Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Separation Media (BGE) and Sample Solutions
2.3. CE Experiments
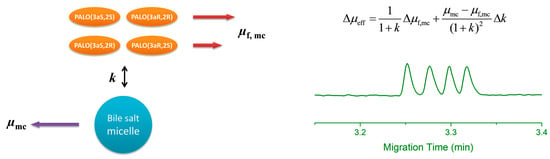
3. Theory and Calculation
3.1. Model of Chiral and Achiral Separation
3.2. Calculation of Retention Factors and Selectivities
4. Results and Discussion
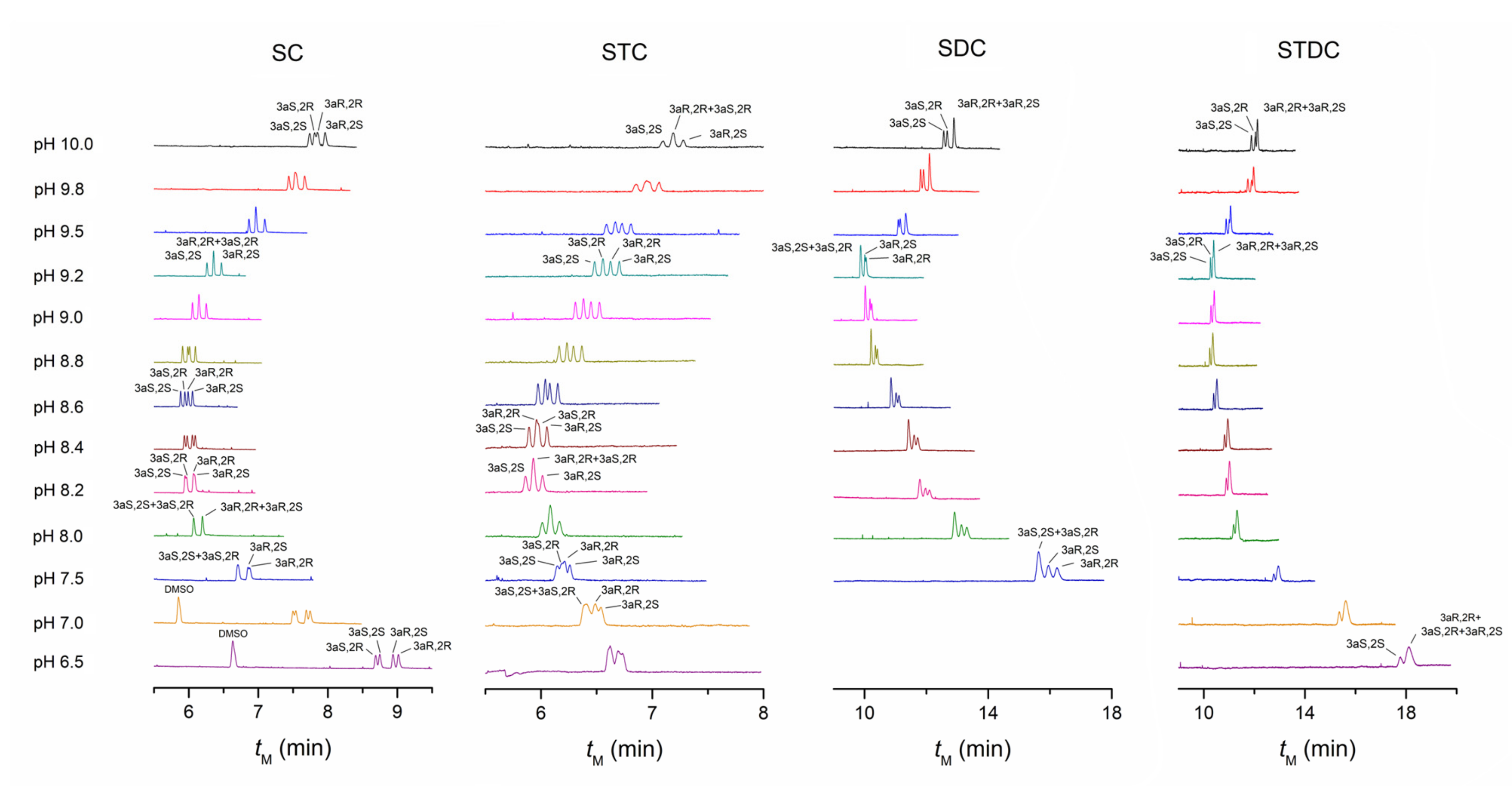
4.1. Comparison of Separation Performance of Different Bile Salts
4.1.1. The Performance of SC and STC
4.1.2. The Performance of SDC and STDC
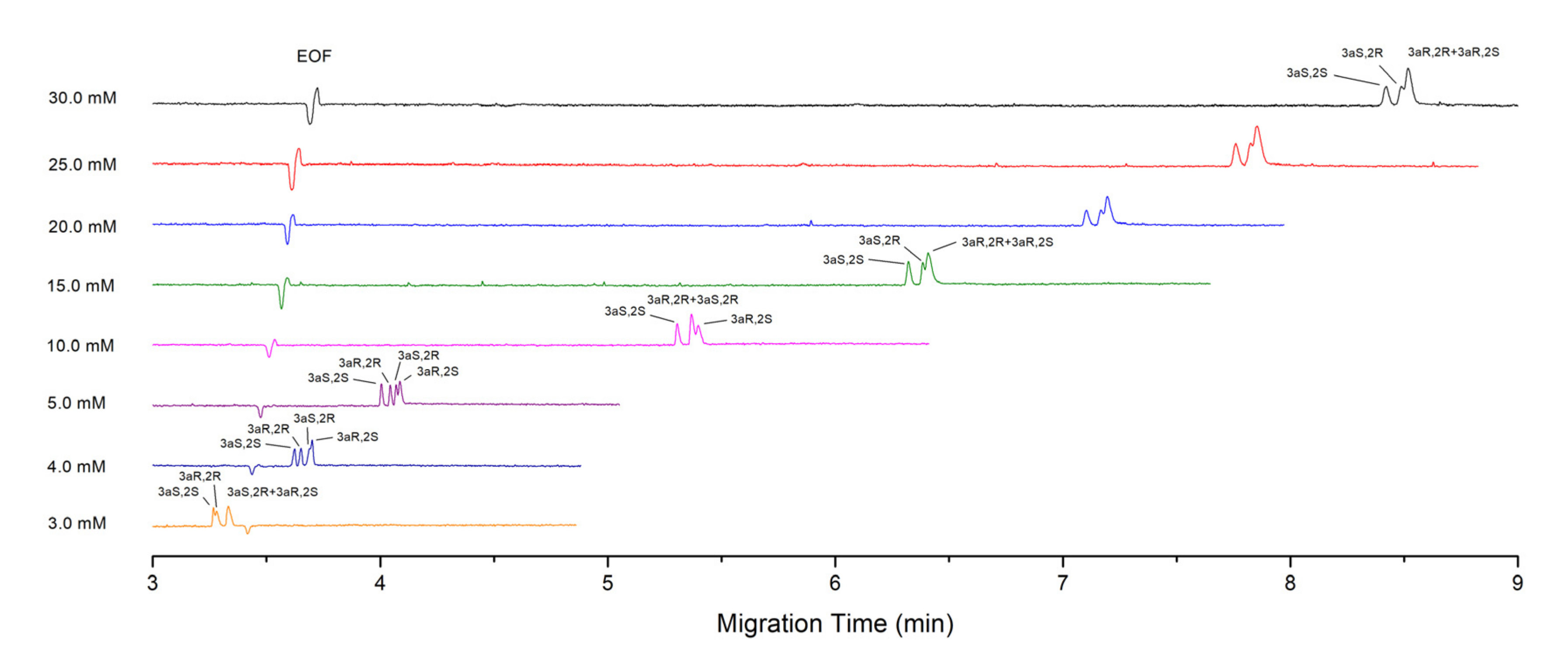
4.2. Effect of SDC and STDC Concentration on the Separation
4.2.1. Theoretical Consideration
4.2.2. Experiment Results
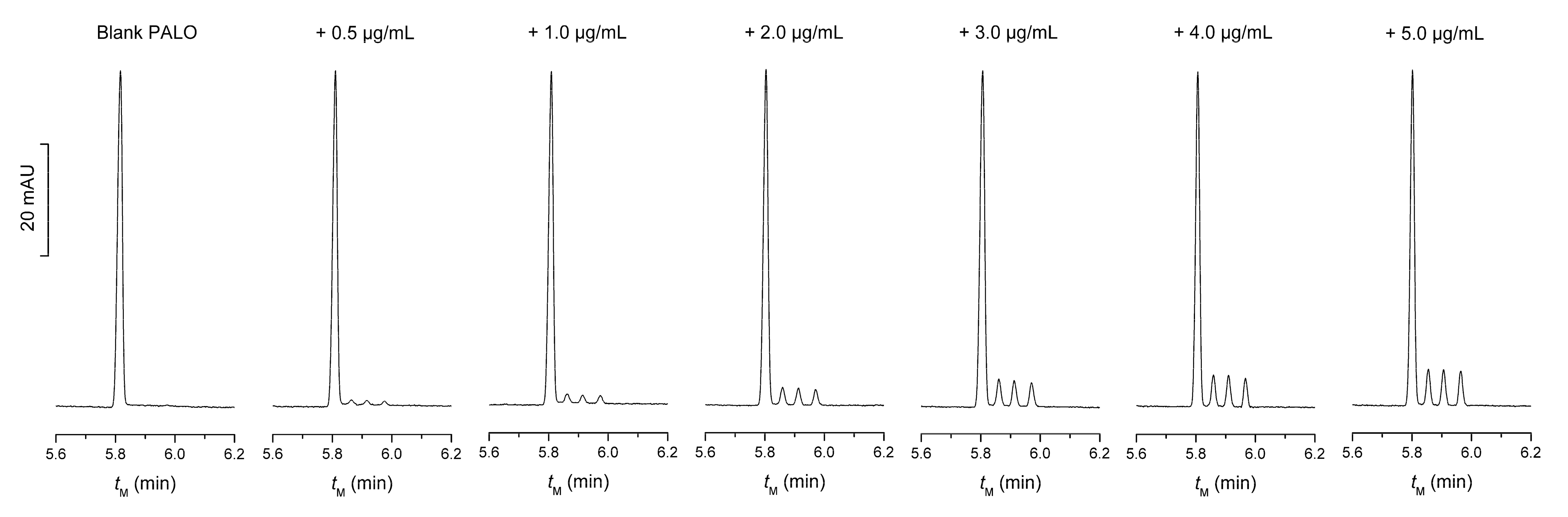
5. Validation of the Method
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Krait, S.; Konjaria, M.-L.; Scriba, G.K.E. Advances of capillary electrophoresis enantioseparations in pharmaceutical analysis (2017–2020). Electrophoresis 2021, 42, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Application of enantioselective separation techniques to bioanalysis of chiral drugs and their metabolites. TrAC Trends Anal. Chem. 2021, 143, 116332. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral liquid chromatography and capillary electrochromatography: Trends from 2017 to 2018. TrAC Trends Anal. Chem. 2019, 118, 779–792. [Google Scholar] [CrossRef]
- Fanali, C. Enantiomers separation by capillary electrochromatography. TrAC Trends Anal. Chem. 2019, 120, 115640. [Google Scholar] [CrossRef]
- Gunjal, P.; Singh, K.S.; Kumar, R.; Kumar, R.; Gulati, M. Role of Chromatograph-based Analytical Techniques in Quantification of Chiral Compounds: An Update. Curr. Anal. Chem. 2021, 17, 355–373. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. History, advancement, bottlenecks, and future of chiral capillary electrochromatography. J. Chromatogr. A 2021, 1637, 461832. [Google Scholar] [CrossRef]
