. [Google Scholar] [CrossRef]
- Taha, M.H. Solid-liquid extraction of uranium from industrial phosphoric acid using macroporous cation exchange resins: MTC1600H, MTS9500, and MTS9570. Sep. Sci. Technol. 2020, 56, 1562–1578. [Google Scholar] [CrossRef]
- Ahmad, M.; Yang, K.; Li, L.; Fan, Y.; Shah, T.; Zhang, Q.; Zhang, B. Modified tubular carbon nanofibers for adsorption of uranium(VI) from water. ACS Appl. Nano Mater. 2020, 3, 6394–6405. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized biobased composite for metal decontamination–Insight on uranium and application to water samples collected from wells in mining areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967. [Google Scholar] [CrossRef]
- Chwastowski, J.; Staroń, P. Influence of Saccharomyces cerevisiae yeast cells immobilized on Cocos nucifera fibers for the adsorption of Pb (II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127735. [Google Scholar] [CrossRef]
- Ma, F.Q.; Dong, B.R.; Gui, Y.Y.; Cao, M.; Han, L.; Jiao, C.S.; Lv, H.T.; Hou, J.J.; Xue, Y. Adsorption of low-concentration uranyl ion by amidoxime polyacrylonitrile fibers. Ind. Eng. Chem. Res. 2018, 57, 17384–17393. [Google Scholar] [CrossRef]
- Wiechert, A.I.; Liao, W.-P.; Hong, E.; Halbert, C.E.; Yiacoumi, S.; Saito, T.; Tsouris, C. Influence of hydrophilic groups and metal-ion adsorption on polymer-chain conformation of amidoxime-based uranium adsorbents. J. Colloid Interface Sci. 2018, 524, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wongjaikham, W.; Wongsawaeng, D.; Hosemann, P. Synthesis of amidoxime polymer gel to extract uranium compound from seawater by UV radiation curing. J. Nucl. Sci. Technol. 2019, 56, 541–552. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime functionalization of algal/polyethyleneimine beads for the sorption of Sr (II) from aqueous solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef] [Green Version]
- Hamza, M.F.; Mubark, A.E.; Wei, Y.; Vincent, T.; Guibal, E. Quaternization of composite algal/PEI beads for enhanced uranium sorption—application to ore acidic leachate. Gels 2020, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Abu Khoziem, H.; Khalafalla, M.; Abdellah, W. Green recovery of uranium from Abu Zeneima mineralised carbonaceous shale, West Central Sinai, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–12. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Khalafalla, M.S.; Abed, N.S.; Fouda, A.; Elwakeel, K.Z.; Guibal, E.; Hamad, N.A. U (VI) and Th (IV) recovery using silica beads functionalized with urea-or thiourea-based polymers–Application to ore leachate. Sci. Total Environ. 2022, 821, 153184. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.; Zhou, K.; Wei, Y.; Khoziem, H.A.A.; Alotaibi, S.H.; Guibal, E. Effect of bi-functionalization of algal/polyethyleneimine composite beads on the enhancement of tungstate sorption: Application to metal recovery from ore leachate. Sep. Purif. Technol. 2022, 290, 120893. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mira, H.; Wei, Y.; Aboelenin, S.M.; Guibal, E.; Salem, W.M. Sulfonation of chitosan for enhanced sorption of Li (I) from acidic solutions–Application to metal recovery from waste Li-ion mobile battery. Chem. Eng. J. 2022, 441, 135941. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Abdel-Rahman, A.A.-H.; Negm, A.S.; Hamad, D.M.; Khalafalla, M.S.; Fouda, A.; Wei, Y.; Amer, H.H.; Alotaibi, S.H.; Goda, A.E.-S. Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment. Polymers 2022, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Chi, F.T.; Zhang, S.; Wen, J.; Xiong, J.; Hu, S. Control of pore chemistry in metal-organic frameworks for selective uranium extraction from seawater. Microporous Mesoporous Mater. 2019, 288, 109567. [Google Scholar] [CrossRef]
