Sample Preparation and Analytical Methods for Identifying Organic Compounds in Bituminous Emissions
Abstract
:1. Introduction
2. Regulations and Past Research
3. Sample Collection and Sample Enrichment Methods
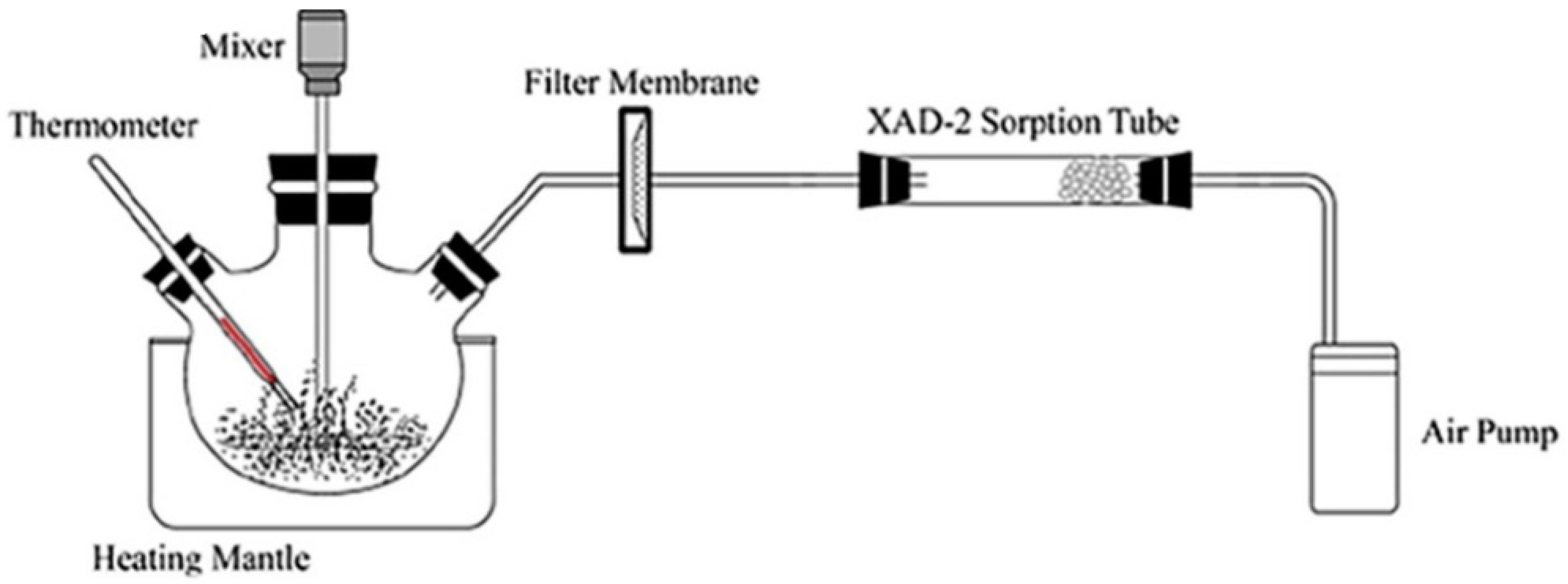
3.1. Filter Sampling
3.2. Headspace Sampling
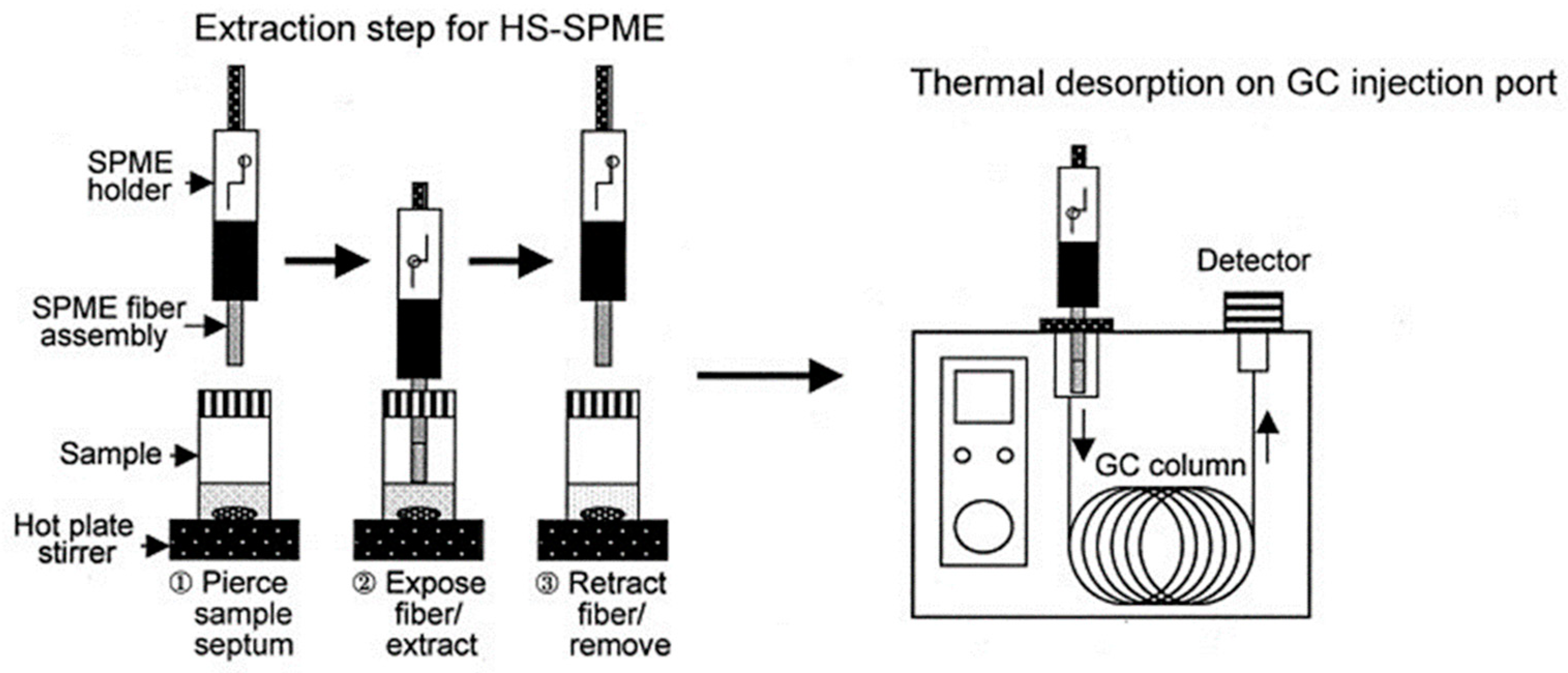
3.3. Headspace Solid Phase Microextraction Sampling
3.4. Nonseparative Real Time Sampling
3.5. Summary of Sample Preparation Techniques
4. Considerations for GC or LC Instruments and Detectors When Analysing Bitumen
5. Concerning Additives for Fume Control in Bitumen
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mundt, D.J.; Adams, R.C.; Marano, K.M. A Historical Review of Additives and Modifiers Used in Paving Asphalt Refining Processes in the United States. J. Occup. Environ. Hyg. 2009, 6, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.N.; Self, A.; Read, J. Shell Bitumen Handbook, 6th ed.; ICE: London, UK, 2014. [Google Scholar]
- Eurobitume; Asphalt institute. IS-230 The Bitumen Industry—A Global Perspective; Asphalt Institute: Lexington, KY, USA, 2015; p. 58. [Google Scholar]
- Weigel, S.; Stephan, D. The prediction of bitumen properties based on FTIR and multivariate analysis methods. Fuel 2017, 208, 655–661. [Google Scholar] [CrossRef]
- Rubio, M.C.; Martinez, G.; Baena, L.; Moreno, F. Warm mix asphalt: An overview. J. Clean. Prod. 2012, 24, 76–84. [Google Scholar] [CrossRef]
- Binet, S.; Bonnet, P.; Brandt, H.; Castegnaro, M.; Delsaut, P.; Fabries, J.F.; Huynh, C.K.; Lafontaine, M.; Morel, G.; Nunge, H.; et al. Development and validation of a new bitumen fume generation system which generates polycyclic aromatic hydrocarbon concentrations proportional to fume concentrations. Ann. Occup. Hyg. 2002, 46, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiss, A.; Koch, W.; Kock, H.; Elend, M.; Raabe, M.; Pohlmann, G. Collection, validation and generation of bitumen fumes for inhalation studies in rats Part 1: Workplace samples and validation criteria. Ann. Occup. Hyg. 2006, 50, 789–804. [Google Scholar] [CrossRef] [Green Version]
- Brandt, H.C.A.; De Groot, P.C. A laboratory rig for studying aspects of worker exposure to bitumen fumes. Am. Ind. Hyg. Assoc. J. 1999, 60, 182–190. [Google Scholar] [CrossRef]
