12/15-Lipoxygenase Regulation of Diabetic Cognitive Dysfunction Is Determined by Interfering with Inflammation and Cell Apoptosis
Abstract
:1. Introduction
2. Results
2.1. MMSE Scores
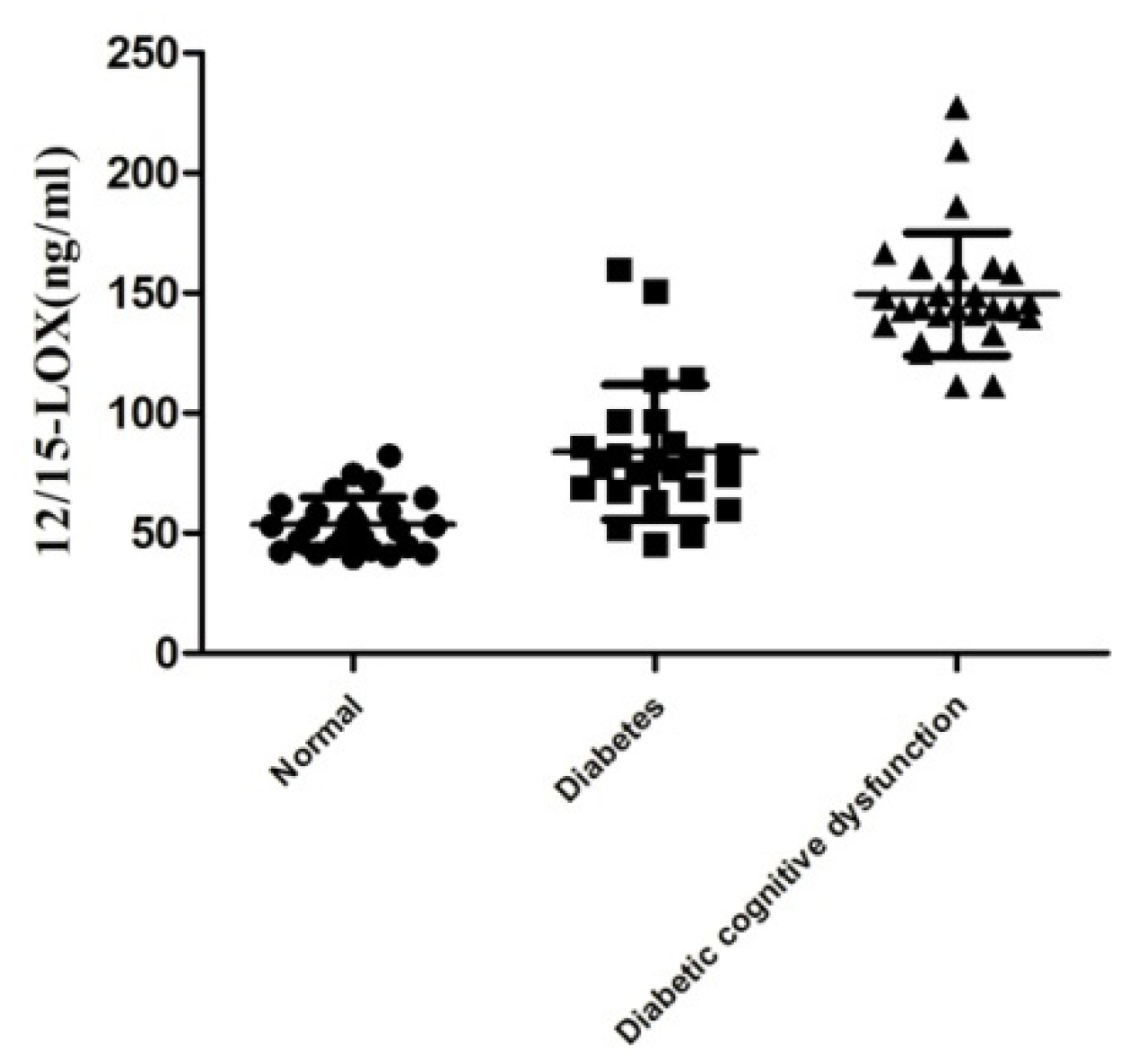
2.2. Level of 12/15-LOX in the Plasma
2.3. Effect of 12/15-LOX on Fasting Blood Glucose and Random Blood Glucose in Diabetic Rats
2.4. Effects of 12/15-LOX on the Changes in Insulin, TG, TC and LDL-C Levels in the Plasma of Rats with Diabetic Cognitive Dysfunction
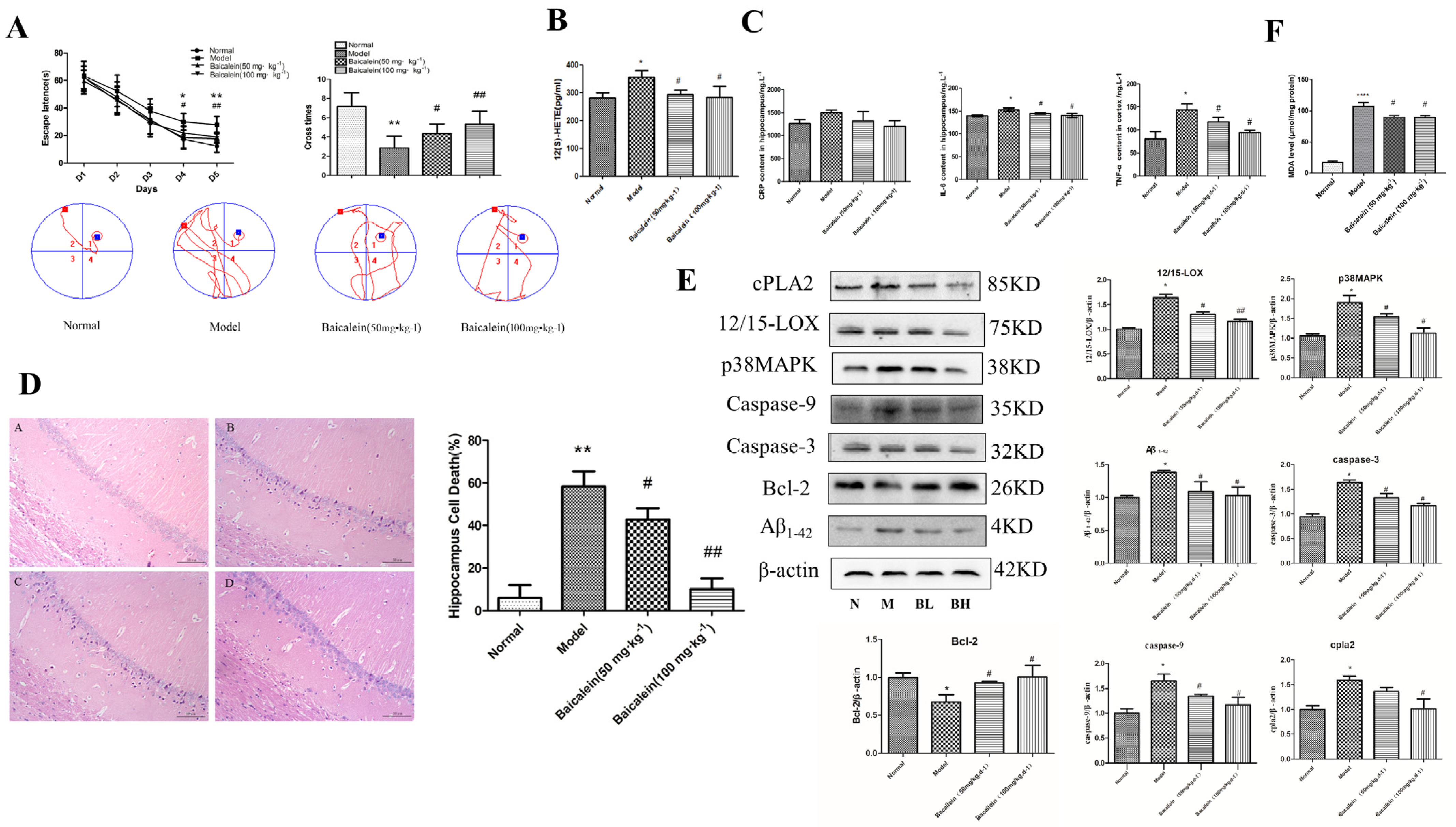
2.5. Effects of 12/15-LOX on Changes in Learning and Memory Functions in Rats with Diabetic Cognitive Dysfunction
2.6. Effects of 12/15-LOX on the Changes in Neuronal Morphology in the Hippocampus of Rats with Diabetic Cognitive Dysfunction
2.7. Effects of 12/15-LOX on Changes in C Reaction Protein (CRP), Tumour Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6) in the Hippocampus of Rats with Diabetic Cognitive Dysfunction
2.8. Changes in 12/15-LOX, p38MAPK, Aβ1-42, Caspase-3, Caspase-9, Bcl-2, Phospho-p38MAPK and cPLA2 Expression in the Hippocampus of Rats with Diabetic Cognitive Dysfunction
2.9. Changes in 12(S)-HETE Levels in the Hippocampus of Rats with Diabetic Cognitive Dysfunction
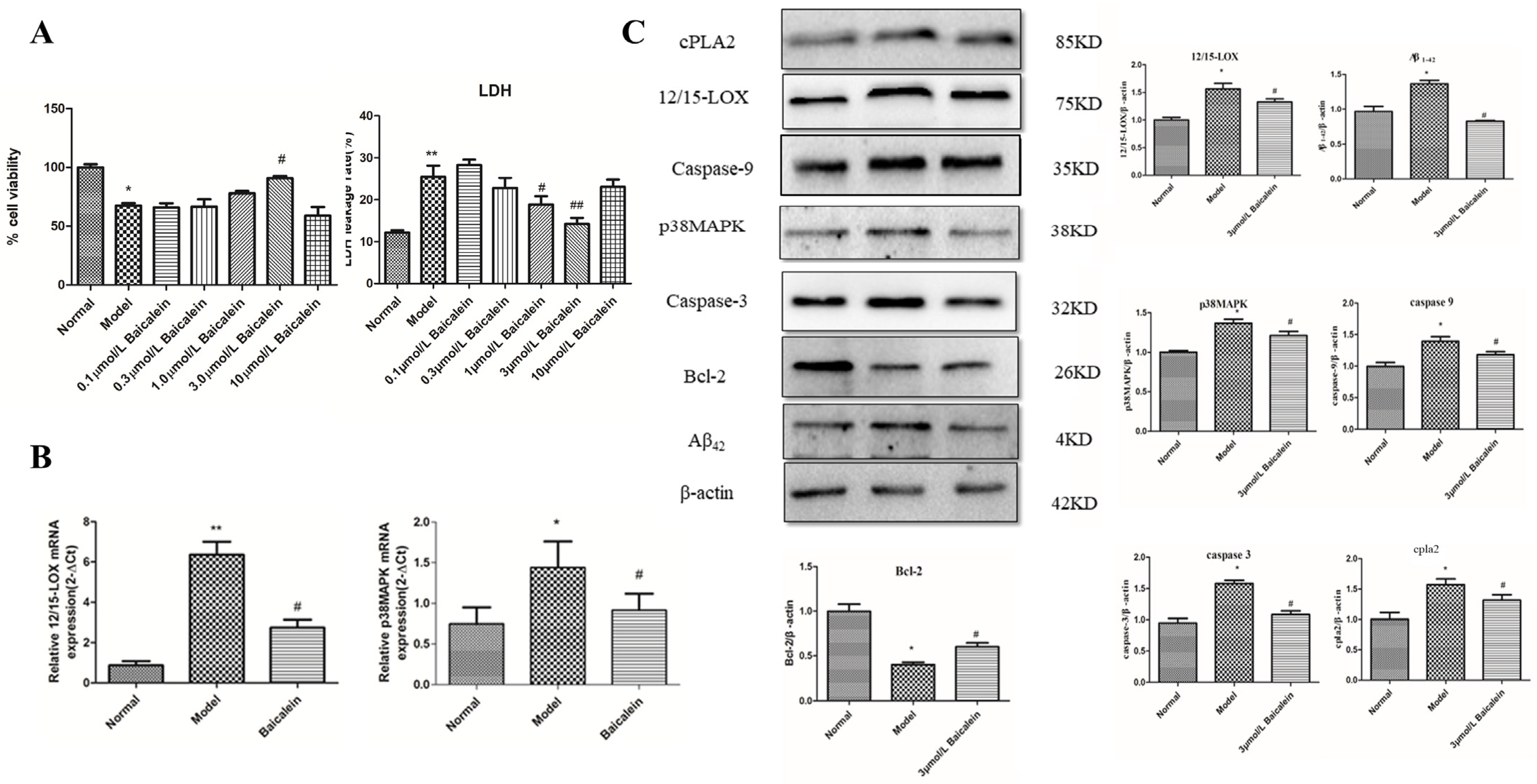
2.10. Effect of 12/15-LOX on HT22 Cells Treated with High-Glucose Medium
2.11. Effect of 12/15-LOX at Different Concentrations on LDH Leakage in HT22 Cells Treated with High Glucose
2.12. Changes in 12/15-LOX and p38 MAPK mRNA Expression in HT22 Cells Treated with High Glucose
2.13. The Protein Expression of 12/15-LOX, p38 MAPK, Aβ42, Caspase-3, Caspase-9, Bcl-2, Phospho-p38 MAPK and cPLA2 in HT22 Cells Treated with High-Glucose Medium
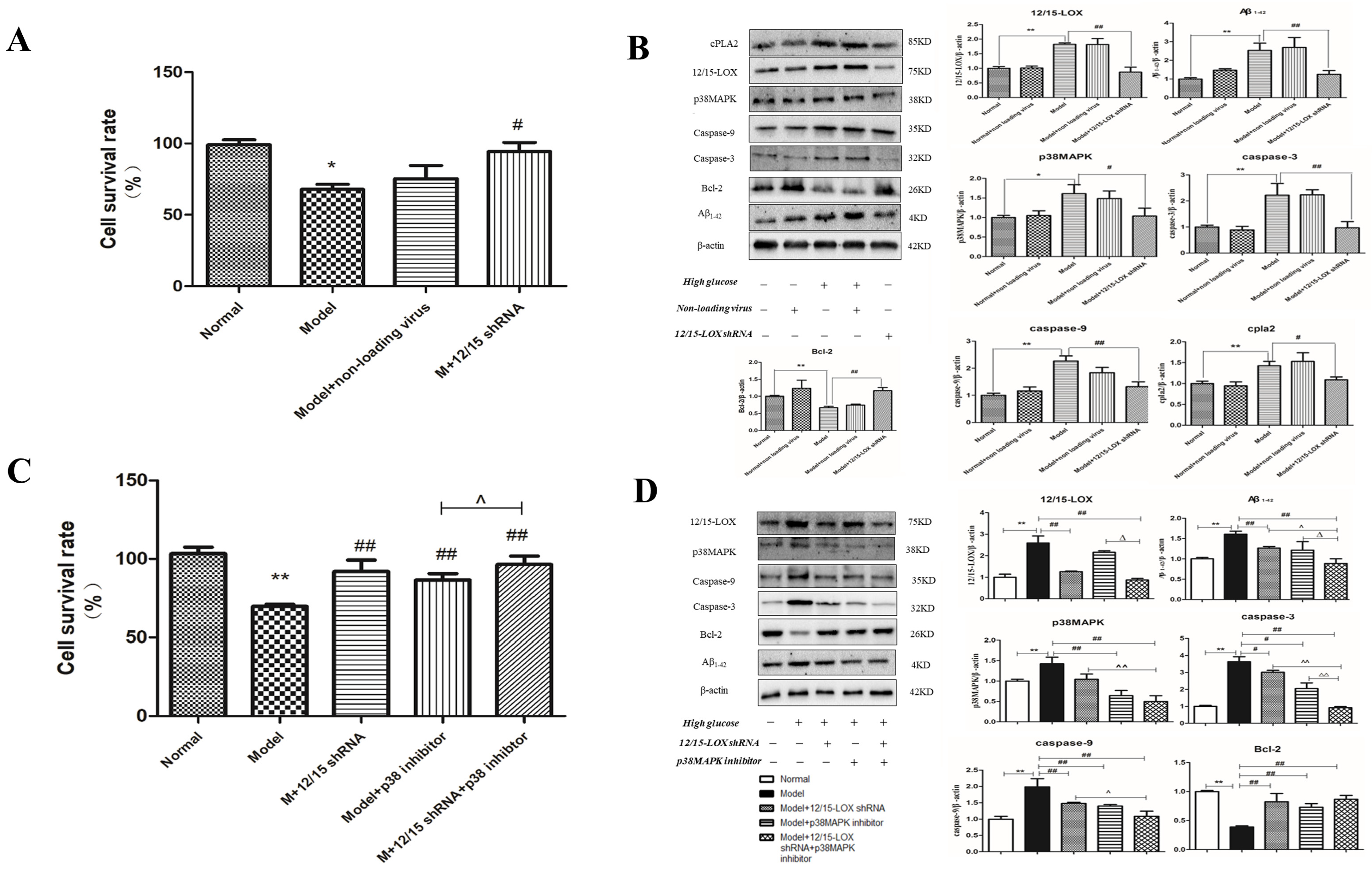
2.14. Effect of 12/15-LOX shRNA Transfection on the Survival Rate of HT22 Cells Treated with High-Glucose Medium
2.15. Effect of 12/15-LOX shRNA on the Expression of 12/15-LOX, p38 MAPK, Aβ, Caspase-3, Caspase-9, Bcl-2, Phospho-p38 MAPK and cPLA2 Protein in HT22 Cells Treated with High Glucose