- Ang, K.L.; Li, D.; Nikoloski, A.N. The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 2. Chelating resins. Miner. Eng. 2018, 123, 8–15. [Google Scholar] [CrossRef]
- Hamza, M.F. Removal of uranium (VI) from liquid waste of calcareous shale, Allouga, southwestern Sinai, Egypt. Desalin. Water Treat. 2015, 54, 2530–2540. [Google Scholar] [CrossRef]
- Hamza, M.F.; El Aassy, I.E. Solid phase extraction of uranium removal from underground water, Wadi Naseib, Southwestern Sinai, Egypt. Desalin. Water Treat. 2014, 52, 331–338. [Google Scholar] [CrossRef]
- Aly, M.M.; Hamza, M.F. A review: Studies on uranium removal using different techniques. Overview. J. Dispers. Sci. Technol. 2013, 34, 182–213. [Google Scholar] [CrossRef]
- Zahra, M.H.; Hamza, M.F.; El-Habibi, G.; Abdel-Rahman, A.A.-H.; Mira, H.I.; Wei, Y.; Alotaibi, S.H.; Amer, H.H.; Goda, A.E.-S.; Hamad, N.A. Synthesis of a Novel Adsorbent Based on Chitosan Magnetite Nanoparticles for the High Sorption of Cr (VI) ions: A Study of Photocatalysis and Recovery on Tannery Effluents. Catalysts 2022, 12, 678. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Hamza, M.F.; El-Aassy, I.E.; Guibal, E. Integrated treatment of tailing material for the selective recovery of uranium, rare earth elements and heavy metals. Miner. Eng. 2019, 133, 138–148. [Google Scholar] [CrossRef]
- Hamza, M.F.; Khalafalla, M.S.; Wei, Y.; Hamad, N.A. Effect of bi-functionalization silica micro beads on uranium adsorption from synthetic and washing pregnant uranyl solutions. J. Radioanal. Nucl. Chem. 2021, 330, 191–206. [Google Scholar] [CrossRef]
- Lai, Y.C.; Lee, W.J.; Huang, K.L.; Wu, C.M. Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process. J. Hazard. Mater. 2008, 154, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Ahmed, F.Y.; El-Aassy, I.; Fouda, A.; Guibal, E. Groundwater purification in a polymetallic mining area (SW Sinai, Egypt) using functionalized magnetic chitosan particles. Water Air Soil Pollut. 2018, 229, 1–14. [Google Scholar]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (Kw). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.; Shehal-Deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Althumayri, K.; Fouda, A.; Hamad, N.A. Synthesis and Characterization of Functionalized Chitosan Nanoparticles with Pyrimidine Derivative for Enhancing Ion Sorption and Application for Removal of Contaminants. Materials 2022, 15, 4676. [Google Scholar] [CrossRef]
- Hamza, M.F.; Alotaibi, S.H.; Wei, Y.; Mashaal, N.M. High-Performance Hydrogel Based on Modified Chitosan for Removal of Heavy Metal Ions in Borehole: A Case Study from the Bahariya Oasis, Egypt. Catalysts 2022, 12, 721. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2020, 55, 4193–4212. [Google Scholar] [CrossRef]
- Hamza, M.F.; Gamal, A.; Hussein, G.; Nagar, M.S.; Abdel-Rahman, A.A.H.; Wei, Y.; Guibal, E. Uranium (VI) and zirconium (IV) sorption on magnetic chitosan derivatives–effect of different functional groups on separation properties. J. Chem. Technol. Biotechnol. 2019, 94, 3866–3882. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.I.; Abdel-Rahman, A.A.H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Haggag, E.S.A.; Khalafalla, M.S.; Masoud, A.M. Leaching kinetics of uranium, rare earth elements and copper using tartaric acid from El Allouga ore material, Southwestern Sinai, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–16. [Google Scholar] [CrossRef]