- Autelitano, F.; Giuliani, F. Analytical assessment of asphalt odor patterns in hot mix asphalt production. J. Clean. Prod. 2018, 172, 1212–1223. [Google Scholar] [CrossRef]
- Lange, C.R.; Stroup-Gardiner, M. Temperature-dependent chemical-specific emission rates of aromatics and polyaromatic hydrocarbons (PAHs) in bitumen fume. J. Occup. Environ. Hyg. 2007, 4, 72–76. [Google Scholar] [CrossRef]
- Yu, M.; Wu, S.; Chen, M.; Zhang, H. Evaluation of volatile organic compounds from asphalt using UV-visible spectrometer. Adv. Mater. Res. 2012, 472–475, 432–436. [Google Scholar] [CrossRef]
- Gasthauer, E.; Maze, M.; Marchand, J.P.; Amouroux, J. Characterization of asphalt fume composition by GC/MS and effect of temperature. Fuel 2008, 87, 1428–1434. [Google Scholar] [CrossRef]
- Autelitano, F.; Bianchi, F.; Giuliani, F. Airborne emissions of asphalt/wax blends for warm mix asphalt production. J. Clean. Prod. 2017, 164, 749–756. [Google Scholar] [CrossRef]
- McCarthy, B.M.; Blackburn, G.R.; Kriech, A.J.; Kurek, J.T.; Wissel, H.L.; Osborn, L.V. Comparison of field- and laboratory-generated asphalt fumes. Transp. Res. Rec. 1999, 1661, 54–59. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Nian, T.; Mao, Y. An overview of studies on the hazards, component analysis and suppression of fumes in asphalt and asphalt mixtures. Constr. Build. Mater. 2021, 289, 123185. [Google Scholar] [CrossRef]
- Bolliet, C.; Kriech, A.J.; Juery, C.; Vaissiere, M.; Brinton, M.A.; Osborn, L.V. Effect of Temperature and Process on Quantity and Composition of Laboratory-generated Bitumen Emissions. J. Occup. Environ. Hyg. 2015, 12, 438–449. [Google Scholar] [CrossRef]
- Mo, S.; Wang, Y.; Xiong, F.; Ai, C. Effects of asphalt source and mixing temperature on the generated asphalt fumes. J. Hazard. Mater. 2019, 371, 342–351. [Google Scholar] [CrossRef]
- Long, Y.; Wu, S.; Xiao, Y.; Cui, P.; Zhou, H. VOCs reduction and inhibition mechanisms of using active carbon filler in bituminous materials. J. Clean. Prod. 2018, 181, 784–793. [Google Scholar] [CrossRef]
- Boczkaj, G.; Przyjazny, A.; Kaminski, M. Characteristics of volatile organic compounds emission profiles from hot road bitumens. Chemosphere 2014, 107, 23–30. [Google Scholar] [CrossRef]