- De Koster, N.; Clark, C.P.; Kohler, I. Past, present, and future developments in enantioselective analysis using capillary electromigration techniques. Electrophoresis 2021, 42, 38–57. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis 2019, 40, 2420–2437. [Google Scholar] [CrossRef]
- West, C. Recent trends in chiral supercritical fluid chromatography. TrAC Trends Anal. Chem. 2019, 120, 115648. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Analytical and preparative scale separation of enantiomers of chiral drugs by chromatography and related methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- El Deeb, S.; Silva, C.F.; Junior, C.S.; Hanafi, R.S.; Borges, K.B. Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling. Molecules 2021, 26, 2841. [Google Scholar] [CrossRef]
- Kodama, S.; Nakajima, S.; Ozaki, H.; Takemoto, R.; Itabashi, Y.; Kuksis, A. Enantioseparation of hydroxyeicosatetraenoic acids by hydroxypropyl-γ-cyclodextrin-modified micellar electrokinetic chromatography. Electrophoresis 2016, 37, 3196–3205. [Google Scholar] [CrossRef]
- Orlandini, S.; Pasquini, B.; Caprini, C.; Del Bubba, M.; Douša, M.; Pinzauti, S.; Furlanetto, S. Enantioseparation and impurity determination of ambrisentan using cyclodextrin-modified micellar electrokinetic chromatography: Visualizing the design space within quality by design framework. J. Chromatogr. A 2016, 1467, 363–371. [Google Scholar] [CrossRef]
- Terabe, S.; Otsuka, K.; Ando, T. Electrokinetic chromatography with micellar solution and open-tubular capillary. Anal. Chem. 1985, 57, 834–841. [Google Scholar] [CrossRef]
- Kaneta, T.; Tanaka, S.; Taga, M.; Yoshida, H. Migration behavior of inorganic anions in micellar electrokinetic capillary chromatography using cationic surfactant. Anal. Chem. 1992, 64, 798–801. [Google Scholar] [CrossRef]
- Deeb, S.E.; Iriban, M.A.; Gust, R. MEKC as a powerful growing analytical technique. Electrophoresis 2011, 32, 166–183. [Google Scholar] [CrossRef]
- Silva, M. Micellar electrokinetic chromatography: A review of methodological and instrumental innovations focusing on practical aspects. Electrophoresis 2013, 34, 141–158. [Google Scholar] [CrossRef]
