A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness
Abstract
1. Introduction
1.1. The Productive Model of Brain Function
1.2. The Transmissive Model of Brain Function
2. Properties of a Transmissive Brain
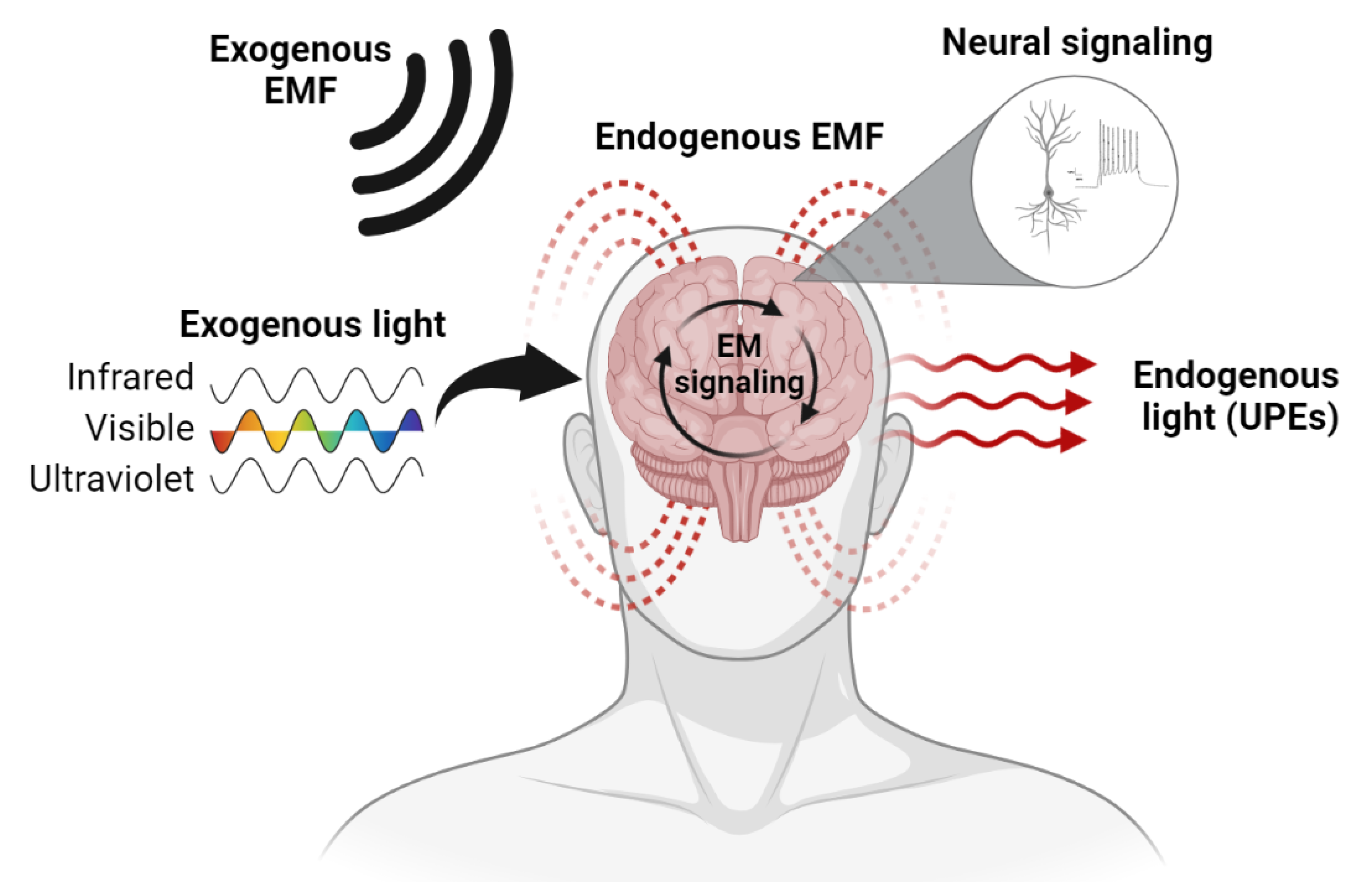
3. The Brain Is an Electromagnetic Organ
3.1. Endogenous EM Fields of the Brain
3.2. Endogenous Light Emissions of the Brain
3.3. EM–Brain Interactions in the Laboratory
3.4. EM–Brain Interactions in Naturalistic Settings
4. Brain Tissue as an EM-Receptive Biomaterial: A Hypothesis
5. Novel In Vitro Approaches to Test EM-Based Transmissive Function
6. Implications for Health and Disease
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santoro, G.; Wood, M.D.; Merlo, L.; Anastasi, G.P.; Tomasello, F.; Germanò, A. The anatomic location of the soul from the heart, through the brain, to the whole body, and beyond: A journey through Western history, science, and philosophy. Neurosurgery 2009, 65, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P. The seat of the soul. Perspect. Biol. Med. 1959, 2, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Tizzard, J. Why Does Kant Think We Must Believe in the Immortal Soul? Can. J. Philos. 2019, 50, 114–129. [Google Scholar] [CrossRef]
- Feldman, R.P.; Goodrich, J.T. The edwin smith surgical papyrus. Child’s Nerv. Syst. 1999, 15, 281–284. [Google Scholar] [CrossRef]
- Clower, W.T.; Finger, S. Discovering trepanation: The contribution of Paul Broca. Neurosurgery 2001, 49, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Hobert, L.; Binello, E. Trepanation in Ancient China. World Neurosurg. 2017, 101, 451–456. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Delgado, J.M.; Roberts, W.W.; Miller, N.E. Learning motivated by electrical stimulation of the brain. Am. J. Physiol. Leg. Content 1954, 179, 587–593. [Google Scholar] [CrossRef]
- Delgado, J.M. Free behavior and brain stimulation. Int. Rev. Neurobiol. 1964, 6, 349–449. [Google Scholar]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Penfield, W. Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proc. Natl. Acad. Sci. USA 1958, 44, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.J.; Thompson-Schill, S.L. Functional Neuroimaging Can Support Causal Claims about Brain Function. J. Cogn. Neurosci. 2010, 22, 2415–2416. [Google Scholar] [CrossRef] [PubMed]
- Aine, C.J. A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/FMRI and PET. Crit. Rev. Neurobiol. 1995, 9, 229–309. [Google Scholar] [PubMed]
- Vaidya, A.R.; Pujara, M.S.; Petrides, M.; Murray, E.A.; Fellows, L.K. Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 2019, 23, 653–671. [Google Scholar] [CrossRef] [PubMed]
- James, W. Human Immortality: Two Supposed Objections to the Doctrine; Houghton: Mifflin, PA, USA, 1898. [Google Scholar]