- Rühl, R.; Musanke, U.; Kolmsee, K.; Priess, R.; Zoubek, G.; Breuer, D. Vapours and aerosols of bitumen: Exposure data obtained by the German Bitumen Forum. Ann. Occup. Hyg. 2006, 50, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Xiu, M.; Wang, X.; Morawska, L.; Pass, D.; Beecroft, A.; Mueller, J.F.; Thai, P. Emissions of particulate matters, volatile organic compounds and polycyclic aromatic hydrocarbons from warm and hot asphalt mixes. J. Clean. Prod. 2020, 275, 123094. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Huang, S.; Yuan, H. Study on asphalt volatile organic compounds emission reduction: A state-of-the-art review. J. Clean. Prod. 2021, 318, 128596. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, P.; Qiu, L.; Wu, S. Influence of VOC emission on asphalt components. Key Eng. Mater. 2014, 599, 178–181. [Google Scholar] [CrossRef]
- Oestman, C.E.; Colmsjoe, A.L.; Sjoeholm, E.A. A laboratory method for the assessment of polycyclic aromatic compound emission from heated bitumen. Fuel 1987, 66, 1720–1726. [Google Scholar] [CrossRef]
- Lei, M.; Wu, S.; Liu, G.; Amirkhanian, S. VOCs characteristics and their relation with rheological properties of base and modified bitumens at different temperatures. Constr. Build. Mater. 2018, 160, 794–801. [Google Scholar] [CrossRef]
- AIOH Exposure Standards Committee. Polycyclic Aromatic Hydricarbons (PAHs) and Occupational Health Issues (Position Paper); Australian Institute of Occupational Hygienists: Gladstone Park, Australia, 2016; pp. 1–17. [Google Scholar]
- Binet, S.; Pfohl-Leszkowicz, A.; Brandt, H.; Lafontaine, M.; Castegnaro, M. Bitumen fumes: Review of work on the potential risk to workers and the present knowledge on its origin. Sci. Total Environ. 2002, 300, 37–49. [Google Scholar] [CrossRef]
- Rajabi, H.; Hadi Mosleh, M.; Mandal, P.; Lea-Langton, A.; Sedighi, M. Emissions of volatile organic compounds from crude oil processing—Global emission inventory and environmental release. Sci. Total Environ. 2020, 727, 138654. [Google Scholar] [CrossRef] [Green Version]