- Otsuka, K.; Terabe, S. Enantiomer separation of drugs by micellar electrokinetic chromatography using chiral surfactants. J. Chromatogr. A 2000, 875, 163–178. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Enhanced Resolution of Chiral Amino Acids with Capillary Electrophoresis for Biosignature Detection in Extraterrestrial Samples. Anal. Chem. 2017, 89, 1329–1337. [Google Scholar] [CrossRef]
- García, M.Á.; Menéndez-López, N.; Boltes, K.; Castro-Puyana, M.; Marina, M.L. A capillary micellar electrokinetic chromatography method for the stereoselective quantitation of bioallethrin in biotic and abiotic samples. J. Chromatogr. A 2017, 1510, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, V.; García, M.Á.; Marina, M.L. Enantiomeric separation of cis-bifenthrin by CD-MEKC: Quantitative analysis in a commercial insecticide formulation. Electrophoresis 2010, 31, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xue, T.; He, T. Investigation on the chiral recognition mechanism between verteporfin and cholate salts by capillary electrophoresis. J. Sep. Sci. 2020, 43, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.B.; Quirino, J.P. Bile Salts in Chiral Micellar Electrokinetic Chromatography: 2000–2020. Molecules 2021, 26, 5531. [Google Scholar] [CrossRef]
- Madenci, D.; Egelhaaf, S.U. Self-assembly in aqueous bile salt solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Meier, A.R.; Yehl, J.B.; Eckenroad, K.W.; Manley, G.A.; Strein, T.G.; Rovnyak, D. Stepwise Aggregation of Cholate and Deoxycholate Dictates the Formation and Loss of Surface-Available Chirally Selective Binding Sites. Langmuir 2018, 34, 6489–6501. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Ch’ng, B.T.; Wallace, R.G. Studies in bile salt solutions: 1. Surface tension evidence for a stepwise aggregation model. J. Colloid Interface Sci. 1983, 95, 410–419. [Google Scholar] [CrossRef]