- Rouleau, N.; Dotta, B.T. Electromagnetic fields as structure-function zeitgebers in biological systems: Environmental orchestrations of morphogenesis and consciousness. Front. Integr. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, H.; Radin, D.; Mossbridge, J.; Vieten, C.; Delorme, A. Exceptional experiences reported by scientists and engineers. Explore 2018, 14, 329–341. [Google Scholar] [CrossRef]
- Mossbridge, J.A.; Radin, D. Precognition as a form of prospection: A review of the evidence. Psychol. Conscious. Theory Res. Pr. 2018, 5, 78–93. [Google Scholar] [CrossRef]
- Myers, F.W.H. Glossary of terms used in psychical research. Proc. Soc. Psych. Res. 1896, 12, 166–174. [Google Scholar]
- Brannigan, A.; Wanner, R.A. Historical distributions of multiple discoveries and theories of scientific change. Soc. Stud. Sci. 1983, 13, 417–435. [Google Scholar] [CrossRef]
- Bennett, M.V. Gap junctions as electrical synapses. J. Neurocytol. 1997, 26, 349–366. [Google Scholar] [CrossRef]
- Krnjevic, K. Chemical Nature of Synaptic Transmission in Vertebrates. Physiol. Rev. 1974, 54, 418–540. [Google Scholar] [CrossRef]
- Eccles, J.C. A unitary hypothesis of mind-brain interaction in the cerebral cortex. Proc. R. Soc. London. B Biol. Sci. 1990, 240, 433–451. [Google Scholar]
- Hari, S.D. Eccles’s Psychons Could Be Zero-Energy Tachyons. Neuro Quantology 2008, 6, 152–160. [Google Scholar] [CrossRef]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef]
- Persinger, M.A.; Dotta, B.T.; Saroka, K.S. Bright light transmits through the brain: Measurement of photon emissions and frequency-dependent modulation of spectral electroencephalographic power. World J. Neurosci. 2013, 3, 10–16. [Google Scholar] [CrossRef]
- John, E.R. A field theory of consciousness. Conscious. Cogn. 2001, 10, 184–213. [Google Scholar] [CrossRef] [PubMed]
- John, E.R. A theory of consciousness. Curr. Dir. Psychol. Sci. 2003, 12, 244–250. [Google Scholar] [CrossRef]
- McFadden, J. The conscious electromagnetic information (cemi) field theory: The hard problem made easy? J. Conscious. Stud. 2002, 9, 45–60. [Google Scholar]
- McFadden, J. The CEMI field theory: Seven clues to the nature of consciousness. In The Emerging Physics of Consciousness; Springer: Berlin/Heidelberg, Germany, 2006; pp. 387–406. [Google Scholar]
- McFadden, J. Integrating information in the brain’s EM field: The cemi field theory of consciousness. Neurosci. Conscious. 2020, 2020, niaa016. [Google Scholar] [CrossRef]
- Hameroff, S.; Penrose, R. Orchestrated reduction of quantum coherence in brain microtubules: A model for consciousness. Math. Comput. Simul. 1996, 40, 453–480. [Google Scholar] [CrossRef]
- Hameroff, S.; Penrose, R. Orchestrated objective reduction of quantum coherence in brain microtubules: The “orch or” model for consciousness. Philos. Trans. R. Soc. Lond. 1998, 356, 1869–1896. [Google Scholar]