- Abdellah, W.M.; Khalafalla, M.S.; Abu Khoziem, H.A.; El Hussain, O.M. Physical and chemical processes of Abu Rusheid cataclastic rocks for recovering niobium, zirconium and uranium compounds. Physicochem. Probl. Miner. Process. 2021, 57, 137–152. [Google Scholar] [CrossRef]
- El Sheikh, R.; El Sheikh, E.M.; Khalafalla, M.S.; Mahfouz, L.I.; El-Gabry, M.M.; Gouda, A.A. Simultaneous preconcentration and determination of zirconium in environmental samples using ultrasound-assisted ionic liquid based dispersive liquid-liquid microextraction combined with spectrophotometry. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Khoziem, A.; Hanaa, A. Processing of Abu Dob mineralized pegmatites, Central Eastern Desert, Egypt: A study on the kinetics of dissolution process and extraction of some valuable metals. J. Radioanal. Nucl. Chem. 2022, 331, 937–951. [Google Scholar] [CrossRef]
- Yeswanth, S.; Sekhar, K.C.; Chaudhary, A.; Sarma, P. Anti-microbial and Anti-biofilm activity of a novel Dibenzyl (benzo d thiazol-2-yl (hydroxy) methyl) phosphonate by inducing protease expression in Staphylococcus aureus. Med. Chem. Res. 2018, 27, 785–795. [Google Scholar] [CrossRef]
- Davies, W.; Gray, U. A rapid and specific titrimetric method for the precise determination of uranium using iron (II) sulphate as reductant. Talanta 1964, 11, 1203–1211. [Google Scholar] [CrossRef]
- Mathew, K.; Bürger, S.; Vogt, S.; Mason, P.; Morales-Arteaga, M.; Narayanan, U. Uranium assay determination using Davies and Gray titration: An overview and implementation of GUM for uncertainty evaluation. J. Radioanal. Nucl. Chem. 2009, 282, 939–944. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Chapter 39—Rare-earth elements. In Analytical Spectroscopy Library; Marczenko, Z., Balcerzak, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 10, pp. 341–349. [Google Scholar]
- Marczenko, Z.; Balcerzak, M. Chapter 54—Uranium. In Analytical Spectroscopy Library; Marczenko, Z., Balcerzak, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 10, pp. 446–455. [Google Scholar]
- Ibrahim, M.; Zalata, A.; Assaf, H.; Ibrahim, I.; Rashed, M. El Sella Shear Zone, South Eastern Desert, Egypt. Example of vein type uranium deposit. In Proceedings of the 9th International Mining, Petroleum, and Metallurgical Engering Conference, Cairo University, Giza, Egypt, 21–24 February 2005; pp. 41–55. [Google Scholar]
- Ibrahim, T.; Amer, T.; Ali, K.; Omar, S. Uranium potentiality and its extraction from El Sela shear zone, south Eastern Desert Egypt. Sci. Fac. Sci. Minufia Univ. 2007, 21, 1–18. [Google Scholar]
- Ali, K.G. Structural control of El Sela granites and associated uranium deposits, Southern Eastern Desert, Egypt. Arab. J. Geosci. 2013, 6, 1753–1767. [Google Scholar] [CrossRef]
- Gaafar, I.M.; Aboelkhair, H.M.; Bayoumi, M.B. Integration of gamma-ray spectrometric and aster data for uranium exploration in Qash Amer-El-Sela area, Southeastern Desert, Egypt. Nucl. Sci. Sci. J. 2017, 6, 17–33. [Google Scholar] [CrossRef]
- Gawad, A.E.A.; Orabi, A.H.; Bayoumi, M.M. Uranium evaluation and its recovery from microgranite dike at G. El Sela area, South Eastern Desert, Egypt. Arab. J. Geosci. 2015, 8, 4565–4580. [Google Scholar] [CrossRef]
