Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study
Abstract
:1. Introduction
2. Overview of Metal Chelates as Corrosion Inhibitors
3. Investigation
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inada, Y.; Mochizuki, K.; Tsuchiya, T.; Tsuji, H.; Funahashi, S. Equilibrium and kinetics of the dinuclear complex formation between N, N′-ethylenebis (salicylideneiminato) copper (II) and metal (II, I) ions in acetonitrile. Inorg. Chim. Acta 2005, 358, 3009–3014. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Nassr, L.A.-M.E. Tailoring, physicochemical characterization, anti-bacterial and DNA binding mode studies of Cu (II) Schiff bases amino acid bioactive agents incorporating 5-bromo-2-hydroxybenzaldehyde. J. Iran. Chem. Soc. 2015, 12, 943–955. [Google Scholar] [CrossRef]
- Khalaf, M.M.; El-Lateef, H.M.A.; Alhadhrami, A.; Sayed, F.N.; Mohamed, G.G.; Gouda, M.; Shaaban, S.; Abu-Dief, A.M. Synthesis, Spectroscopic, Structural and Molecular Docking Studies of Some New Nano-Sized Ferrocene-Based Imine Chelates as Antimicrobial and Anticancer Agents. Materials 2022, 15, 3678. [Google Scholar] [CrossRef] [PubMed]
- Abosadiya, H.M.; Hasbullah, S.A.; Yamin, B.M. Synthesis, X-ray, NMR, FT-IR, UV/vis, DFT and TD-DFT studies of N-(4-chlorobutanoyl)-N′-(2-, 3-and 4-methylphenyl) thiourea derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 144, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. A robust in vitro anticancer, antioxidant and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II) and VO (II) cations: Probing the aspects of DNA interaction. Appl. Organomet. Chem. 2020, 34, e5373. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Sagher, H.M.; Shehata, M.R. Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu (II), Pd (II), Zn (II) and Cr (III) complexes. Appl. Organomet. Chem. 2019, 33, e4943. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, A. Spectral studies on Co (II), Ni (II) and Cu (II) complexes with thiosemicarbazone (L1) and semicarbazone (L2) derived from 2-acetyl furan. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 1347–1351. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Alzahrani, S.O.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1242, 130693. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT Calculation, and Molecular Docking of Two Fe(III) Imine Chelates as Anti-COVID-19 and Pharmaceutical Drug Candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef]
- Kızılcıklı, İ.; Ülküseven, B.; Daşdemir, Y.; Akkurt, B. Zn (II) and Pd (II) Complexes of Thiosemicarbazone-S-alkyl Esters Derived from 2/3-Formylpyridine. Synth. React. Inorg. Met.-Org. Chem. 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M.; Adam, A.M.; Ibrahim, E.M.M. Sonochemical synthesis, structural inspection and semiconductor behavior of three new nano sized Cu (II), Co (II) and Ni (II) chelates based on tri-dentate NOO imine ligand as precursors for metal oxides. Appl. Organomet. Chem. 2018, 32, e4174. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. New Cd (II), Mn (II) and Ag (I) Schiff base complexes: Synthesis, characterization, DNA binding and antimicrobial activity. Inter. J. Nano Chem. 2016, 2, 83–91. [Google Scholar] [CrossRef]
- Liberta, A.E.; West, D.X. Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: Current status. Biometals 1992, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, S.I.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Fatah, S.M.; Alotaibi, T.M.; Alsalme, A.M.; Nafady, A. Catalytic Oxidation of Benzyl Alcohol Using Nanosized Cu/Ni Schiff-Base Complexes and Their Metal Oxide Nanoparticles. Catalysts 2018, 8, 452. [Google Scholar] [CrossRef]
- Al-Abdulkarim, H.A.; El-Khatib, R.M.; Aljohani, F.S.; Mahran, A.; Alharbi, A.; Mersal, G.A.M.; El-Metwaly, N.M.; Abu-Dief, A.M. Optimization for synthesized quinoline-based Cr3+, VO2+, Zn2+ and Pd2+ complexes: DNA interaction, bio-logical assay and in-silico treatments for verification. J. Mol. Liq. 2021, 339, 116797. [Google Scholar] [CrossRef]
- Zangrando, E.; Casanova, M.; Alessio, E. Trinuclear metallacycles: Metallatriangles and much more. Chem. Rev. 2008, 108, 4979–5013. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Abu-Dief, A.M. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+ and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl. Organomet. Chem. 2021, 35, e6169. [Google Scholar] [CrossRef]
- Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Al-Farraj, E.S.; Attar, R.M.S.; El-Metwaly, N.M.; Abu-Dief, A.M. Tailoring of some novel bis-hydrazone metal chelates, spectral based characterization and DFT calculations for pharmaceutical applications and in-silico treatments for verification. J. Mol. Struct. 2022, 1264, 133263. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Shehata, M.R.; Abdel-Mawgoud, A.A.H. Novel azomethine Pd (II)-and VO (II)-based metallo-pharmaceuticals as anticancer, antimicrobial, and antioxidant agents: Design, structural inspection, DFT investigation, and DNA interaction. J. Phys. Org. Chem. 2019, 32, e4009. [Google Scholar] [CrossRef]
- Dalgarno, S.J.; Power, N.P.; Atwood, J.L. Metallo-supramolecular capsules. Coord. Chem. Rev. 2008, 252, 825–841. [Google Scholar] [CrossRef]
- Ibrahim, E.M.M.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Elshafaie, A.; Hamdan, S.K.; Ahmed, A.M. The synthesis of CuO and NiO nanoparticles by facile thermal decomposition of metal-Schiff base complexes and an examination of their electric, thermoelectric and magnetic Properties. Mater. Res. Bull. 2018, 107, 492–497. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Al-Abdulkarim, H.A.; Alzahrani, S.; El-Sarrag, G.; Ismael, M. Synthesis, structuralelucidation, DFT calculation, biological studies and DNA inter-action of some aryl hydrazone Cr3+, Fe3+, and Cu2+ chelates. Comput. Biol. Chem. 2022, 97, 107643. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, H.M. Synergistic effect of polyethylene glycols and rare earth Ce4+ on the corrosion inhibition of carbon steel in sulfuric acid solution: Electrochemical, computational, and surface morphology studies. Res. Chem. Intermed. 2016, 42, 3219–3240. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Ismael, M.; Mohamed, I. Novel Schiff base amino acid as corrosion inhibitors for carbon steel in CO2-saturated 3.5% NaCl solution: Experimental and computational study. Corros. Rev. 2015, 33, 77–97. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion Inhibitors for Carbon Steel in Acidic/Chloride Media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Soliman, K.A.; Tantawy, A.H. Novel synthesized Schiff Base-based cationic Gemini surfactants: Electrochemical investigation, theoretical modeling and applicability as biodegradable inhibitors for mild steel against acidic corrosion. J. Mol. Liq. 2017, 232, 478–498. [Google Scholar] [CrossRef]
- Wu, H.; Kou, F.; Jia, F.; Liu, B.; Yuan, J.; Bai, Y. A V-shaped ligand 1, 3-bis (1-methylbenzimidazol-2-yl)-2-oxapropane and its Cu (II) complex: Synthesis, crystal structure, antioxidation and DNA-binding properties. J. Photochem. Photobiol. B Biol. 2011, 105, 190–197. [Google Scholar] [CrossRef]
- Sun, W.; Nesic, S. A mechanistic model of H2S corrosion of mild steel. Paper No. 07655. In Proceedings of the NACE International Corrosion Conference & Expo., Houstan, TX, USA, 11–15 March 2007. [Google Scholar]
- Marshall, K.C.; Stout, R.; Mitchell, R. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 1971, 68, 337–348. [Google Scholar] [CrossRef]
- Aly, K.I.; Moustafa, A.H.; Ahmed, E.K.; Abd El-Lateef, H.M.; Gamal, M.M.; Mohamed, S.M. New Polymer Syntheses part 60*: A Facile Synthetic Route to Novel Polyamides Based on Thieno[2,3-b]thiophene and Their Application for Corrosion Inhibition Behavior. Chin. J. Polym. Sci. 2018, 36, 835–847. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M. Novel dispersed Tl2O3-SiO2/polyaniline nano composites: In-sit polymerization, characterization and enforcement as a corrosion protective layer for carbon-steel in acidic chloride medium. Colloids Surf. A 2019, 573, 95–111. [Google Scholar] [CrossRef]
- Gupta, N.K.; Verma, C.; Quraishi, M.A.; Mukhrajee, A.K. Schiff’s Bases Derived from L-lysine and Aromatic Aldehydes as Green Corrosion Inhibitors for Mild Steel: Experimental and theoretical studies. J. Mol. Liq. 2016, 215, 47–57. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Alnajjar, A.O. Enhanced the protection capacity of poly(o-toluidine) by synergism with zinc or lanthanum additives at C-steel/HCl interface: A combined DFT, molecular dynamic simulations and experimental methods. J. Mol. Liq. 2020, 303, 112641. [Google Scholar] [CrossRef]