2.16. Effect of 12/15-LOX shRNA and p38MAPK Inhibitor on the Cell Survival Rate of HT22 Cells Treated with High Glucose
2.17. Effect of 12/15-LOX shRNA and p38MAPK Inhibitor on the Expression of 12/15-LOX, p38MAPK, Aβ1-42, Caspase-3, Caspase-9, Bcl-2 and Phospho-p38MAPK Protein in HT22 Cells Treated with High Glucose
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. The MMSE
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Animal
4.5. Establishment of Animal Models
| HFD | |
| Sugar | 20% |
| Lard | 10% |
| Egg yolk | 10% |
| Basal (protein 20.6%, fat 12.0%, carbohydrate 67.4%) | 60% |
4.6. Fasting Blood Glucose and Random Blood Glucose Test
4.7. The Morris Water Maze Test
4.8. Biochemical Assays
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Histopathological Observation
4.11. Western Blotting
4.12. Cell Culture
4.13. Lentivirus Transfection
4.14. MTT Assay
4.15. LDH leakage
4.16. Quantitative Real-Time PCR (qPCR)
4.17. Chemicals
4.18. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Reagan, L.P. Diabetes as a chronic metabolic stressor: Causes, consequences and clinical complications. Exp. Neurol. 2012, 233, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Surkova, E.V. Diabetes mellitus and the central nervous system. Ter. Arkh. 2016, 88, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Rauch, G.; Rauch, R.A.; Haque, A. Risk factors for cerebral hypoperfusion, mild cognitive dysfunction, and dementia. Neurobiol. Aging. 2000, 21, 161–169. [Google Scholar] [CrossRef]
- Akaishi, T.; Homma, S. Baroreceptor control of vasopressin-producing cells in streptozotocin diabetic rats. Brain Res. Bull. 1993, 31, 719–722. [Google Scholar] [CrossRef]