- Karim, M.A.; Gafaar, I.; El-Halim, A.; Hanfi, M.; El-Dine, N.W. Natural radioactivity and radiological implications of granite rocks, El-Sela area, Southeastern Desert, Egypt. J. Radioanal. Nucl. Chem. 2021, 330, 707–720. [Google Scholar] [CrossRef]
- Gaafar, I.; Cuney, M.; Gawad, A.A. Mineral chemistry of two-mica granite rare metals: Impact of geophysics on the distribution of uranium mineralization at El Sela shear zone, Egypt. Open J. Geol. 2014, 4, 137. [Google Scholar] [CrossRef]
- Shahin, H.; Bahige, M. Column Percolation Leaching of Uranium from El-Sela Area, South Eastern Desert, Egypt. J. Chem. 2016, 5, 32–41. [Google Scholar]
- Fan, G.; Liao, C.; Fang, T.; Luo, S.; Song, G. Amberlyst 15 as a new and reusable catalyst for the conversion of cellulose into cellulose acetate. Carbohydr. Polym. 2014, 112, 203–209. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.H.; Qian, G.F.; Huang, G.H.; Tang, X.Z.; Lin, B.F. Adsorption of Cu(II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef] [Green Version]
- Akkoz, Y.; Coskun, R.; Delibas, A. Preparation and characterization of sulphonated bio-adsorbent from waste hawthorn kernel for dye (MB) removal. J. Mol. Liq. 2019, 287, 11. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdel-Rahman, A.A.H. Extraction studies of some hazardous metal ions using magnetic peptide resins. J. Dispers. Sci. Technol. 2015, 36, 411–422. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)—Hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grondahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 1–23. [Google Scholar]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nistico, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stabil. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Caetano, C.S.; Caiado, M.; Farinha, J.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of free fatty acids over chitosan with sulfonic acid groups. Chem. Eng. J. 2013, 230, 567–572. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Urbano, B.; Rivas, B.L. Poly(sodium 4-styrene sulfonate) and poly(2-acrylamido glycolic acid) polymer–clay ion exchange resins with enhanced mechanical properties and metal ion retention. Polym. Int. 2012, 61, 23–29. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 45. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Amesh, P.; Venkatesan, K.A.; Suneesh, A.S.; Samanta, N. Diethylenetriamine tethered mesoporous silica for the sequestration of uranium from aqueous solution and seawater. J. Environ. Chem. Eng. 2020, 8, 103995. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Alphantoniou, E.; Terpilowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A.; et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef]
- He, D.X.; Tan, N.; Luo, X.M.; Yang, X.C.; Ji, K.; Han, J.W.; Chen, C.; Liu, Y.Q. Preparation, uranium (VI) absorption and reuseability of marine fungus mycelium modified by the bis-amidoxime-based groups. Radiochim. Acta 2020, 108, 37–49. [Google Scholar] [CrossRef]
- Nezhad, M.M.; Semnani, A.; Tavakkoli, N.; Shirani, M. Efficient removal and recovery of uranium from industrial radioactive wastewaters using functionalized activated carbon powder derived from zirconium carbide process waste. Environ. Sci. Pollut. Res. 2021, 28, 57073–57089. [Google Scholar] [CrossRef] [PubMed]