- Borinelli, J.B.; Blom, J.; Portillo-Estrada, M.; De Maeijer, P.K.; den Bergh, W.V.; Vuye, C. VOC emission analysis of bitumen using proton-transfer reaction time-of-flight mass spectrometry. Materials 2020, 13, 3659. [Google Scholar] [CrossRef]
- IARC Working Group; WHO. Benzene; International Agency for Research on Cancer: Lyon, France, 2018; Volume 120. [Google Scholar]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Vu-Duc, T.; Huynh, C.-K.; Binet, S. Laboratory generated bitumen fumes under standardized conditions. clean-up scheme and ion trap GC-MS analysis of VOC, semi-volatile and particulate PAH and PASH. J. Occup. Environ. Hyg. 2007, 4, 245–248. [Google Scholar] [CrossRef]
- Trumbore, D.; Osborn, L.; Blackburn, G.; Niebo, R.; Kriech, A.; Maxim, L.D. Effect of oxidation and extent of oxidation on biologically active PACs in asphalt products. Inhal. Toxicol. 2011, 23, 745–761. [Google Scholar] [CrossRef]
- Huynh, C.K.; Duc, T.V.; Deygout, F.; Le Coutaller, P.; Surmont, F. Identification and quantification of PAH in bitumen by GC-ion-Trap MS and HPLC-Fluorescent detectors. Polycycl. Aromat. Compd. 2007, 27, 107–121. [Google Scholar] [CrossRef]
- Blomberg, J.; De Groot, P.C.; Brandt, H.C.A.; Van der Does, J.J.B.; Schoenmakers, P.J. Development of an on-line coupling of liquid-liquid extraction, normal-phase liquid chromatography and high-resolution gas chromatography producing an analytical marker for the prediction of mutagenicity and carcinogenicity of bitumen and bitumen fumes. J. Chromatogr. A 1999, 849, 483–494. [Google Scholar] [CrossRef]
- Brandt, H.; Lafontaine, M.; Kriech, A.J.; De Groot, P.; Bonnet, P.; Binet, S.; Wissel, H.; Morele, Y.; Nunge, H.; Castegnaro, M. Inhalation study on exposure to bitumen fumes Part 2: Analytical results at two exposure levels. Ann. Occup. Hyg. 2000, 44, 31–41. [Google Scholar] [CrossRef]
- Bonnet, P.; Binet, S.; Brandt, H.; Kriech, A.J.; Lafontaine, M.; Nunge, H.; Morele, Y.; De Groot, P.; Wissel, H.; Castegnaro, M. Inhalation study on exposure to bitumen fumes Part 1: Development and validation of the equipment. Ann. Occup. Hyg. 2000, 44, 15–29. [Google Scholar] [CrossRef]
- NIOSH. Hazard Review: Health Effects of Occupational Exsposure to Asphalt; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2000.