- Hebling, C.M.; Thompson, L.E.; Eckenroad, K.W.; Manley, G.A.; Fry, R.A.; Mueller, K.T.; Strein, T.G.; Rovnyak, D. Sodium Cholate Aggregation and Chiral Recognition of the Probe Molecule (R,S)-1,1′-Binaphthyl-2,2′-diylhydrogenphosphate (BNDHP) Observed by 1H and 31P NMR Spectroscopy. Langmuir 2008, 24, 13866–13874. [Google Scholar] [CrossRef]
- De Wit, R.; Aapro, M.; Blower, P. Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients? Cancer Chemother. Pharmacol. 2005, 56, 231–238. [Google Scholar] [CrossRef]
- Aapro, M.; Blower, P. 5-hydroxytryptamine type-3 receptor antagonists for chemotherapy-induced and radiotherapy-induced nausea and emesis. Cancer 2005, 104, 1–18. [Google Scholar] [CrossRef]
- Eisenberg, P.; Figueroa-Vadillo, J.; Zamora, R.; Charu, V.; Hajdenberg, J.; Cartmell, A.; Macciocchi, A.; Grunberg, S. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Cancer 2003, 98, 2473–2482. [Google Scholar] [CrossRef]
- El-Wahab Mubarak, M.A.; El-Bagary, R.I.; Abdul-Azim Mohammad, M.; Abo-Talib, N.F.; Elkady, E.F. Chiral and achiral liquid chromatographic separation of palonosetron hydrochloride and its related impurities utilizing two different stationary phases: Polysaccharide and alkyl amide columns. Microchem. J. 2021, 168, 106398. [Google Scholar] [CrossRef]
- Murthy, M.V.; Krishnaiah, C.; Jyothirmayi, K.; Srinivas, K.; Mukkanti, K.; Kumar, R.; Samanta, G. Enantioseparation of Palonosetron Hydrochloride and Its Related Enantiomeric Impurities by Computer Simulation and Validation. Am. J. Anal. Chem. 2011, 2, 437–466. [Google Scholar] [CrossRef]
- Wang, M.; Ding, X.; Chen, H.; Chen, X. Enantioseparation of Palonosetron Hydrochloride by Capillary Zone Electrophoresis with High-concentrationβ-CD as Chiral Selector. Anal. Sci. 2009, 25, 1217–1220. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Q.; Hu, S.; Guo, W.; Yang, Z.; Zhang, Y. Thermodynamic models to elucidate the enantioseparation of drugs with two stereogenic centers by micellar electrokinetic chromatography. J. Chromatogr. A 2017, 1512, 133–142. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Guo, X.-M.; Shi, H.-J.; Luo, R.-J. Separation mechanisms for palonosetron stereoisomers at different chiral selector concentrations in MEKC. Electrophoresis 2015, 36, 825–829. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Shi, H.-J.; Yu, H.; Dong, J.; Guo, X.-M. Solvent-modified MEKC for the enantioseparation of palonosetron hydrochloride and related enantiomeric impurities. Electrophoresis 2015, 36, 2762–2767. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Wang, G.-X.; Guo, W.-B.; Guo, X.-M.; Zhao, M. Effect of low concentration sodium dodecyl sulfate on the electromigration of palonosetron hydrochloride stereoisomers in micellar electrokinetic chromatography. J. Chromatogr. A 2014, 1342, 86–91. [Google Scholar] [CrossRef]
- Tian, K.; Chen, H.-L.; Tang, J.-H.; Chen, X.-G.; Hu, Z.-D. Enantioseparation of palonosetron hydrochloride by micellar electrokinetic chromatography with sodium cholate as chiral selector. J. Chromatogr. A 2006, 1132, 333–336. [Google Scholar] [CrossRef]
- Østergaard, J.; Jensen, H.; Holm, R. Use of correction factors in mobility shift affinity capillary electrophoresis for weak analyte—Ligand interactions. J. Sep. Sci. 2009, 32, 1712–1721. [Google Scholar] [CrossRef]
- Dubský, P.; Dvořák, M.; Ansorge, M. Affinity capillary electrophoresis: The theory of electromigration. Anal. Bioanal. Chem. 2016, 408, 8623–8641. [Google Scholar] [CrossRef]
- Britz-McKibbin, P.; Chen, D.D.Y. Accurately describing weak analyte-additive interactions by capillary electrophoresis. Electrophoresis 2002, 23, 880–888. [Google Scholar] [CrossRef]
- Valkó, I.E.; Billiet, H.A.H.; Frank, J.; Luyben, K.C.A.M. Factors affecting the separation of mandelic acid enantiomers by capillary electrophoresis. Chromatographia 1994, 38, 730–736. [Google Scholar] [CrossRef]
- Kawaoka, J.; Gomez, F.A. Use of mobility ratios to estimate binding constants of ligands to proteins in affinity capillary electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1998, 715, 203–210. [Google Scholar] [CrossRef]