- Zidan, I.H.; Cheira, M.F.; Bakry, A.R.; Atia, B.M. Potentiality of uranium recovery from G.Gattar leach liquor using Duolite ES-467 chelating resin: Kinetic, thermodynamic and isotherm features. Int. J. Environ. Anal. Chem. 2020, 102, 2102–2124. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, D.; Zhang, Q.; Wang, Y.; Liu, Y.; Zhao, J.; Chen, B. Highly efficient removal of uranium from highly acidic media achieved using a phosphine oxide and amino functionalized superparamagnetic composite polymer adsorbent. J. Mater. Chem. A 2020, 8, 10925–10934. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, M.; Zhang, M.; Wang, M.; Chen, J.; Li, R.; Qiu, L.; Feng, X.; Hu, J.; Wu, G. Efficient removal of uranium from diluted aqueous solution with hydroxypyridone functionalized polyethylene nonwoven fabrics. Radiat. Phys. Chem. 2020, 171, 108742. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, N.; Dai, Y.; Wang, B.; Liu, Y.; Chen, C.; Huang, D. Effective biosorption of uranium from aqueous solution by cyanobacterium Anabaena Flos-aquae. Environ. Sci. Pollut. Res. 2020, 27, 44306–44313. [Google Scholar] [CrossRef]
- Yousef, L.A.; Bakry, A.R.; Ahmad, A.A. Uranium(VI) recovery from acidic leach liquor using manganese oxide coated zeolite (MOCZ) modified with amine. J. Radioanal. Nucl. Chem. 2020, 324, 409–421. [Google Scholar] [CrossRef]
- Xu, Z.; Xing, Y.; Ren, A.; Ma, D.; Li, Y.; Hu, S. Study on adsorption properties of water hyacinth-derived biochar for uranium (VI). J. Radioanal. Nucl. Chem. 2020, 324, 1317–1327. [Google Scholar] [CrossRef]
- Wen, Z.; Huang, K.; Niu, Y.; Yao, Y.; Wang, S.; Cao, Z.; Zhong, H. Kinetic study of ultrasonic-assisted uranium adsorption by anion exchange resin. Colloids Surf. A 2020, 585, 124021. [Google Scholar] [CrossRef]
- Tuzen, M.; Saleh, T.A.; Sari, A. Naeemullah Interfacial polymerization of trimesoyl chloride with melamine and palygorskite for efficient uranium ions ultra-removal. Chem. Eng. Res. Des. 2020, 159, 353–361. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, L.; Le, Z.; Wang, Y.; Liu, Z.; Huang, G.; Adesina, A.A. Preparation of porous chitosan/carboxylated carbon nanotube composite aerogels for the efficient removal of uranium(VI) from aqueous solution. Int. J. Biol. Macromol. 2020, 160, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Basu, H.; Rout, S.; Pimple, M.V.; Singhal, R.K. Nano-hydroxyapatite coated activated carbon impregnated alginate: A new hybrid sorbent for uranium removal from potable water. J. Environ. Chem. Eng. 2020, 8, 103999. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Zhao, Y.; Chen, Y.; Tang, S. The removal of uranium using novel temperature sensitive urea-formaldehyde resin: Adsorption and fast regeneration. Sci. Total Environ. 2020, 735, 139399. [Google Scholar] [CrossRef]
- Lu, W.; Dai, Z.; Li, L.; Liu, J.; Wang, S.; Yang, H.; Cao, C.; Liu, L.; Chen, T.; Zhu, B.; et al. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium(VI). J. Mol. Liq. 2020, 303, 112604. [Google Scholar] [CrossRef]
- Liang, L.; Lin, X.; Liu, Y.; Sun, S.; Chu, H.; Chen, Y.; Liu, D.; Luo, X.; Zhang, J.; Shang, R. Carboxymethyl konjac glucomannan mechanically reinforcing gellan gum microspheres for uranium removal. Int. J. Biol. Macromol. 2020, 145, 535–546. [Google Scholar] [CrossRef]
- Kolhe, N.; Zinjarde, S.; Acharya, C. Removal of uranium by immobilized biomass of a tropical marine yeast Yarrowia lipolytica. J. Environ. Radioact. 2020, 223–224, 106419. [Google Scholar] [CrossRef]