- IARC. Occupational Exposures to Bitumens and Their Emissions; WHO: Lyon, France, 2011. [Google Scholar]
- NIOSH. NIOSH Manual of Analytical Methods (NMAM), Method 5042, 4th ed.; NIOSH: Washington, DC, USA, 1998; Issue 1.
- Sutter, B.; Ravera, C.; Hussard, C.; Langlois, E. Alternatives for benzene in the extraction of bitumen fume from exposure sample media. Ann. Occup. Hyg. 2016, 60, 101–112. [Google Scholar] [CrossRef]
- Moo, A.; Bywood, P.; Silva, D.; McMillan, J. Bitumen Contents and Fumes Health Effects Associated with Exposure to Bitumen, Enviromental Scan 232; Institute for Safety, Compensation and Recovery Research: Melbourne, Australia, 2019. [Google Scholar]
- Trumbore, D.C. The magnitude and source of air emissions from asphalt blowing operations. Environ. Prog. 1998, 17, 53–59. [Google Scholar] [CrossRef]
- Kriech, A.J.; Emmel, C.; Osborn, L.V.; Breuer, D.; Redman, A.P.; Hoeber, D.; Bochmann, F.; Ruehl, R. Side-by-Side Comparison of Field Monitoring Methods for Hot Bitumen Emission Exposures: The German IFA Method 6305, U.S. NIOSH Method 5042, and the Total Organic Matter Method. J. Occup. Environ. Hyg. 2010, 7, 712–725. [Google Scholar] [CrossRef]
- Nies, E.; Brüning, T.; Steinhausen, M.; Welge, P.; Werner, S.C.M.; Pallapies, D.; Bartsch, R.; Brinkmann, B.; Schriever-Schwemmer, G.; Hartwig, A.; et al. Bitumen (Dampf und Aerosol bei der Heißverarbeitung) [MAK value documentation in German language, 2019]. In The MAK-Collection for Occupational Health and Safety; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 1253–1371. [Google Scholar]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [Green Version]
- Brudi, L.C.; Adolfo, F.R.; do Nascimento, P.C.; Cargnin, R.S.; Bohrer, D.; de Carvalho, L.M.; Burgo, T.A.L.; Cravo, M.C.; do Nascimento, L.A.H. Emission and Collection of Polycyclic Aromatic Hydrocarbons From Raw Asphalt Samples Heated at 130 °C. Energy Fuels 2020, 34, 11248–11257. [Google Scholar] [CrossRef]
- Mundt, K.A.; Dell, L.D.; Crawford, L.; Sax, S.N.; Boffetta, P. Cancer risk associated with exposure to bitumen and bitumen fumes: An updated systematic review and meta-analysis. J. Occup. Environ. Med. 2018, 60, e6–e54. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Rong, H.; Meng, Y.; Liu, X.; Liu, Y.; Peng, C. Review of fume-generation mechanism, test methods, and fume suppressants of asphalt materials. J. Clean. Prod. 2022, 347, 131240. [Google Scholar] [CrossRef]
- Schulte, P.A. Gaps in scientific knowledge about the carcinogenic potential of asphalt/bitumen fumes. J. Occup. Environ. Hyg. 2007, 4, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Calzavara, T.S.; Carter, C.M.; Axten, C. Air Sampling Methodology for Asphalt Fume in Asphalt Production and Asphalt Roofing Manufacturing Facilities: Total Particulate Sampler versus Inhalable Particulate Sampler. Appl. Occup. Environ. Hyg. 2003, 18, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Deygout, F.; Le Coutaller, P. Field Sampling Investigations Within the Road Paving Industry. J. Occup. Environ. Hyg. 2010, 7, 103–108. [Google Scholar] [CrossRef]
- Sutter, B.; Pelletier, E.; Blaskowitz, M.; Ravera, C.; Stolze, C.; Reim, C.; Langlois, E.; Breuer, D. Sampling and analysis of bitumen fumes: Comparison of German and French methods to determine a conversion formula. Ann. Work Expo. Health 2018, 62, 721–732. [Google Scholar] [CrossRef]
- McClean, M.D.; Rinehart, R.D.; Ngo, L.; Eisen, E.A.; Kelsey, K.T.; Herrick, R.F. Inhalation and dermal exposure among asphalt paving workers. Ann. Occup. Hyg. 2004, 48, 663–671. [Google Scholar] [CrossRef]
- Gaudefroy, V.; Viranaiken, V.; Paranhos, R.; Jullien, A.; De la Roche, C. Laboratory assessment of fumes generated by bituminous mixtures and bitumen. Road Mater. Pavement Des. 2010, 11, 83–100. [Google Scholar] [CrossRef]