- Dexter, S.C.; Sullivan, J.D., Jr.; Williams, J., III; Watson, S.W. Influence of substrate wettability on the attachment of marine bacteria to various surfaces. Appl. Microbiol. 1975, 30, 298–308. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Okafor, P.C.; Offiong, O.E.; Ita, B.I.; Ibok, U.J.; Ekpe, U.J. Comparative investigation into the kinetics of corrosion inhibition of aluminium alloy AA 1060 in acidic medium. Bull. Elect. Chem. 2001, 17, 459–464. [Google Scholar]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2 -3.5% NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Sayed, A.R.; Shalabi, K. Studying the effect of two isomer forms thiazole and thiadiazine on the inhibition of acidic chloride-induced steel corrosion: Empirical and Computer simulation explorations. J. Mol. Liq. 2022, 356, 119044. [Google Scholar] [CrossRef]
- Olivares, O.; Likhanova, N.V.; Gomez, B.; Navarrete, J.; Llanos-Serrano, M.E.; Arce, E.; Hallen, J.M. Electrochemical and XPS studies of decylamides of α-amino acids adsorption on carbon steel in acidic environment. Appl. Surf. Sci. 2006, 252, 2894. [Google Scholar] [CrossRef]
- Fu, J.J.; Li, S.N.; Cao, L.H.; Wang, Y.; Yan, L.H.; Lu, L.D. L-Tryptophan as green corrosion inhibitor for low carbon steel in hydrochloric acid solution. J. Mater. Sci. 2010, 45, 979–986. [Google Scholar] [CrossRef]
- Cui, R.; Gu, N.; Li, C. Polyaspartic acid as a green corrosion inhibitor for carbon steel. Mater. Corros. 2011, 62, 362–369. [Google Scholar] [CrossRef]
- Fu, J.J.; Li, S.N.; Wang, Y.; Cao, L.H.; Lu, L.D. Computational and electrochemical studies of some amino acid compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. J. Mater. Sci. 2010, 45, 6255–6265. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Majidi, M.R.; Seyyedi, K. Investigation of inhibition effect of some amino acids against steel corrosion in HCl solution. Appl. Surf. Sci. 2004, 225, 176–185. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Synthesis and corrosion inhibition of α-amino acids alkylamides for mild steel in acidic environment. Mater. Chem. Phys. 2008, 110, 344–351. [Google Scholar] [CrossRef]
- Morad, M.S. Corrosion inhibition of mild steel in sulfamic acid solution by S-containing amino acids. J. Appl. Electrochem. 2008, 38, 1509–1518. [Google Scholar] [CrossRef]
- Özcan, M. AC impedance measurement of cystine adsorption at mild steel/sulfuric acid interface as corrosion inhibitor. J. Solid State Electrochem. 2008, 12, 1653–1661. [Google Scholar] [CrossRef]
- Morad, M.S. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions containing Cl−, F− and Fe3+ ions: Behavior under polarization conditions. J. Appl. Electrochem. 2005, 35, 889–895. [Google Scholar] [CrossRef]
- Silva, A.B.D.; Agostinho, S.M.L.; Barcia, O.E.; Cordeiro, G.G.; D’elia, E. The effect of cysteine on the corrosion of 304L stainless steel in sulphuric acid. Corros. Sci. 2006, 48, 3668–3674. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F. Tryptophan as copper corrosion inhibitor in 0.5 M aerated sulfuric acid. Corros. Sci. 2002, 44, 1995–2011. [Google Scholar] [CrossRef]
- Barouni, K.; Bazzi, L.; Salghi, R.; Mihit, M.; Hammouti, B.; Albourine, A.; El Issami, S. Some amino acids as corrosion inhibitors for copper in nitric acid solution. Mater. Lett. 2008, 62, 3325–3327. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. A theoretical study on the inhibition efficiencies of some amino acids as corrosion inhibitors of nickel. Corros. Sci. 2010, 52, 3435–3443. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghasemi, Z.; Seifzadeh, D. The inhibition effect of some amino acids towards the corrosion of aluminum in 1 M HCl+ 1 M H2SO4 solution. Appl. Surf. Sci. 2005, 249, 408–418. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Tizpar, A. The inhibition effect of some amino acids towards Pb–Sb–Se–As alloy corrosion in sulfuric acid solution. Appl. Surf. Sci. 2006, 252, 3667–3672. [Google Scholar] [CrossRef]
- Kiani, M.A.; Mousavi, M.F.; Ghasemi, S.; Shamsipur, M.; Kazemi, S.H. Inhibitory effect of some amino acids on corrosion of Pb–Ca–Sn alloy in sulfuric acid solution. Corros. Sci. 2008, 50, 1035–1045. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Lei, S.; Ma, H.; Yu, R.; Liu, D. Investigation on some Schiff bases as HCl corrosioninhibitors for copper. Corros. Sci. 1999, 41, 1273–1287. [Google Scholar] [CrossRef]
- Quan, Z.; Chen, S.; Li, S. Protection of copper corrosion by modification of self-assembled films of Schiff bases with alkanethiol. Corros. Sci. 2001, 43, 1071–1080. [Google Scholar] [CrossRef]