- Basu, H.; Pimple, M.V.; Saha, S.; Patel, A.; Dansena, C.; Singhal, R.K. TiO2 microsphere impregnated alginate: A novel hybrid sorbent for uranium removal from aquatic bodies. New J. Chem. 2020, 44, 3950–3960. [Google Scholar] [CrossRef]
- Shelar-Lohar, G.; Joshi, S. Comparative study of uranium and thorium metal ion adsorption by gum ghatti grafted poly(acrylamide) copolymer composites. RSC Adv. 2019, 9, 41326–41335. [Google Scholar] [CrossRef] [Green Version]
- Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Hosseini, S.H.; Kharghani, K.; Zarei, H.; Rastegar, A. Kinetic, equilibrium and thermodynamic studies on sorption of uranium and thorium from aqueous solutions by a selective impregnated resin containing carminic acid. J. Hazard. Mater. 2015, 286, 152–163. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Liu, T.; Wu, W. Recovery of uranium(VI) from aqueous solution by 2-picolylamine functionalized polystyrene-co-maleic anhydride) resin. J. Colloid Interface Sci. 2017, 497, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Li, L.; Kong, F.; Lin, S.; Wang, Z.; Li, W. Preparation of three-dimensional fiber-network chitosan films for the efficient treatment of uranium-contaminated effluents. Water Sci. Technol. 2020, 81, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ma, J.; Shi, Y.; Du, Z.; Zhao, Y.; Tuo, X.; Leng, Y. Uranium(VI) adsorption on montmorillonite colloid. J. Radioanal. Nucl. Chem. 2020, 324, 541–549. [Google Scholar] [CrossRef]
- Salah, B.A.; Gaber, M.S.; Kandil, A.H.T. The removal of uranium and thorium from their aqueous solutions by 8-hydroxyquinoline immobilized bentonite. Minerals 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Yousef, L.A.; Ahmad, A.A.; Bakry, A.R. Separation of uranium ions from acetate medium by Dowex50WX8/Alizarin Red-S and its application on granitic samples, South Um Tawat, Eastern Desert. Int. J. Environ. Anal. Chem. 2020, 8, 667–681. [Google Scholar] [CrossRef]
- Orabi, A.H.; Abdelhamid, A.E.-S.; Salem, H.M.; Ismaiel, D.A. New adsorptive composite membrane from recycled acrylic fibers and Sargassum dentifolium marine algae for uranium and thorium removal from liquid waste solution. J. Radioanal. Nucl. Chem. 2020, 326, 1233–1247. [Google Scholar] [CrossRef]
- Liu, H.-J.; Jing, P.-F.; Liu, X.-Y.; Du, K.-J.; Sun, Y.-K. Synthesis of beta-cyclodextrin functionalized silica gel and its application for adsorption of uranium(VI). J. Radioanal. Nucl. Chem. 2016, 310, 263–270. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. MCM-41 silica particles grafted with polyacrylonitrile: Modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater. 2016, 226, 117–124. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Elwakeel, K.Z.; Wei, Y.; Guibal, E.; Hamad, N.A. Phosphorylation of guar gum/magnetite/chitosan nanocomposites for uranium (VI) sorption and antibacterial applications. Molecules 2021, 26, 1920. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Amer. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar]
- Freundlich, H.M.F. Uber die adsorption in lasungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Newton, MA, USA, 1994; p. 243. [Google Scholar]
- Kegl, T.; Kosak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nano-materials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M.J. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical ad-sorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]



| Major Oxides (%) | Wt. (%) | Trace Elements | ppm |
|---|---|---|---|
| SiO2 | 76.59 | U | 1158 |
| Al2O3 | 8.6 | REE | 1330 |
| TiO2 | 1.03 | Th | 16 |