- Ragy, M.M.; Kamal, N.N. Linking senile dementia to type 2 diabetes: Role of oxidative stress markers, C-reactive protein and tumor necrosis factor-α. Neurol. Res. 2017, 39, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Borkowska, A.; Ciebiada, M.; Loba, J. Serum levels of inflammatory markers in depressed elderly patients with diabetes and mild cognitive dysfunction. PLoS ONE 2015, 20, e0120433. [Google Scholar]
- Jawale, A.; Datusalia, A.K.; Bishnoi, M.; Sharma, S.S. Reversal of diabetes-induced behavioral and neurochemical deficits by cinnamaldehyde. Phytomedicine 2016, 15, 923–930. [Google Scholar] [CrossRef]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef]
- Dzamko, N.; Geczy, C.L.; Halliday, G.M. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 2015, 302, 89–102. [Google Scholar] [CrossRef]
- Herder, C.; Fürstos, J.F.; Nowotny, B.; Begun, A.; Strassburger, K.; Müssig, K.; Szendroedi, J.; Icks, A.; Roden, M.; GDS Group. Associations between inflammation-related biomarkers and depressive symptoms in individuals with recently diagnosed type 1 and type 2 diabetes. Brain. Behav. Immun. 2017, 61, 137–145. [Google Scholar] [CrossRef]
- Hajebrahimi, B.; Kiamanesh, A.; Asgharnejad Farid, A.A.; Asadikaram, G. Type 2 diabetes and mental disorders; a plausible link with inflammation. Cell Mol. Biol. 2016, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Nadler, J.L. Inflammatory mechanisms of diabetic complications. Curr. Diab. Rep. 2007, 7, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Rumzhum, N.N.; Ammit, A.J. Cyclooxygenase 2: Its regulation, role and impact in airway inflammation. Clin. Exp. Allergy. 2016, 46, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta. 2015, 1851, 331–339. [Google Scholar] [CrossRef]
- Luu, L.; Dai, F.F.; Prentice, K.J.; Huang, X.; Hardy, A.B.; Hansen, J.B.; Liu, Y.; Joseph, J.W.; Wheeler, M.B. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia 2013, 56, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tang, L.; Qu, Z.; Lei, S.H.; Li, W.; Wang, Y.H. Hippocampal insulin resistance and the Sirtuin 1 signaling pathway in diabetes-induced cognitive dysfunction. Neural. Regen. Res. 2021, 16, 2465–2474. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022. [Google Scholar] [CrossRef]