- Liu, J.; Wu, B.W.; Zhang, B.; Liu, Y. Synthesis and characterization of metal complexes of Cu (II), Ni (II), Zn (II), Co (II), Mn (II) and Cd (II) with tetradentate Schiff bases. Turk. J. Chem. 2006, 30, 41–48. [Google Scholar]
- Jamil, D.M.; Al-Okbi, A.K.; Al-Baghdadi, S.B.; Al-Amiery, A.A.; Kadhim, A.; Gaaz, T.S.; Kadhum, A.A.H.; Mohamad, A.B. Experimental and theoretical studies of Schif bases as corrosion inhibitors. Chem. Cent. J. 2018, 12, 7. [Google Scholar] [CrossRef]
- Bhatia, P.K.; Guar, Y.D.; Rao, N.S.S. Plant. Phy. Biochem. 1993, 19, 30. [Google Scholar]
- Deshpande, M.M.; Junne, S.B.; Saraf, D.V.; Kulkarni, P.A. Synthesis and spectral analysis of some new lanthanide complexes derived from 2, 4 and 2, 5-dihydroxy acetophenones and screened their antimicrobial activity. J. Chem. Pharm. Res. 2010, 2, 453–458. [Google Scholar]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Experimental and computational approaches of sustainable quaternary bisammonium fluorosurfactants for corrosion inhibition as protective films at mild steel/H2SO4 interface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126141. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shaaban, S.; Shalabi, K.; Khalaf, M.M. Novel organoselenium-based N-mealanilic acids as efficacious corrosion inhibitors for 6061 aluminum alloy in molar HCl: In-silico modeling, electrochemical, and surface morphology studies. J. Taiwan Inst. Chem. Eng. 2022, 133, 104258. [Google Scholar] [CrossRef]
- Nassar, A.M.; Hassan, A.M.; Shoeib, M.A. Synthesis, characterization and anticorrosion studies of new homobimetallic Co (II), Ni (II), Cu (II), and Zn (II) Schiff base complexes. J. Bio. Tribo-Corros. 2015, 1, 1–16. [Google Scholar] [CrossRef]
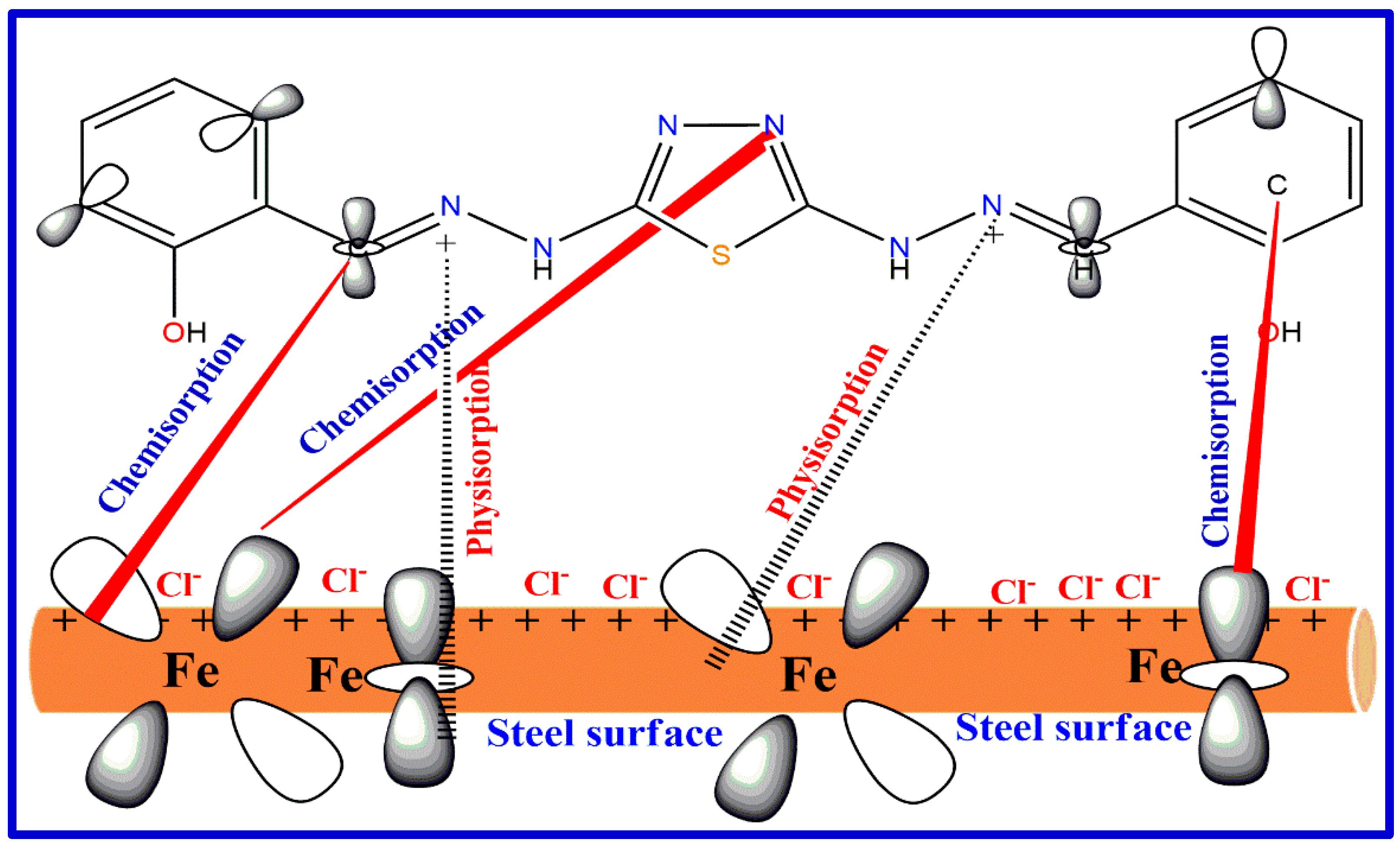
- Jawad, Q.A.; Zinad, S.D.; Dawood Salim, R.; A. Al-Amiery, A.; Sumer Gaaz, T.; Takriff, M.S.; H. Kadhum, A.A. Synthesis, characterization, and corrosion inhibition potential of novel thiosemicarbazone on mild steel in sulfuric acid environment. Coatings 2019, 9, 729. [Google Scholar] [CrossRef]
- Danaee, I.; RameshKumar, S.; RashvandAvei, M.; Vijayan, M. Electrochemical and Quantum Chemical Studies on Corrosion Inhibition Performance of 2,2′-(2-Hydroxyethylimino)bis[N-(alphaalphadimethylphenethyl)-N-methylacetamide] on Mild Steel Corrosion in 1M HCl Solution. Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Kubba, R.M. Experimental Evaluation for the Inhibition of Carbon Steel Corrosion in Salt and Acid Media by New Derivative of Quinolin-2-One. Iraqi J. Sci. 2020, 61, 1861–1873. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.; Quraishi, M.A. 2,4-Diamino-5-(phenylthio)-5H-chromeno [2,3-b] pyridine3-carbonitriles as green and effective corrosion inhibitors: Gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 2016, 6, 53933–53948. [Google Scholar] [CrossRef]
- Lawal, O.J.; Potgieter, J.H.; Billing, C.; Whitefield, D.J. The Influence of REE β-Diketone Complexes on the Corrosion Behaviour of Mild Steel and 304 SS in 3.5% NaCl Solution. Minerals 2022, 12, 416. [Google Scholar] [CrossRef]
- Musa, H.; Bishir, U.; Adamu, I.M.; Bashir, I.M. Effect of Aniline as Corrosion Inhibitor on the Corrosion of Aluminium in Hydrochloric Acid Solution. Res. J. Chem. Environ. 2020, 24, 2. [Google Scholar]
- Mahross, M.H.; Efil, K.; El-Nasr, T.A.S.; Abbas, O.A. Synthesis, characterization and corrosion inhibition of N′-phenylbenzohydrazide derivative metal complexes: Experimental and quantum chemical studies. Z. Phys. Chem. 2019, 233, 949–972. [Google Scholar] [CrossRef]
- Fekry, A.M.; Ameer, M.A. Corrosion inhibition of mild steel in acidic media using newly synthesized heterocyclic organic molecules. Int. J. Hydrog. Energy 2010, 35, 7641–7651. [Google Scholar] [CrossRef]
- Haruna, A.; Rumah, M.M.; Sani, U.; Ibrahim, A.K. Synthesis, Characterization and corrosion Inhibition Studies on Mn (II) and Co (II) Complexes Derived from 1-{(Z)-[(2-hydroxyphenyl) imino] methyl} naphthalen-2-ol in 1M HCl Solution. Int. J. Biol. Chem. Sci. 2021, 3, 9–18. [Google Scholar] [CrossRef]
- Hamak, K.F.; Eissa, H.H. Synthesis, characterization, biological evaluation and anti corrosion activity of some heterocyclic compounds oxazepine derivatives from Schiff bases. Organic. Chem. Curr. Res. 2013, 2, 1. [Google Scholar]