| Fe2O3total | 3.76 | Mn | 36 |
| CaO | 1.22 | V | 48 |
| MgO | 0.46 | Zn | 79 |
| Na2O | 0.42 | Pb | 139 |
| K2O | 1.27 | Zr | 177 |
| P2O5 | 0.51 | Hf | 5.4 |
| L.O.I | 5.7 | Nb | 58 |
| Total | 99.56 | Ta | 2.7 |
| Assignment | CH | CH-S | CH-S + U | CH-S (5th Cycle) | Ref. |
|---|---|---|---|---|---|
| O-H and N-H (stretching) | 3288 | 3442 | 3444 | 3448 | [65,66] |
| C-H (stretching) | 2867 | 2923, 2852 | 2924, 2856 | 2921, 2852 | [66,67] |
| -NCS group | 2096 | 2073 | 2075 | [68] | |
| C=O (stretching) and NH of amide | 1642, 1575 | 1628 | 1631 | 1692, 1631 | [66,69] |
| -CH2 (bending) and C-N (stretching) | 1415 | 1457 | 1458 | 1457 | [66,69] |
| CH3 (symmetric deformation) | 1371, 1305 | 1395 | 1397 | 1395, 1321 | [66,67,69] |
| -N-C- (stretching) and sulfonamide group | 1228 | 1216 | 1107 | [68] | |
| C-O-C (stretching) | 1143 | ||||
| C-O (skeletal stretching) and C-H out-of-plane (bending) | 1041, 1022 | 1058, 1029 | 1029 | 1027 | [65,66] |
| C-O- of epoxy ring | 892 | ||||
| C-O-S (stretching) and -(CH2)n- rocking | 649 | 670 | 670 | 670, 616 | [68] |
| O-H out of plane (bending), C-S and/or C-O-S (stretching). | 525 | 525 | 498 | [70,71,72] | |
| Polysulfides(S-S stretching) | 466 | our |
| Sorbent | C | N | H | O | S |
|---|---|---|---|---|---|
| CH (%) | 45.94 | 4.83 | 6.98 | 42.25 | 0 |
| CH (mmol g−1) | 38.248 | 3.449 | 69.253 | 26.408 | 0 |
| CH-S (%) | 41.56 | 6.91 | 6.22 | 43.12 | 2.19 |
| CH-S (mmol g−1) | 34.602 | 4.934 | 61.712 | 26.952 | 0.683 |
| Model | Parameter | 1 | 2 | 3 |
|---|---|---|---|---|
| Exp. | qeq (mmol U g−1) | 0.968 | 0.966 | 0.959 |
| PFORE | qeq,1 (mmol U g−1) | 0.978 | 0.974 | 0.966 |
| k1 × 102 (min−1) | 3.27 | 3.71 | 3.28 | |
| R2 | 0.995 | 0.979 | 0.989 | |
| AIC | −136.6 | −146.8 | −144.9 | |
| PSORE | qeq,2 (mmol U g−1) | 0.802 | 0.796 | 0.817 |
| k2 × 102 (L mmol−1 min−1) | 11.7 | 9.87 | 10.68 | |
| R2 | 0.797 | 0.815 | 0.856 | |
| AIC | −48.3 | −39.7 | −49.3 | |
| RIDE | De × 108 (m2 min−1) | 2.19 | 1.97 | 2.05 |
| R2 | 0.992 | 0.989 | 0.973 | |
| AIC * | −137 | −143.3 | −144.5 |
| Model | Parameter | 1 | 2 | 3 | |
|---|---|---|---|---|---|
| Experimental | qm,exp. | mmol U g−1 | 1.543 | 1.477 | 1.43 |
| Langmuir | qm,L | mmol U g−1 | 1.572 | 1.483 | 1.451 |
| bL | L mmol−1 | 1.32 | 1.29 | 1.31 | |
| R2 | - | 0.993 | 0.984 | 0.979 | |
| AIC | - | −144 | −147 | −169 | |
| Freundlich | kF | mmol1−1/n g−1 L1/n | 0.85 | 0.79 | 0.68 |
| nF | - | 2.1 | 2.51 | 35 | |
| R2 | - | 0.894 | 0.911 | 0.928 | |
| AIC | - | −74.3 | −69.3 | −58.4 | |
| Sips | qm,S | mmol U g−1 | 1.59 | 1.481 | 1.447 |
| bS | L mmol−1 | 1.17 | 1.08 | 1.14 | |
| nS | - | 1.16 | 1.23 | 1.27 | |
| R2 | - | 0.993 | 0.989 | 0.981 | |
| AIC | - | −152 | −176 | −185 | |
| Temkin | AT × 10−3 | L mmol−1 | 22.56 | 30.2 | 28.1 |
| bT | kJ mol−1 | 17.4 | 16.4 | 15.3 | |
| R2 | - | 0.786 | 0.769 | 0.832 | |
| AIC | - | −36 | −46 | −25 |
| Sorbent | pH | teq | qm,exp | qm,L | bL | Ref. |
|---|---|---|---|---|---|---|
| Functionalized of the activated carbon | 5 | 140 | - | 0.808 | 81.4 | [79] |
| Phosphonated mesoporous silica | 4 | 10 | 1.48 | 1.15 | 12.6 | [77] |
| Duolite (ES-467) | 3 | 90 | - | 0.326 | 10.9 | [80] |
| Functionalized magnetic with amino and phosphine oxide composite | 0.5 | 180 | 0.727 | 0.825 | 5.47 | [81] |
| Functionalized polyethylene non-woven fabrics | 4 | 720 | 0.084 | 0.087 | 119 | [82] |
| Cyanobacterium | 5 | 60 | 0.708 | 0.799 | 28.6 | [83] |
| Manganese oxide functionalized with amine | 4 | 20 | - | 0.416 | 19.0 | [84] |