- Morgan, A.H.; Hammond, V.J.; Sakoh-Nakatogawa, M.; Ohsumi, Y.; Thomas, C.P.; Blanchet, F.; Piguet, V.; Kiselyov, K.; O’Donnell, V.B. A novel role for 12/15-lipoxygenase in regulating autophagy. Redox. Biol. 2015, 4, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid. Res. 2006, 45, 334–356. [Google Scholar] [CrossRef]
- Hernandez-Perez, M.; Chopra, G.; Fine, J.; Conteh, A.M.; Anderson, R.M.; Linnemann, A.K.; Benjamin, C.; Nelson, J.B.; Benninger, K.S.; Nadler, J.L.; et al. Inhibition of 12/15-Lipoxygenase Protects Against β-Cell Oxidative Stress and Glycemic Deterioration in Mouse Models of Type 1 Diabetes. Diabetes 2017, 66, 2875–2887. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Taylor-Fishwick, D.A.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 2011, 50, 115–131. [Google Scholar] [CrossRef]
- Conteh, A.M.; Reissaus, C.A.; Hernandez-Perez, M.; Nakshatri, S.; Anderson, R.M.; Mirmira, R.G.; Tersey, S.A.; Linnemann, A.K. Platelet-type 12-lipoxygenase deletion provokes a compensatory 12/15-lipoxygenase increase that exacerbates oxidative stress in mouse islet β cells. J. Biol. Chem. 2019, 294, 6612–6620. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Natarajan, R.; LaPage, J.; Lanting, L.; Kim, N.; Becerra, D.; Clemmons, B.; Nast, C.C.; Surya Prakash, G.K.; Mandal, M.; et al. 12/15-lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 13–20. [Google Scholar] [CrossRef]
- Suzuki, H.; Kayama, Y.; Sakamoto, M.; Iuchi, H.; Shimizu, I.; Yoshino, T.; Katoh, D.; Nagoshi, T.; Tojo, K.; Minamino, T.; et al. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes 2015, 64, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.; Pye, C.; Al-Shabrawey, M.; Elmarakby, A.A. Inhibition of 12/15-Lipoxygenase Reduces Renal Inflammation and Injury in Streptozotocin-Induced Diabetic Mice. J. Diabetes Metab. 2015, 6, 555. [Google Scholar]