- Eissa, H.H. Synthesis, Characterization, Anticorrosion Activity and Antibacterial Activity of Macrocyclic Schiff Bases Based on 1, 3-Dithiocarbonyl Phenyl Dihydrazide. Org. Chem. Curr. Res. 2015, 4, 151–163. [Google Scholar]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.M.; Ebenso, E.E. Comparative Investigation of Corrosion-Mitigating Behavior of Thiadiazole-Derived Bis-Schiff Bases for Mild Steel in Acid Medium: Experimental, Theoretical, and Surface Study. ACS Omega 2020, 5, 13503–13520. [Google Scholar] [CrossRef]
- Al-Joborry, N.M.; Kubba, R.M. Theoretical and Experimental Study for Corrosion Inhibition of Carbon Steel in Salty and Acidic Media by A New Derivative of Imidazolidine 4-One. Iraqi J. Sci. 2020, 61, 1842–1860. [Google Scholar] [CrossRef]
- Jaafar, W.A.; Saeed, R.S. Synthesis, characterization and corrosion inhibition study of new heterocyclic compounds and schiff base with [Co (II), Ni (II), Cu (II) and Hg (II)] complexes. Syst. Rev. Pharm. 2020, 11, 134–143. [Google Scholar]
- Ade, S.B.; Jadhav, B.U.; Lonkar, S.M.; Shitole, N.V. A role of Schiff base and their metal complexes used as corrosion inhibitor in different corrosive medium. JETIR 2020, 7, 167–174. [Google Scholar]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Sanap, S.V.; Patil, R.M.; Dubey, R.S. Corrosion inhibition of mild steel by using mixed ligand metal complexes. Int. J. Chem. Sci. 2013, 11, 503–517. [Google Scholar]
- Ating, E.I.; Umoren, S.A.; Udousoro, I.I.; Ebenso, E.E.; Udoh, A.P. Leaves extract of ananas sativum as green corrosion inhibitor for aluminium in hydrochloric acid solutions. Green Chem. Lett. Rev. 2010, 3, 61–68. [Google Scholar] [CrossRef]
- Taha, R.H.; Gaber, G.A.; Mohamed, L.Z.; Ghanem, W.A. Corrosion inhibition of two Schiff base complexes on the mild steel in 1M HCl solution. Egypt. J. Chem. 2019, 62, 367–381. [Google Scholar] [CrossRef]
- Hachelef, H.; Benmoussat, A.; Khelifa, A.; Athmani, D.; Bouchareb, D. Study of corrosion inhibition by electrochemical impedance spectroscopy method of 5083 aluminum alloy in 1 M HCl solution containing propolis extract. J. Mater. Environ. Sci. 2016, 7, 1751–1758. [Google Scholar]
- Kashyap, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Om, H.; Narasimhan, B. Synthesis, biological evaluation and corrosion inhibition studies of transition metal complexes of Schiff base. Chem. Central J. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4 [4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Synergism between red tetrazolium and uracil on the corrosion of cold rolled steel in H2SO4 solution. Corros. Sci. 2009, 51, 1344–1355. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Çulha, M.; Yazıcı, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Mahross, M.H.; Efil, K.; El-Nasr, T.A.; Abbas, O.A. Experimental and Theoretical Study on Corrosion Inhibition of Mild Steel in Oilfield Formation Water Using Some Schiff Base Metal Complexes. J. Electrochem. Sci. Techol. 2017, 8, 222–235. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, K.; Singh, A.K.; Singh, V.P. Synthesis, structural and corrosion inhibition studies on Mn (II), Cu (II) and Zn (II) complexes with a Schiff base derived from 2-hydroxypropiophenone. Polyhedron 2014, 77, 57–65. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Chen, J.; Zhang, M.; Ye, G.; Lin, H. Anodic desorption of inhibitors. J. Chin. Soc. Corros. Pro. 1995, 15, 241–248. [Google Scholar]
- Kassim, K.; Nordin, N.A.; Zulkifli, N.Z.; Hashim, N.Z.N. Synthesis of Ni (II) and Cu (II) complexes with N-(benzylcarbamothioyl) benzamide as corrosion inhibitors for mild steel in 1M HCl. In AIP Conferece Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1904, p. 020024. [Google Scholar]
- Veni, K.; Karthik, A.D.; Geetha, K. Characterization Corrosion Inhibition and Biological Applications of Schiff Base Transition Metal Complexes. IOSR J. Pharm. 2017, 1, 62–68. [Google Scholar]
- Baboukani, A.R.; Sharifi, E.; Akhavan, S.; Saatchi, A. Co complexes as a corrosion inhibitor for 316 l stainless steel in H2SO4 solution. J. Mater. Sci. Chem. Eng. 2016, 4, 28–35. [Google Scholar]