- Vu-Duc, T.; Huynh, C.K.; Lafontaine, M.; Bonnet, P.; Binet, S. A spectrophotometric method for the determination of organic soluble matter in bitumen fumes. Appl. Occup. Environ. Hyg. 2002, 17, 495–500. [Google Scholar] [CrossRef]
- Kurek, J.T.; Kriech, A.J.; Wissel, H.L.; Osborn, L.V.; Blackburn, G.R. Laboratory generation and evaluation of paving asphalt fumes. Transp. Res. Rec. 1999, 1661, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Isacsson, U.; Edwards, Y. Chemical Characterization and Screening of Emission Profiles of Bituminous Sealants Using Solid-Phase Microextraction. Energy Fuels 2006, 20, 1528–1535. [Google Scholar] [CrossRef]
- Tang, B.; Isacsson, U. Determination of aromatic hydrocarbons in bituminous emulsion sealants using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1137, 15–21. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Wan, M.; Cui, P. Inhibiting effect of Layered Double Hydroxides on the emissions of volatile organic compounds from bituminous materials. J. Clean. Prod. 2015, 108, 987–991. [Google Scholar] [CrossRef]
- Wang, J.; Lewis, D.M.; Castranova, V.; Frazer, D.G.; Goldsmith, T.; Tomblyn, S.; Simpson, J.; Stone, S.; Afshari, A.; Siegel, P.D. Characterization of Asphalt Fume Composition under Simulated Road Paving Conditions by GC/MS and Microflow LC/Quadrupole Time-of-Flight MS. Anal. Chem. 2001, 73, 3691–3700. [Google Scholar] [CrossRef]
- Boom, Y.J.; Enfrin, M.; Grist, S.; Giustozzi, F. Recycled plastic modified bitumen: Evaluation of VOCs and PAHs from laboratory generated fumes. Sci. Total Environ. 2022, 832, 155037. [Google Scholar] [CrossRef] [PubMed]
- Law, B.F.; Stone, S.; Frazer, D.; Siegel, P.D. Characterization of laboratory simulated road paving-like asphalt by high-performance liquid chromatography and gas chromatography-mass spectrometry. J. Occup. Environ. Hyg. 2006, 3, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Ettre, L.S. Static Headspace—Gas Chromatography: Theory and Practice, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- McNair, H.M.; Miller, J.M.; Settle, F.A. Basic Gas Chromatography, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar]
- Hagghi, A.; Dalali, N.; Abolghasemi, M.M. Synthesis of graphitic carbon nitride on 3D porous anodized aluminum wire as new fiber for microextraction of polycyclic aromatic hydrocarbons in water and wastewater samples. Sep. Sci. Technol. 2021, 56, 2398–2406. [Google Scholar] [CrossRef]
- Hagghi, A.; Dalali, N.; Abolghasemi, M.M. Selectively determination trace amounts of polycyclic aromatic hydrocarbons from water and wastewater matrices using graphitic carbon nitride/layered double hydroxide nanocomposite on porous anodized aluminum wire as SPME fiber. Polycycl. Aromat. Compd. 2021, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Lian, L.; Wang, X.; Yue, B.; Ding, L.; Lou, D. Velvet-like carbon nitride as a solid-phase microextraction fiber coating for determination of polycyclic aromatic hydrocarbons by gas chromatography. J. Chromatogr. A 2022, 1671, 462993. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Hu, Y.; Peng, S.; Peng, X.; Li, Z.-W.; Zheng, J.; Zhu, F.; Ouyang, G. Convenient synthesis of a hyper-cross-linked polymer via knitting strategy for high-performance solid phase microextraction of polycyclic aromatic hydrocarbons. Microchem. J. 2022, 179, 107535. [Google Scholar] [CrossRef]
- Sutter, B.; Pelletier, E.; Ravera, C.; Langlois, E. Performances of a Bitumen Fume and Condensate Generation System for Sampling Method Development. J. Environ. Prot. 2016, 7, 973–984. [Google Scholar] [CrossRef] [Green Version]
- De Coning, P.; Swinley, J. A Practical Guide to Gas Analysis by Gas Chromatography; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Harris, D.C.; Lucy, C.A. Quantitative Chemical Analysis, 10th ed.; W.H. Freeman: New York, NY, USA, 2020. [Google Scholar]
- EPA, U.S. Method 8260D (SW-846): Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); EPA: Washington, DC, USA, 2006.