- Obrosova, I.G.; Stavniichuk, R.; Drel, V.R.; Shevalye, H.; Vareniuk, I.; Nadler, J.L.; Schmidt, R.E. Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am. J. Pathol. 2010, 177, 1436–1447. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Giannopoulos, P.F.; Praticò, D. The 12/15-lipoxygenase as an emerging therapeutic target for Alzheimer’s disease. Trends. Pharmacol. Sci. 2015, 36, 181–186. [Google Scholar] [CrossRef]
- Di Meco, A.; Li, J.G.; Blass, B.E.; Abou-Gharbia, M.; Lauretti, E.; Praticò, D. 12/15-Lipoxygenase Inhibition Reverses Cognitive dysfunction, Brain Amyloidosis, and Tau Pathology by Stimulating Autophagy in Aged Triple Transgenic Mice. Biol. Psychiatry 2017, 81, 92–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Karatas, H.; Yigitkanli, K.; Holman, T.R.; van Leyen, K. Contributions of 12/15-Lipoxygenase to Bleeding in the Brain Following Ischemic Stroke. Adv. Exp. Med. Biol. 2019, 1161, 125–131. [Google Scholar] [PubMed]
- Yigitkanli, K.; Zheng, Y.; Pekcec, A.; Lo, E.H.; van Leyen, K. Increased 12/15-Lipoxygenase Leads to Widespread Brain Injury Following Global Cerebral Ischemia. Transl. Stroke Res. 2017, 8, 194–202. [Google Scholar] [CrossRef]
- Karatas, H.; Eun Jung, J.; Lo, E.H.; van Leyen, K. Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models. Brain Res. 2018, 1678, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gaberel, T.; Gakuba, C.; Zheng, Y.; Lépine, M.; Lo, E.H.; van Leyen, K. Impact of 12/15-Lipoxygenase on Brain Injury After Subarachnoid Hemorrhage. Stroke 2019, 50, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Huyck, R.W.; Glenn, L.; Gottipati, V.; Haynes, B.A.; Hansson, G.I.; Marley, A.; McPheat, W.L.; Nadler, J.L. Activation of the 12/15 lipoxygenase pathway accompanies metabolic decline in db/db pre-diabetic mice. Prostaglandins Lipid Mediat. 2018, 136, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Brünner, Y.F.; Kofoet, A.; Benedict, C.; Freiherr, J. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J. Clin. Endocrinol. Metab. 2015, 100, 212–219. [Google Scholar] [CrossRef]