- Das, M.; Biswas, A.; Kundu, B.K.; Mobin, S.M.; Udayabhanu, G.; Mukhopadhyay, S. Targeted synthesis of cadmium (II) Schiff base complexes towards corrosion inhibition on mild steel. RSC Adv. 2017, 7, 48569–48585. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Abdel-Rahman, L.H.; Sañudo, E.C.; Aliaga-Alcalde, N. Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J. Electroanal. Chem. 2015, 743, 120–133. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; El-Gendy, B.E.D.M. Investigation of adsorption and inhibition effects of some novel anil compounds towards mild steel in H2SO4 solution: Electrochemical and theoretical quantum studies. J. Electroanal. Chem. 2015, 758, 135–147. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Adam, M.S.S.; Khalaf, M.M. Synthesis of polar unique 3d metal-imine complexes of salicylidene anthranilate sodium salt. Homogeneous catalytic and corrosion inhibition performance. J. Taiwan Inst. Chem. Eng. 2018, 88, 286–304. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Soliman, K.A.; Abd El-Lateef, H.M. Homo-dinuclear VO2+ and Ni2+ dihydrazone complexes: Synthesis, characterization, catalytic activity and CO2-corrosion inhibition under sustainable conditions. Inorg. Chim. Acta 2020, 499, 119212. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Soliman, K.A.; Al-Omair, M.A.; Adam, M.S.S. A combination of modeling and experimental approaches to investigate the novel nicotinohydrazone Schiff base and its complexes with Zn (II) and ZrO (II) as inhibitors for mild-steel corrosion in molar HCl. J. Taiwan Inst. Chem. Eng. 2021, 120, 391–408. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Desoky, M.; Mohamad, A.; Shehata, M.R.; Abu-Dief, A.M. Targeted synthesis of two iron (III) tetradentate dibasic chelating Schiff base complexes towards inhibition of acidic induced steel corrosion: Empirical and DFT insights. Appl. Organomet. Chem. 2022, 36, e6718. [Google Scholar] [CrossRef]
- El-Tabesh, R.N.; Abdel-Gaber, A.M.; Hammud, H.H.; Al-Oweini, R. Correction to: Effect of Mixed-Ligands Copper Complex on the Corrosion Inhibition of Carbon Steel in Sulfuric Acid Solution. J. Bio Tribo-Corros. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- El-Baradie, K.Y.; El-Wakiel, N.A.; El-Ghamry, H.A. Synthesis, characterization and corrosion inhibition in acid medium of l-histidine Schiff base complexes. Appl. Organomet. Chem. 2015, 29, 117–125. [Google Scholar] [CrossRef]
- Ade, S.B.; Deshpande, M.N.; Kolhatkar, D.G. Corrosion a universal environmental problem: A role of Schiff base metal complexes as inhibitors. J. Chem. Pharm. Res. 2012, 4, 1033–1035. [Google Scholar]
- Ali, O.A. Palladium (II) and zinc (II) complexes of neutral [N2O2] donor Schiff bases derived from furfur aldehyde: Synthesis, characterization, fluorescence and corrosion inhibitors of ligands. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ouf, A.E.F.M.; Ali, M.S.; Soliman, M.S.; El-Defrawy, A.M.; Mostafa, S.I. Synthesis and characterization of new transition metal complexes of Schiff-base derived from 2-aminopyrimidine and 2, 4-dihydroxybenzaldehyde and its applications in corrosion inhibition. J. Korean Chem. Soc. 2010, 54, 402–410. [Google Scholar]
- Singh, V.P.; Singh, P.; Singh, A.K. Synthesis, structural and corrosion inhibition studies on cobalt (II), nickel (II), copper (II) and zinc (II) complexes with 2-acetylthiophene benzoylhydrazone. Inorg. Chim. Acta 2011, 379, 56–63. [Google Scholar] [CrossRef]
- Shokry, H.; Yuasa, M.; Sekine, I.; Issa, R.M.; El-Baradie, H.Y.; Gomma, G.K. Corrosion inhibition of mild steel by Schiff base compounds in various aqueous solutions. Corros. Sci. 1998, 39, 2173–2186. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K.; Singh, V.P. Synthesis, structural and corrosion inhibition properties of some transition metal (II) complexes with o-hydroxyacetophenone-2-thiophenoyl hydrazone. Polyhedron 2013, 65, 73–81. [Google Scholar] [CrossRef]
- Mahdavian, M.; Naderi, R. Corrosion inhibition of mild steel in sodium chloride solution by some zinc complexes. Corros. Sci. 2011, 53, 1194–1200. [Google Scholar] [CrossRef]
- Negm, N.A.; Kandile, N.G.; Aiad, I.A.; Mohammad, M.A. New eco-friendly cationic surfactants: Synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surf. A Phys. Eng. Asp. 2011, 391, 224–233. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Migahed, M.A.; Nassar, I.F. Corrosion Inhibition of Tubing Steel during Acidization of Oil and Gas Wells. Electrochim. Acta 2008, 53, 2877–2882. [Google Scholar] [CrossRef]
- Bentiss, F.; Gassama, F.; Barbary, D.; Gengembre, L.; Vezin, H.; Laggrenee, M.; Traisnel, M. Enhanced Corrosion Resistance of Mild Steel in Molar Hydrochloric Acid Solution by 1,4-bis(2-pyridyl)-5H-Pyridazino[4,5-b]indole: Electrochemical, Theoretical and XPS Studies. Appl. Surf. Sci. 2006, 252, 2684–2691. [Google Scholar] [CrossRef]