| Water hyacinth-biochar | 6 | 720 | 0.571 | 0.663 | 407 | [85] |
| 201X8 ion exchanger | 1.57 | 120 | - | 0.282 | 5.71 | [86] |
| Trimesoyl chloride-melamine-palygorskite composite | 6 | 75 | - | 1.16 | 0.95 | [87] |
| Carboxylated chitosan nanotubes aerogels | 5 | 150 | 1.07 | 1.29 | 23.6 | [88] |
| activated carbon-Nano-HAP-alginate | 6 | 480 | 0.042 | 0.078 | 0.95 | [89] |
| Urea-formaldehyde resin | 6 | 180 | 0.412 | 0.417 | 381 | [90] |
| Chitosan/Amidoxime PAN/GO | 6 | 120 | 1.04 | 1.24 | 169 | [91] |
| Carboxymethyl konjac-glucomannan-gellan gum | 6 | 720 | 0.411 | 0.428 | 65.0 | [92] |
| Yarrowia lipolytica-alginate beads | 7.5 | 90 | - | 0.102 | 2.86 | [93] |
| Amidoxime-marine fungus | 5 | 120 | 1.56 | 1.56 | 0.38 | [78] |
| TiO2-alginate | 5 | 1440 | 0.105 | 0.132 | - | [94] |
| Gum-ghatti polyacrylamide | 6 | 240 | - | 1.54 | 29.8 | [95] |
| Resin of carminic acid | 5 | 120 | 0.807 | 0.808 | 36.3 | [96] |
| Functionalized picolylamine resin | 5.3 | 120 | 2.10 | 2.31 | 164 | [97] |
| Chitosan film | 5 | 600 | 0.735 | 0.827 | 223 | [98] |
| Colloid montmorillonite | 6 | 60 | - | 0.076 | 187 | [99] |
| Impregnated bentonite | 4 | 15 | - | 0.268 | 40.0 | [100] |
| Dowex 50W X8/Alizarin Red-S | 3 | 30 | - | 0.512 | 141 | [101] |
| Acrylic Membrane/Algal fiber | 3 | 60 | 0.260 | 0.268 | 9.05 | [102] |
| Urea/SiO2 | 4 | 90 | 1.14 | 1.17 | 21.3 | [18] |
| Thiourea/SiO2 | 4 | 90 | 1.02 | 1.16 | 5.98 | [18] |
| Silica gel with β-cyclodextrin/ | 4.5 | 60 | - | 0.070 | 50.3 | [103] |
| silica Functionalized MCM-41 | 5 | 40 | 1.89 | 2.00 | 850 | [104] |
| PGG@C | 4 | 60 | 1.28 | 1.32 | 47.1 | [105] |
| PGG@MC | 4 | 60 | 1.15 | 1.22 | 22.0 | [105] |
| CH-S | 4 | 30 | 1.53 | 1.55 | 1.3 | This work |
| Sorption | Desorption | |||
|---|---|---|---|---|
| Cycles | Removal Effeciency (%) | S.D. (Re. %) | Desorption (%) | S.D. (De %) |
| 1 | 97.6857 | 0.73187 | 99.846 | 0.22026 |
| 2 | 97.003 | 0.72855 | 99.893 | 0.14502 |
| 3 | 96.4471 | 0.79283 | 99.989 | 0.17285 |
| 4 | 96.1154 | 0.71282 | 99.672 | 0.12039 |
| 5 | 95.6361 | 0. 7312 | 99.864 | 0.17931 |
| Constituants | Conc. (mgL−1) | Constituants | Conc. (mgL−1) |
|---|---|---|---|
| U | 386 | Al2O3 | 2540 |
| REE | 448 | Pb | 20 |
| Fe | 2700 | Zr | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Abu Khoziem, H.A.; Khalafalla, M.S.; Abdellah, W.M.; Zaki, D.I.; Althumayri, K.; Wei, Y. Ecofriendly Composite as a Promising Material for Highly-Performance Uranium Recovery from Different Solutions. Toxics 2022, 10, 490. https://doi.org/10.3390/toxics10090490
Hamza MF, Abu Khoziem HA, Khalafalla MS, Abdellah WM, Zaki DI, Althumayri K, Wei Y. Ecofriendly Composite as a Promising Material for Highly-Performance Uranium Recovery from Different Solutions. Toxics. 2022; 10(9):490. https://doi.org/10.3390/toxics10090490
Chicago/Turabian StyleHamza, Mohammed F., Hanaa A. Abu Khoziem, Mahmoud S. Khalafalla, Walid M. Abdellah, Doaa I. Zaki, Khalid Althumayri, and Yuezhou Wei. 2022. "Ecofriendly Composite as a Promising Material for Highly-Performance Uranium Recovery from Different Solutions" Toxics 10, no. 9: 490. https://doi.org/10.3390/toxics10090490
APA StyleHamza, M. F., Abu Khoziem, H. A., Khalafalla, M. S., Abdellah, W. M., Zaki, D. I., Althumayri, K., & Wei, Y. (2022). Ecofriendly Composite as a Promising Material for Highly-Performance Uranium Recovery from Different Solutions. Toxics, 10(9), 490. https://doi.org/10.3390/toxics10090490