- Somfai, G.M.; Knippel, B.; Ruzicska, E.; Stadler, K.; Tóth, M.; Salacz, G.; Magyar, K.; Somogyi, A. Soluble semicarbazide-sensitive amine oxidase (SSAO) activity is related to oxidative stress and subchronic inflammation in streptozotocin-induced diabetic rats. Neurochem. Int. 2006, 48, 746–752. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Gu, J.; Xiong, Z.; Wang, F.; Wang, J.; Wang, W.; Chen, J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol. Biochem. Behav. 2007, 86, 423–430. [Google Scholar] [CrossRef]
- Pallast, S.; Arai, K.; Pekcec, A.; Yigitkanli, K.; Yu, Z.; Wang, X.; Lo, E.H.; van Leyen, K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: A 12/15-lipoxygenase-dependent organelle damage pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1157–1167. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, R.; Cao, L. Neuroprotection of taurine through inhibition of 12/15 lipoxygenase pathway in cerebral ischemia of rats. Neurol. Res. 2017, 39, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Stavniichuk, R.; Obrosov, A.A.; Drel, V.R.; NadlerJ, L.; Obrosova, I.G.; Yorek, M.A. 12/15-Lipoxygenase inhibition counteracts MAPK phosphorylation in mouse and cell culture models of diabetic peripheral neuropathy. J. Diabetes Mellitus 2013, 3. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, X.; Yang, R.; Wang, L.; Liu, L.; Li, M.; Du, W. Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: Down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res. 2010, 1325, 164–173. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Zhang, W.; Sima, A.A. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes 2007, 56, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K. Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by β-Amyloid in rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef]