| SC | STC | SDC | STDC | |
|---|---|---|---|---|
| k3aS,2S | 1.971 | 1.720 | 11.090 | 16.235 |
| k3aR,2R | 2.113 | 1.795 | 12.469 | 18.244 |
| k3aS,2R | 2.004 | 1.770 | 10.718 | 17.086 |
| k3aR,2S | 2.186 | 1.850 | 11.692 | 17.632 |
| α3aR,2R/3aS,2S | 1.072 | 1.043 | 1.214 | 1.124 |
| α3aR,2S/3aS,2R | 1.091 | 1.045 | 1.091 | 1.032 |
| α3aR,2R/3aS,2R | 1.054 | 1.014 | 1.163 | 1.068 |
| α3aR,2S/3aS,2S | 1.109 | 1.075 | 1.054 | 1.086 |
| α3aR,2R/3aR,2S | 0.966 | 0.970 | 1.067 | 1.035 |
| α3aS,2R/3aS,2S | 1.017 | 1.029 | 0.966 | 1.052 |
| t0/tmc (min) | 4.283/9.659 | 4.519/11.236 | 4.092/11.924 | 4.285/11.612 |
| PALO (3aR, 2R) | PALO (3aS, 2R) | PALO (3aR, 2S) | |
|---|---|---|---|
| Calibration range (μg∙mL−1) | 0.5–5.0 | 0.5–5.0 | 0.5–5.0 |
| Regression equation | y = ax + b y: peak area (mV∙s) x: concentration of enantiomeric impurities (μg∙mL−1) | ||
| Slope, a | 1.421 | 1.407 | 1.391 |
| Intercept, b | 0.008 | −0.014 | 0.030 |
| Correlation coefficient, R2 | 0.999 | 0.994 | 0.996 |
| Limit of detection (LOD, μg∙mL−1) | 0.09 | 0.10 | 0.10 |
| Limit of quantification (LOQ, μg∙mL−1) | 0.31 | 0.32 | 0.32 |
| Recovery (%) b | 99.1 | 95.6 | 96.7 |
| Repeatability (RSD, %) b | |||
| Intra-day c | 3.3 | 3.7 | 2.2 |
| Inter-day d | 6.9 | 4.5 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Sun, T.; Li, R.; Zhang, D.; Zhang, Y.; Yang, Z.; Feng, G.; Guo, X. Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography. Molecules 2022, 27, 5233. https://doi.org/10.3390/molecules27165233
Hu S, Sun T, Li R, Zhang D, Zhang Y, Yang Z, Feng G, Guo X. Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography. Molecules. 2022; 27(16):5233. https://doi.org/10.3390/molecules27165233
Chicago/Turabian StyleHu, Shaoqiang, Tao Sun, Rui Li, Dongdong Zhang, Yonghua Zhang, Zhuo Yang, Ge Feng, and Xuming Guo. 2022. "Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography" Molecules 27, no. 16: 5233. https://doi.org/10.3390/molecules27165233