- Abdel-Gabar, A.M.; Abd-El-Nabey, B.A.; Sidahmed, I.M.; El-Zayady, A.M.; Saadawy, M.I. Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media. Corros. Sci. 2006, 48, 2765–2779. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johanson, W.B. Theory in Impedance Spectroscopy; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Abd El-Maksoud, S.A.; Hassan, H.H. Electrochemical Studies on the Effect of (2E)-3-amino-2-phenylazobut-2-enenitrile and Its Derivative on the Behaviour of Copper in Nitric Acid. Mater. Corros. 2007, 58, 369–375. [Google Scholar] [CrossRef]
- Bahkahk, C.K.; Hadi, J.S. New unsymmetrical Schiff base as inhibitor of carbon steel corrosion and antibacterial activity. Res. J. Chem. Sci. 2015, 5, 64–70. [Google Scholar]
- Afak, S.S.; Duran, B.; Yurt, A.; Türkoglu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Muralidharan, S.; Syed Azim, S.; John Berchmans, L.; Iyer, S.V.K. Synergistic influence of iodide ions on the inhibition of corrosion of mild steel in sulphuric acid by n-hexyl amine. Anti-Corros. Methods Mater. 1997, 44, 30. [Google Scholar] [CrossRef]
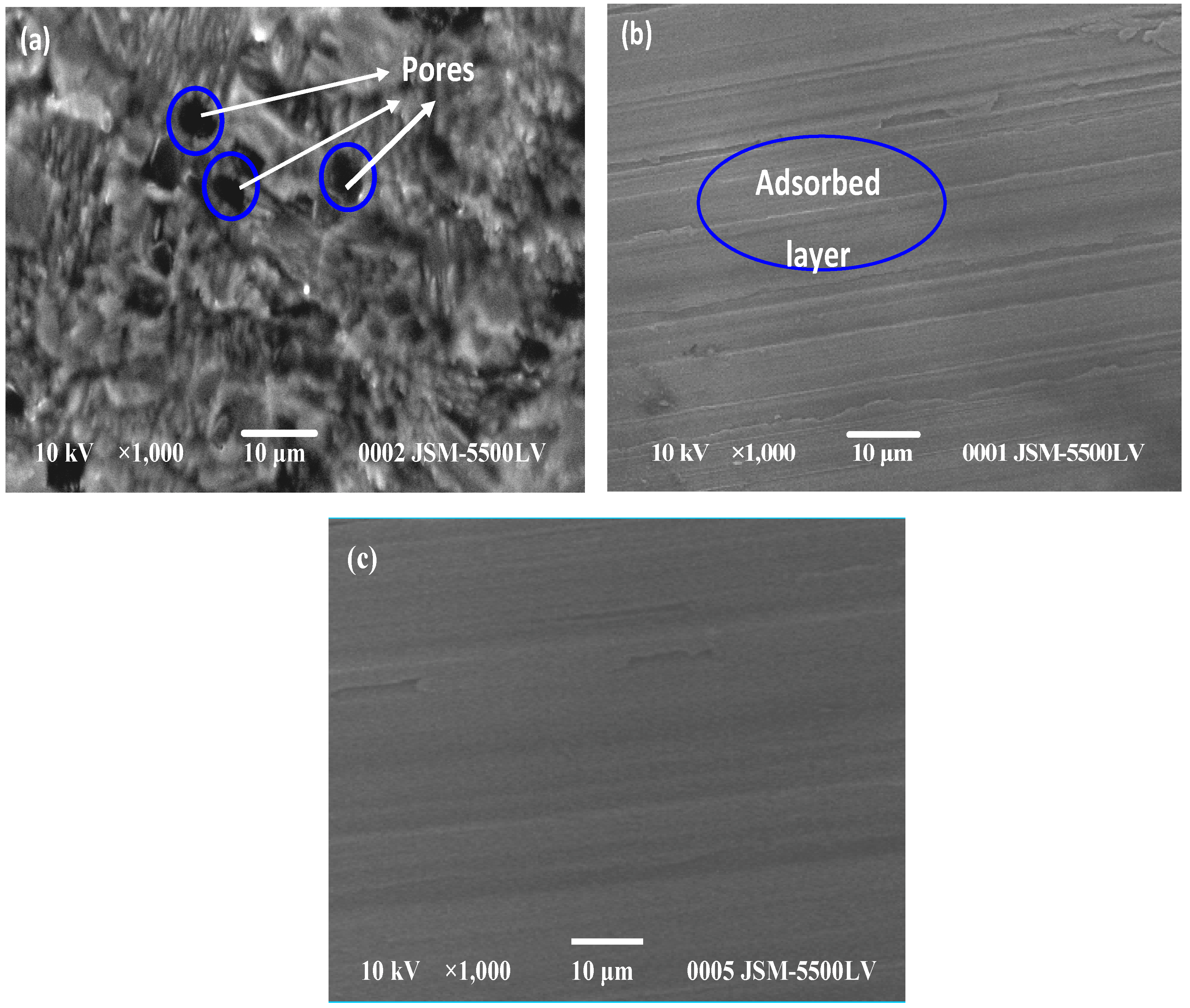
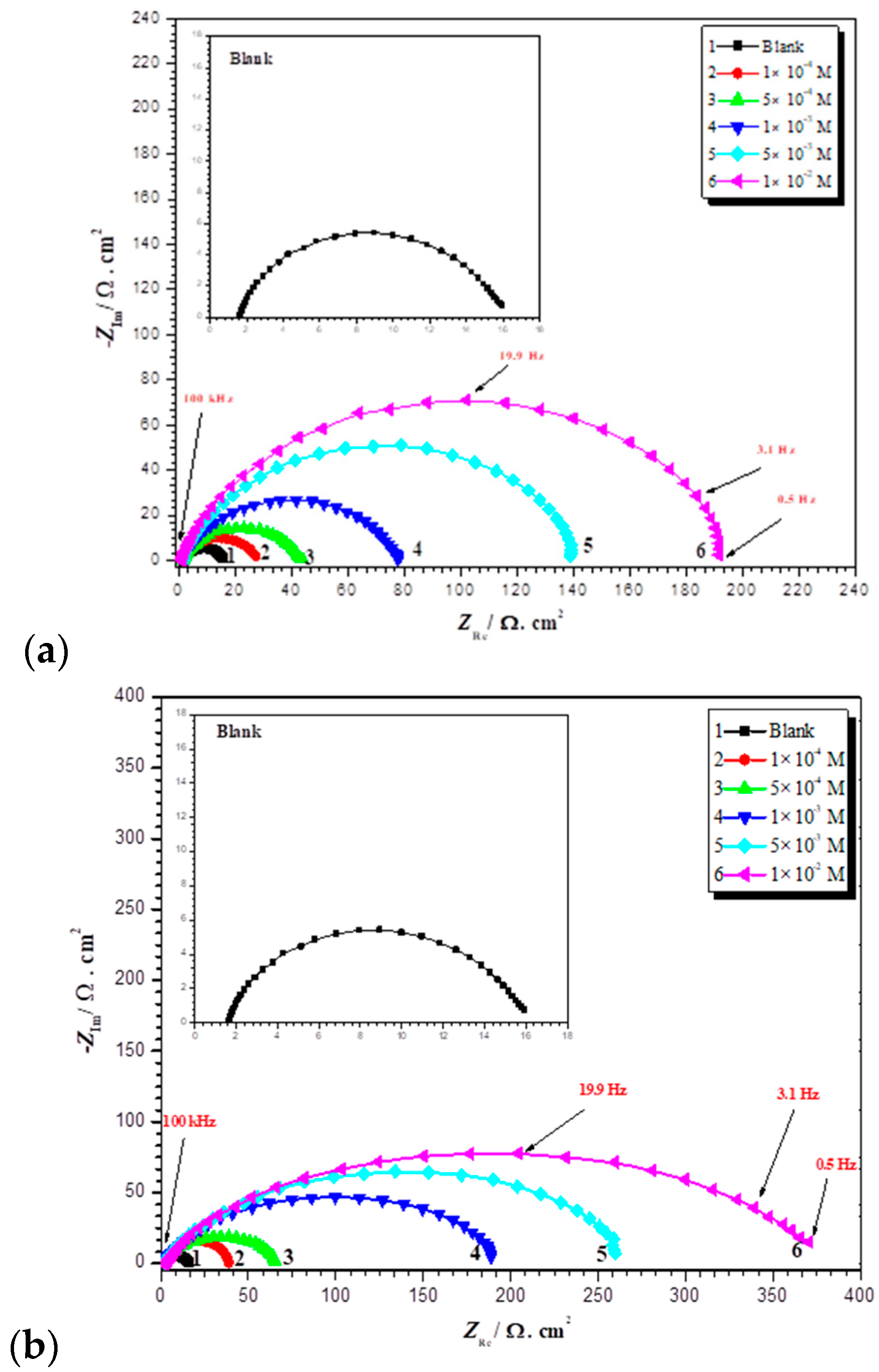
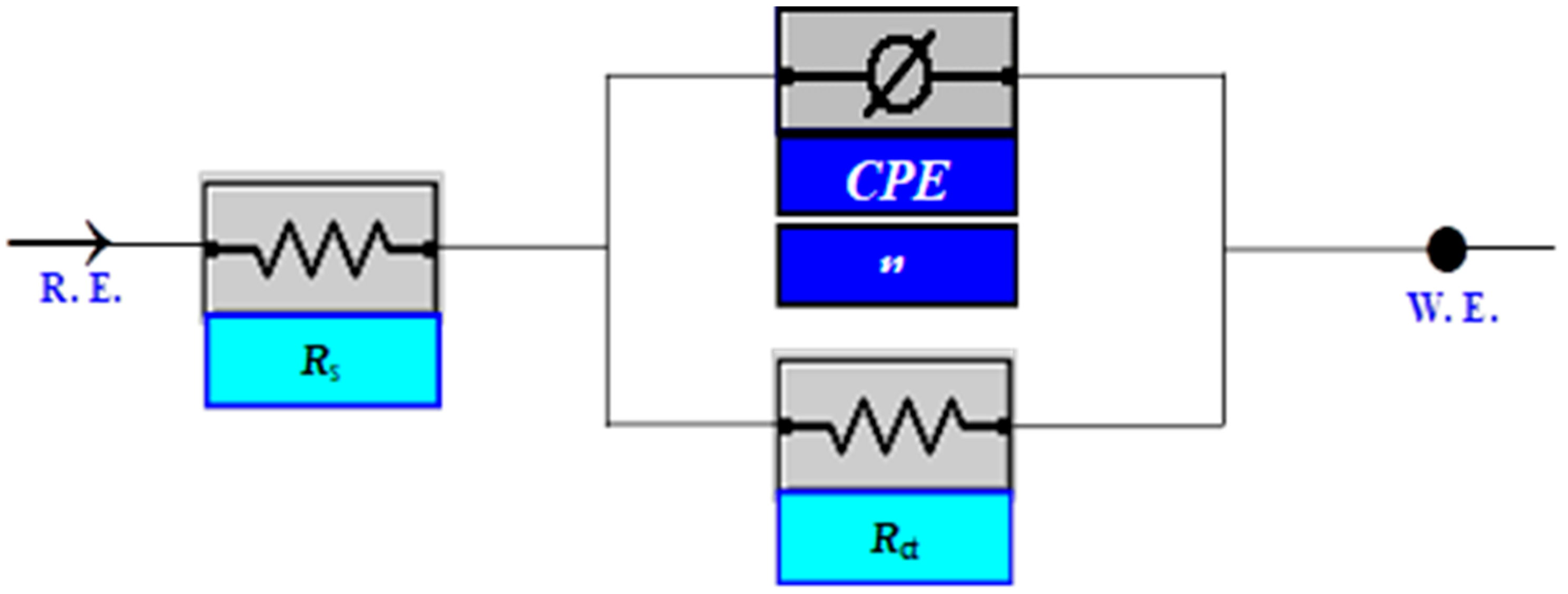

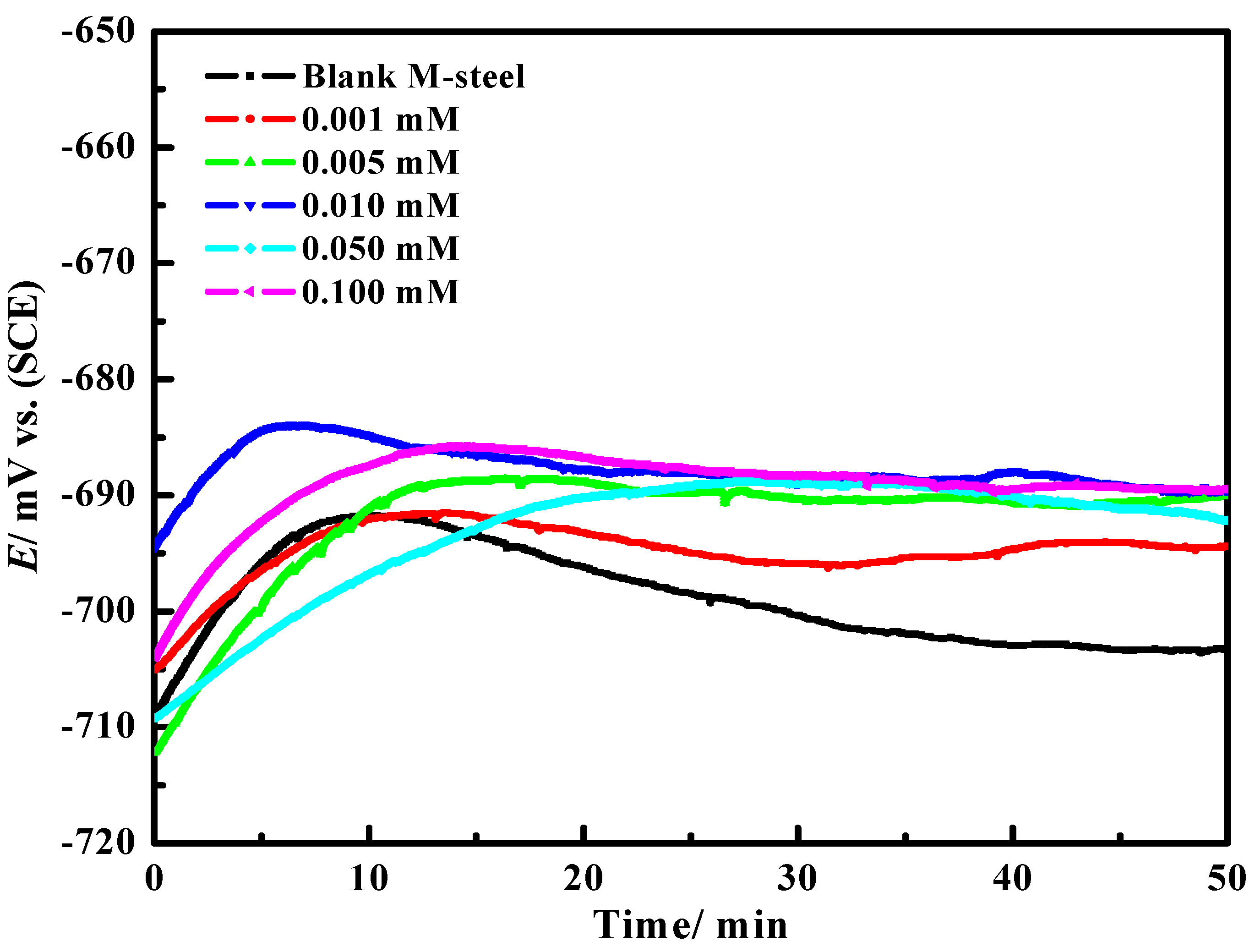
| Compound | AMPIA | [Cd(II)AMPIA] | [Ti(IV)AMPIA] | [Hg(II)AMPIA] | [Zr(IV)AMPIA] |
|---|---|---|---|---|---|
| I.E. (%) | 42.64 | 82.35 | 19.11 | 73.52 | 14.70 |
| 0.1 N HCl | |||||
| I.E. (%) | 88.23 | 82.35 | 11.76 | 76.47 | 64.70 |
| 0.01 N HCl | |||||
| I.E. (%) | 50.00 | 75.00 | 83.30 | 83.30 | 75.00 |
| 0.001 N HCl | |||||
| I.E. (%) | 29.03 | 45.16 | 12.90 | 22.58 | 16.12 |
| 0.1 N HNO3 | |||||
| I.E. (%) | 50.00 | 75 | 50 | 66.66 | 41.66 |
| 0.01 N HNO3 | |||||
| I.E. (%) | 60.00 | 60.00 | 40.00 | 40.00 | 20.00 |
| 0.001 N HNO3 | |||||
| I.E. (%) | 61.11 | 75.92 | 7.40 | 81.48 | 11.11 |
| 0.1 N H2SO4 | |||||
| I.E. (%) | 37.5 | 37.5 | 37.5 | 12.5 | 25 |
| 0.01 N H2SO4 | |||||
| I.E. (%) | 66.66 | 66.66 | 33.33 | 66.66 | 16.66 |
| in 0.001 N H2SO4 |
| No | Compounds | Media | Metal | Ref No. |
|---|---|---|---|---|
| 1 | 2-hydroxy-benzoic acid [1-(2-hydroxy-phenyl)-propylidene]-hydrazide. (H2hbpp) * | 1.0 M HCl | Mild steel | [90] |
| H2hbpp Mn(II) complex * | ||||
| H2hbpp Cu(II) complex * | ||||
| H2hbpp Zn(II) complex * | ||||
| 2 | N-(benzylcarbamothioyl)benzamide (A1) * | 1 M HCl | Mild steel | [92] |
| [N-(benzylcarbamothioyl)benzamide] copper(II) acetate(B1) * | ||||
| [N-(benzylcarbamothioyl) benzamide] nickel(II) acetate (B2) * | ||||
| 3 | Schiff base Ligand derived from p-chlorobenzldehyde and | 0.1 M HNO3 | Mild steel | [93] |
| o-amino phenol. (L) | ||||
| Cu(II) complex. (CuL) * | ||||
| Co(II) complex. (CoL) * | ||||
| 4 | Co-phenanthroline. (PhCo) * | 0.1 M H2SO4 | 316 L stainless steel | [94] |
| 5 | Thiazine Schiff base ligand and [Ni (II), Co (II), Cu (II) and Hg (II)] Metal Complexes. (3) * | 0.1 M HCl | Mild steel | [78] |
| tetrazole Schiff base ligand and [Ni (II), Co (II), Cu (II) and Hg (II)] Metal Complexes. (4) * | ||||
| 1,3-oxazepine Schiff base ligand and [Ni (II), Co (II), Cu (II) and Hg (II)] Metal Complexes. (5, 6) * | ||||
| 6 | 1-{(Z)-[(2-hydroxyphenyl) imino]methyl}naphthalen-2-ol Schiff base ligand. (L) * | 0.1 M HCl | Copper metal surface | [73] |
| 1-{(Z)-[(2-hydroxyphenyl) imino]methyl}naphthalen-2-ol and Mn (II) Complex. (MnL2) * | ||||
| 1-{(Z)-[(2-hydroxyphenyl) imino]methyl}naphthalen-2-ol and Co (II) Complex. (CoL2) * | ||||
| 7 | [N,N-dimethyl-N′-(1-pyridin-2-yl-ethylidene)-ethane1,2-diamine] (L1). * | 15% HCl | Mild steel | [95] |
| [2-morpholino-N-(1-(pyridin-2-yl)ethylidene) ethanamine] (L2) * | ||||
| [(2-(piperidin-1-yl)-N-(1-(pyridin-2-yl)ethylidene) ethanamine)] (L3) * | ||||
| [Cd(L1)2](ClO4)2 * | ||||
| [Cd(L1)(cyanoacetate)(OAc)] * | ||||
| [Cd2(L1)2(N3)4] * | ||||
| [Cd(L2)(N3)2]n * | ||||
| [Cd2(L3)2(N3)4]n * | ||||
| 8 | N-carbamimidoyl-4-((4-chlorobenzylidene)-amino) benzenesulfonamid Schiff base ligand * | 1 M HCl | Mild steel | [83] |
| Cd(II) complex * | ||||
| UO2 (II) complex * | ||||
| 9 | Salicylaldehyde thiosemicarbazone Schiff base ligand (STSC) * | Oilfield formation | Mild steel | [89] |
| STSC Cu(II) complex * | ||||
| STSC Ni(II) complex * | ||||
| STSC Zn(II) complex * | ||||
| 10 | 2-[(1H-indol-3-ylmethylene)-amino]-4-methyl-phenol (AMPIA) * | (0.1 and 0.01 and 0.001) N (HCl and HNO3 and H2SO4) | Carbon and mild steel | [79] |