- Hernandez, C.M.; Kayed, R.; Zheng, H.; Sweatt, J.D.; Dineley, K.T. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2010, 30, 2442–2453. [Google Scholar] [CrossRef]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, S.; Masheta, D.; Guildford, A.L.; Phillips, G.; Santin, M. Dendrimeric Poly (Epsilon-Lysine) Delivery Systems for the Enhanced Permeability of Flurbiprofen across the Blood-Brain Barrier in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 3224. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Ma, B.; Nussinov, R. Synergistic interactions between repeats in tau protein and Aβ amyloids may be responsible for accelerated aggregation via polymorphic states. Biochemistry 2011, 50, 5172–5181. [Google Scholar] [CrossRef]
- Pan, X.D.; Zhu, Y.G.; Lin, N.; Zhang, J.; Ye, Q.Y.; Huang, H.P.; Chen, X.C. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 45. [Google Scholar] [CrossRef]
- Nito, C.; Kamada, H.; Endo, H.; Niizuma, K.; Myer, D.; Chan, P. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2008, 28, 1686–1696. [Google Scholar] [CrossRef]
- Choi, A.Y.; Choi, J.H.; Lee, J.Y.; Yoon, K.S.; Choe, W.; Ha, J.; Yeo, E.J.; Kang, I. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem. Int. 2010, 57, 143–152. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Suo, W.Z. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci. 2009, 84, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ou, S.; Zhou, L.; Tang, H.; Xu, J.; Guo, K.H. Formononetin attenuates Aβ25-35-induced cytotoxicity in HT22 cells via PI3K/Akt signaling and non-amyloidogenic cleavage of APP. Neurosci. Lett. 2017, 639, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Yamabe, N.; Wen, Y.; Fukui, M.; Zhu, B.T. Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration. Brain Res. 2013, 1497, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gliyazova, N.S.; Ibeanu, G.C. The chemical molecule B355252 is neuroprotective in an in vitro model of parkinson’s Disease. Cell Mol. Neurobiol. 2016, 36, 1109–1122. [Google Scholar] [CrossRef] [PubMed]