- EPA, U.S. Method 8275A: Semivolatile Organic Compounds (PAHs and PCBs) in Soils/Sludges and Solid Wastes Using Thermal Extraction/Gas Chromatography/Mass Spectrometry (TE/GC/MS); US Enviromental Protection Agency: Washington, DC, USA, 1996.
- Neumeister, C.E.; Olsen, L.D.; Dollberg, D.D. Development of a flow-injection fluorescence method for estimation of total polycyclic aromatic compounds in asphalt fumes. AIHA J. 2003, 64, 618–624. [Google Scholar] [CrossRef]
- EPA, U.S. Apendix A to Part 136 Methods for Organic Chemical Analysis of Municipal and Indutrial Wastewater, Method 610—Polynuclear Aromatic Hydrocarbons; US Enviromental Protection Agency: Washington, DC, USA, 1984.
- Mousavi, M.; Fini, E.H. Preventing emissions of hazardous organic compounds from bituminous composites. J. Clean. Prod. 2022, 344, 131067. [Google Scholar] [CrossRef]
| Chemical Class | References |
|---|---|
| Polycyclic aromatic hydrocarbons | [7,8,17,33,34,35,36,37,38] |
| Nitrogen-containing polycyclic aromatic hydrocarbons | [34] |
| Oxygen containing polycyclic aromatic hydrocarbons | [37] |
| Sulfur-containing polycyclic aromatic hydrocarbons | [7,33,34,37] |
| Nitrogen-containing volatile organic compounds | [20] |
| Volatile organic compounds | [20,30] |
| Sulfur-containing volatile organic compounds | [20,30] |
| Sample Technique | Comment |
|---|---|
| Filter sampling | The most representative of industrial application Requires solvent extraction of analytes |
| Headspace sampling | Fast sampling Suited for large sample sets Solventless |
| Headspace solid-phase microextraction | Fast sampling Suited for large sample sets Solventless |
| Nonseparative real-time sampling | Solventless Possible to measures organic emissions rates over time |
| GC Detectors | Comment |
|---|---|
| Mass spectrometer (MS) | Good for identifying many unknown compounds |
| Flame ionization detector (FID) | Good for quantitative analysis of known compounds |
| Flame photometric detector (FPD) | Highly sensitive for sulfur containing compounds |
| Nitrogen phosphorus detector (NPD) | Highly sensitive for nitrogen or phosphorous compounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deller, Z.; Maniam, S.; Giustozzi, F. Sample Preparation and Analytical Methods for Identifying Organic Compounds in Bituminous Emissions. Molecules 2022, 27, 5068. https://doi.org/10.3390/molecules27165068
Deller Z, Maniam S, Giustozzi F. Sample Preparation and Analytical Methods for Identifying Organic Compounds in Bituminous Emissions. Molecules. 2022; 27(16):5068. https://doi.org/10.3390/molecules27165068
Chicago/Turabian StyleDeller, Zachary, Subashani Maniam, and Filippo Giustozzi. 2022. "Sample Preparation and Analytical Methods for Identifying Organic Compounds in Bituminous Emissions" Molecules 27, no. 16: 5068. https://doi.org/10.3390/molecules27165068
APA StyleDeller, Z., Maniam, S., & Giustozzi, F. (2022). Sample Preparation and Analytical Methods for Identifying Organic Compounds in Bituminous Emissions. Molecules, 27(16), 5068. https://doi.org/10.3390/molecules27165068