| [Ti(IV)AMPIA] complex * | ||||
| [Zr(IV)AMPIA] complex * | ||||
| [Cd(II)AMPIA] complex * | ||||
| [Hg(II)AMPIA] complex * | ||||
| 11 | (E)-4-(3-Hydroxybenzylideneamino)-2,3-dimethyl-1-phenyl-1,2-dihydropyrazol-5-one (Intermediate) schiff base ligand (TMCSB) * | 1.0 M HCl | Mild steel | [85] |
| Zinc metal complex (MC1): TMCSBZn * | ||||
| Nickel metal complex (MC2): TMCSBNi * | ||||
| Cobalt metal complex (MC3): TMCSBCo * | ||||
| Copper metal complex (MC4): TMCSBCu * | ||||
| 12 | cerium acetylacetone. Ce(acac)3 * | 3.5% NaCl solution | Mild steel and 304 stainless | [69] |
| cerium hexafluoroacetylacetone. Ce(hfac)3 * | ||||
| lanthanum acetylacetone. La(acac)3 * | ||||
| lanthanum hexafluoroacetylacetone. La(hfac)3 * | ||||
| 13 | Bis(di-acetylmonoxime)biphenyl-3,30-dimethoxy-4,40-diamine * | 0.5 M HCl | Mild steel | [96] |
| Co2L(H2O)2(Cl)2·2H2O * | ||||
| Ni2L(H2O)2(Cl)2·2H2O * | ||||
| Cu2L(H2O)2(Cl)2·2H2O * | ||||
| Zn2L(H2O)2(Cl)2·2H2O * | ||||
| 14 | 5-bromo-2-[(E)-(pyridin-3-ylimino)methyl]phenol (HBSAP) * | 3.5% NaCl + 0.1 M HCl | carbon steel in | [97] |
| 5-bromo-2-[(E)-(quinolin-8-ylimino)methyl]phenol (HBSAQ) * | ||||
| 15 | 1-{(Z)-[(3,5dimethylphenyl) imino]methyl}naphthalen-2-ol (HNMA) * | 0.5 M H2SO4 | mild steel in | [98] |
| 5-(diethylamino)-2-{(Z)-[(3,5-dimethylphenyl)imino] methyl} phenol (DMSMA), * | ||||
| 16 | H2SSA (2-[(2-Hydroxy-5-sodium sulfonate-benzylidene)-amino]-benzoate) * | 1.0 M HCl | carbon steel corrosion (CS) | [99] |
| (Cu-SSA and Ni-SSA and Zn-SSA) * | ||||
| 17 | terephthaloyl salicylidene dihydrazone (H2PHL) * | 3.5% NaCl in NaCl | Mild steel | [100] |
| VOPHL and NiPHL * | C-steel | |||
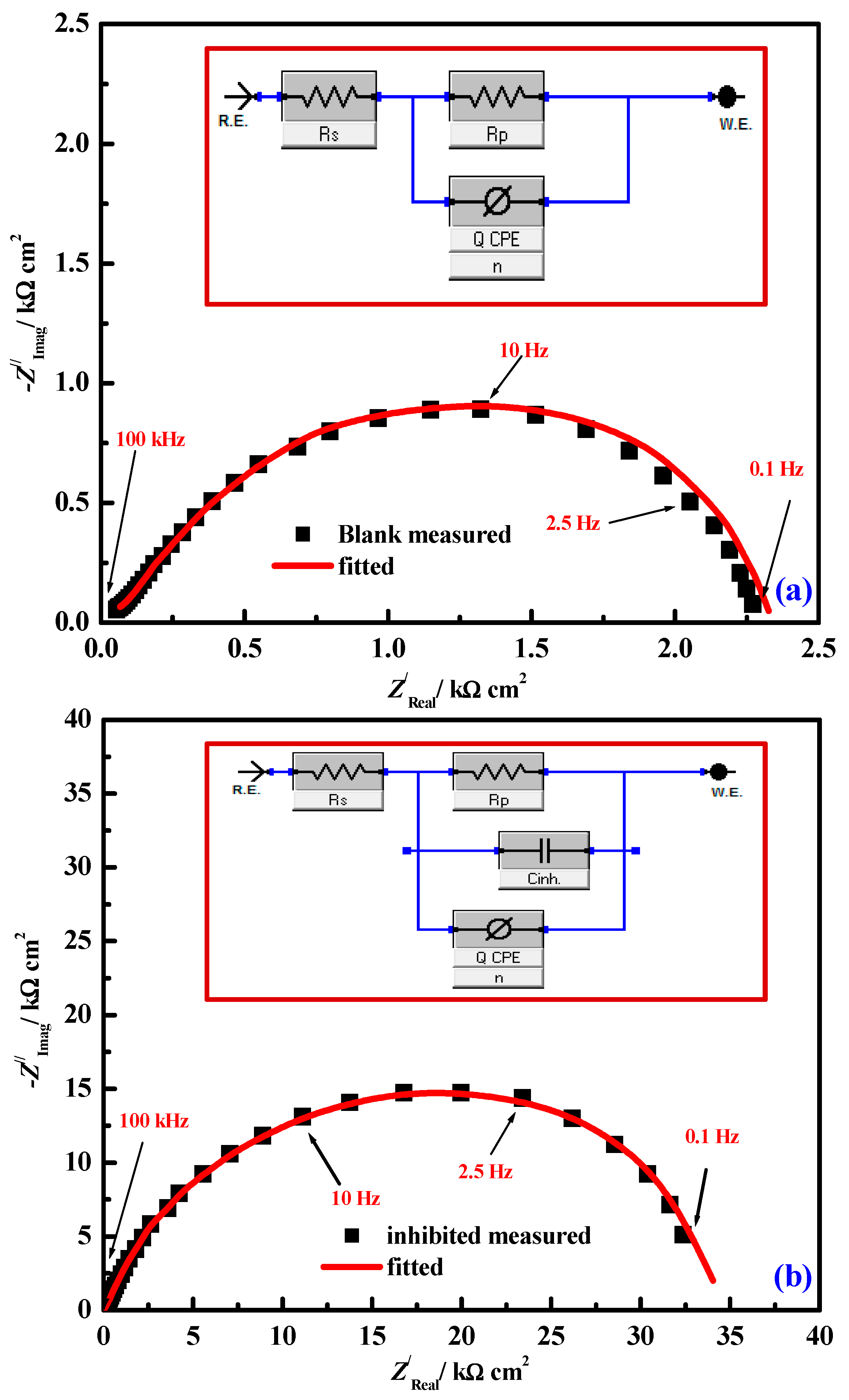
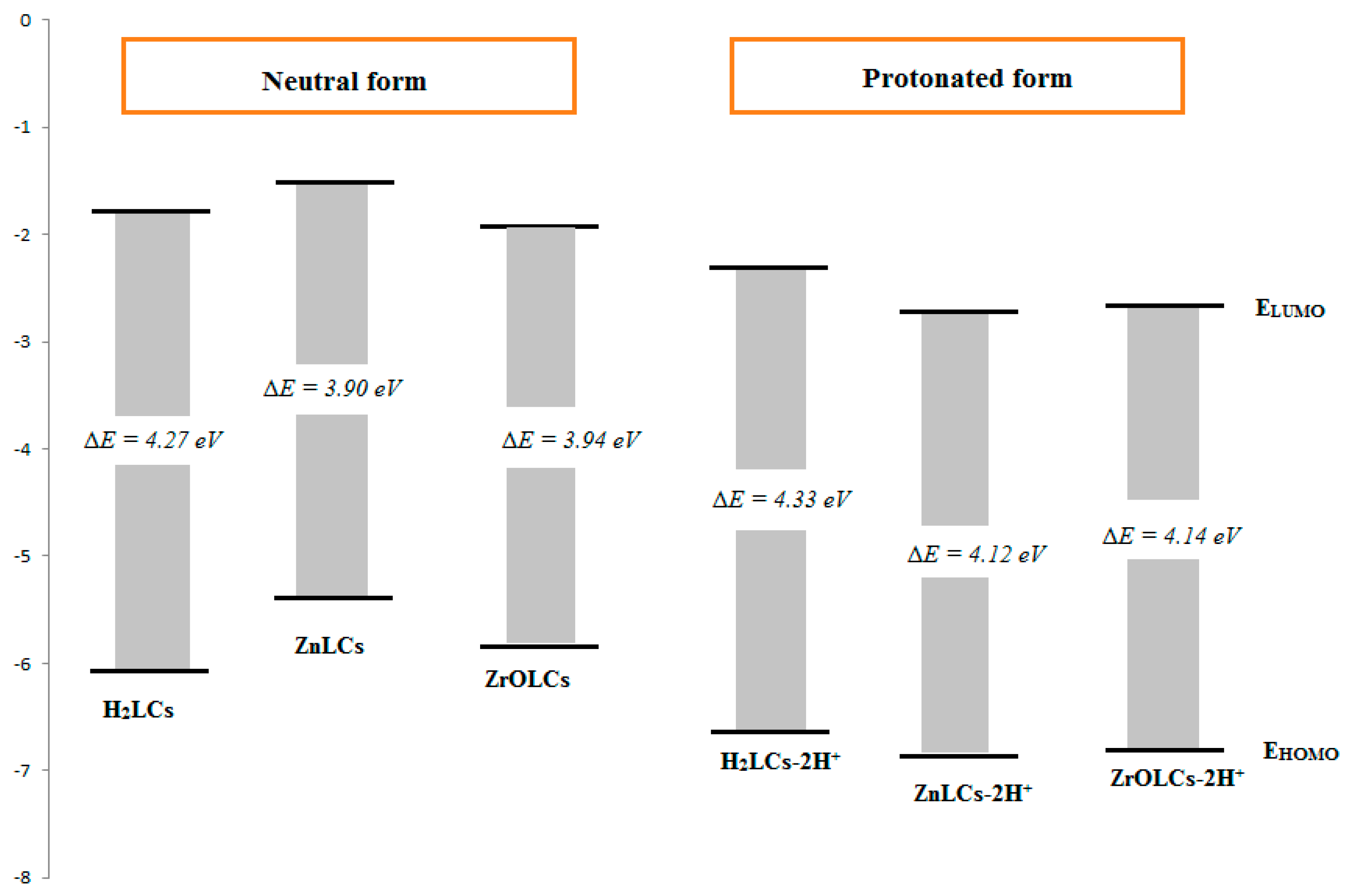
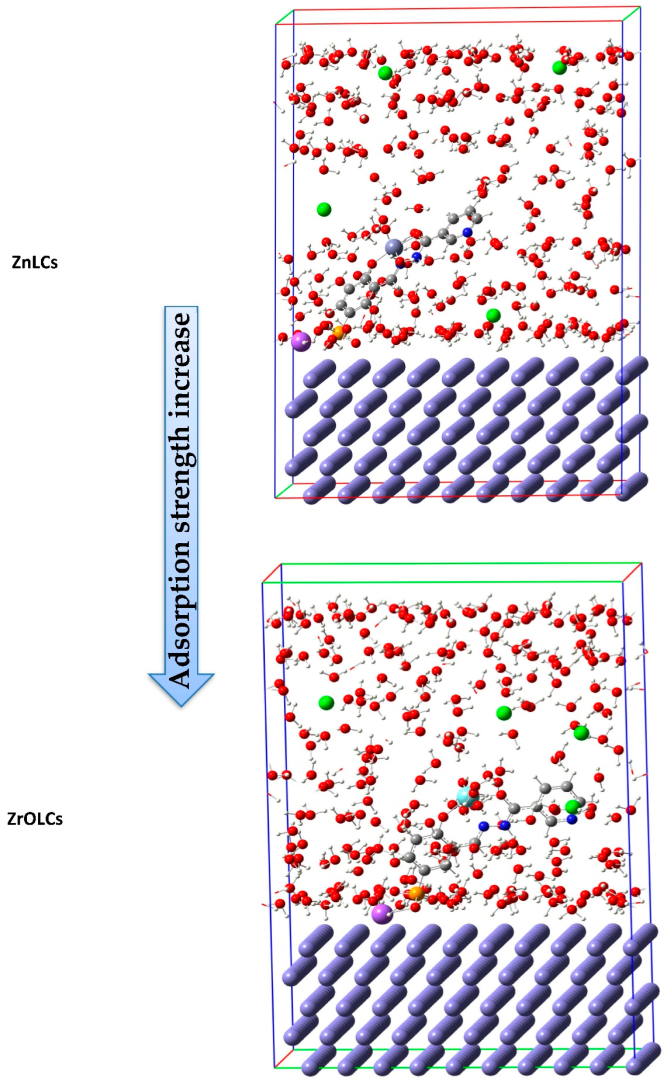
| 18 | 5-sodium sulfonate-2-hydroxybenzylidene)nicotinohydrazone (H2LCs) * | 1.0 M HCl | Mild steel | [101] |
| (ZnLCs) and (ZrOLCs) * | ||||
| 19 | 2,2′-((1E,1’E)-((4-nitro-1,2-phenylene)bis(azanylylidene))bis(methanylylidene))bis(4-bromophenol) (NABS) * | 1.0 N HCl | Carbon steel | [102] |
| 2,2′-((1E,1’E)-((4,5-dimethyl-1,2-phenylene)bis(azanylylidene))bis(methanylylidene))bis(4-bromophenol) (MABS). * | ||||
| 20 | 1-{(Z)-[(2-hydroxyphenyl)imino]methyl} naphthalen-2-ol * | 0.5 M H2SO4 | Carbon steel | [103] |
| Bis-phenanthroline chloro copper (II) chloride di-para-aminobenzoic acid tetrahydrate complex * | ||||
| [Cu(Phen)2Cl]Cl (pABz)2·4H2O (CuPAB) | ||||
| 21 | L-histidine (L1) and (L2) Schiff base ligands * | 2.0 M H2SO4 | Aluminum steel | [104] |
| [CuL1·EtOH] and [CuL2·EtOH] * | ||||
| [NiL1·(H2O)3] and [NiL2·EtOH] * | ||||
| [CoL1·(H2O)3] and [CoL2·H2O] * | ||||
| 22 | 4-Chloro-2-(2-oxo-1, 2-dihydro-indol-3-ylidene amino)-benzoic acid Schiff base (ACBAI) * | 0.1 NHNO3 | Mild steel | [105] |
| [Ti(IV) ACBAI] complex * | ||||
| [Zr(IV) ACBAI] complex * | ||||
| [Cd(II) ACBAI] complex * | ||||
| [Hg(II) ACBAI] complex * | ||||
| 23 | furfuraldehyde and 4,5-dimethyl-1,2-phenylendiamine (L1) * | 1 M HCl | (410 and 304) stainless steel | [106] |
| furfuraldehyde and 4,5-dichloro-1,2-phenylendiamine (L2) * | ||||
| [ZnL1](AcO)2·H2O and [ZnL2](AcO)2·H2O * | ||||
| [PdL1]Cl2 and [PdL2]Cl2 * | ||||
| 24 | Hapdhba * | 0.5 M HCl | Copper steel | [107] |
| cis-[Mo2O5(Hapdhba)2]·H2O * | ||||
| trans-[UO2(Hapdhba)2] * | ||||
| [Pd(Hapdhba)Cl(H2O)]·H2O * | ||||
| [Pd(bpy)(Hapdhba)]Cl·H2O * | ||||
| [Ag(bpy)(Hapdhba)]: * | ||||
| [Ru(Hapdhba)2(H2O)2]·2H2O * | ||||
| [Rh(Hapdhba)2(H2O)Cl]·3H2O * | ||||
| [Au(Hpadhba)Cl2]·H2O * |
| Inhibitor | Blank | Co Complex | ||
|---|---|---|---|---|
| Cinh (ppm) | 0 | 50 | 100 | 200 |
| Rct (Ω) | 124.40 | 407.40 | 490.80 | 480.60 |
| CPE-T (F) | 1.1 × 10−4 | 7.4 × 10−5 | 7.8 × 10−5 | 9.4 × 10−5 |
| IE% | - | 68.90 | 74.40 | 73.80 |
| Rs (Ω) | 2.36 | 1.98 | 1.90 | 1.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study. Int. J. Mol. Sci. 2022, 23, 9360. https://doi.org/10.3390/ijms23169360
El-Lateef HMA, El-Dabea T, Khalaf MM, Abu-Dief AM. Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study. International Journal of Molecular Sciences. 2022; 23(16):9360. https://doi.org/10.3390/ijms23169360
Chicago/Turabian StyleEl-Lateef, Hany M. Abd, Tarek El-Dabea, Mai M. Khalaf, and Ahmed M. Abu-Dief. 2022. "Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study" International Journal of Molecular Sciences 23, no. 16: 9360. https://doi.org/10.3390/ijms23169360