| Item | Normal (n = 26) | Diabetic Patients MMSE > 27(n = 24) | Diabetic Patients MMSE < 27 (n = 27) |
|---|---|---|---|
| Orientation | 9.81 ± 0.39 | 9.67 ± 0.48 | 9.19 ± 0.57 * |
| Memory | 2.75 ± 0.44 | 2.67 ± 0.48 | 2.15 ± 0.47 * |
| Calculation and attention | 4.54 ± 0.51 | 4.00 ± 0.51 * | 3.33 ± 0.55 ** # |
| Recall | 2.69 ± 0.47 | 2.42 ± 0.50 | 2.15 ± 0.46 |
| Language | 9.42 ± 0.50 | 9.04 ± 0.46 | 8.70 ± 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zheng, Q.; Yang, Y.; Luo, Y.; Wang, H.; Li, H.; Yang, L.; Hu, C.; Zhang, J.; Li, Y.; et al. 12/15-Lipoxygenase Regulation of Diabetic Cognitive Dysfunction Is Determined by Interfering with Inflammation and Cell Apoptosis. Int. J. Mol. Sci. 2022, 23, 8997. https://doi.org/10.3390/ijms23168997
Chen Q, Zheng Q, Yang Y, Luo Y, Wang H, Li H, Yang L, Hu C, Zhang J, Li Y, et al. 12/15-Lipoxygenase Regulation of Diabetic Cognitive Dysfunction Is Determined by Interfering with Inflammation and Cell Apoptosis. International Journal of Molecular Sciences. 2022; 23(16):8997. https://doi.org/10.3390/ijms23168997
Chicago/Turabian StyleChen, Qi, Qixue Zheng, Yang Yang, Ying Luo, Hong Wang, Huan Li, Lu Yang, Congli Hu, Jiahua Zhang, Yuke Li, and et al. 2022. "12/15-Lipoxygenase Regulation of Diabetic Cognitive Dysfunction Is Determined by Interfering with Inflammation and Cell Apoptosis" International Journal of Molecular Sciences 23, no. 16: 8997. https://doi.org/10.3390/ijms23168997
APA StyleChen, Q., Zheng, Q., Yang, Y., Luo, Y., Wang, H., Li, H., Yang, L., Hu, C., Zhang, J., Li, Y., Xia, H., Chen, Z., Ma, J., Tian, X., & Yang, J. (2022). 12/15-Lipoxygenase Regulation of Diabetic Cognitive Dysfunction Is Determined by Interfering with Inflammation and Cell Apoptosis. International Journal of Molecular Sciences, 23(16), 8997. https://doi.org/10.3390/ijms23168997