- Hameroff, S.; Penrose, R. Reply to criticism of the ‘Orch OR qubit’–‘Orchestrated objective reduction’is scientifically justified. Phys. Life Rev. 2014, 11, 94–100. [Google Scholar] [CrossRef]
- Persinger, M.A. Electromagnetic bases of the universality of the characteristics of consciousness: Quantitative support. J. Cosmol. 2011, 14, 1096–1103. [Google Scholar]
- Persinger, M. The (Sum of n)= n Concept And the Quantitative Support for the Cerebral-Holographic and Electromagnetic Configuration of Consciousness. J. Conscious. Stud. 2012, 19, 128–153. [Google Scholar]
- Tang, R.; Dai, J. Biophoton signal transmission and processing in the brain. J. Photochem. Photobiol. B Biol. 2014, 139, 71–75. [Google Scholar] [CrossRef]
- Georgiev, D.D. Electric and magnetic fields inside neurons and their impact upon the cytoskeletal microtubules. In Rhythmic Oscillations in Proteins to Human Cognition; Springer: Singapore, 2021; pp. 51–102. [Google Scholar]
- Laufs, H. A personalized history of EEG–fMRI integration. NeuroImage 2012, 62, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, F.; McCormick, D.A. Endogenous electric fields may guide neocortical network activity. Neuron 2010, 67, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Radman, T.; Su, Y.; An, J.H.; Parra, L.C.; Bikson, M. Spike Timing Amplifies the Effect of Electric Fields on Neurons: Implications for Endogenous Field Effects. J. Neurosci. 2007, 27, 3030–3036. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Iyengar, A.K.; Foulser, A.A.; Boyle, M.R.; Fröhlich, F. Endogenous Cortical Oscillations Constrain Neuromodulation by Weak Electric Fields. Brain Stimul. 2014, 7, 878–889. [Google Scholar] [CrossRef]
- Francis, J.T.; Gluckman, B.J.; Schiff, S.J. Sensitivity of neurons to weak electric fields. J. Neurosci. 2003, 23, 7255–7261. [Google Scholar] [CrossRef]
- Opitz, A.; Falchier, A.; Yan, C.G.; Yeagle, E.M.; Linn, G.S.; Megevand, P.; Schroeder, C.E. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci. Rep. 2016, 6, 31236. [Google Scholar] [CrossRef]
- Dierks, T.; Jelic, V.; Julin, P.; Maurer, K.; Wahlund, L.O.; Almkvist, O.; Winblad, B. EEG-microstates in mild memory impairment and Alzheimer’s disease: Possible association with disturbed information processing. J. Neural Transm. 1997, 104, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Y.; Dzirasa, K. Is depression a disorder of electrical brain networks? Neuropsychopharmacology 2020, 45, 230–231. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Magnetoencephalography: From SQUIDs to neuroscience: Neuroimage 20th Anniversary Special Edition. NeuroImage 2012, 61, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Caruso, L.; Wunderle, T.; Lewis, C.M.; Valadeiro, J.; Trauchessec, V.; Rosillo, J.T.; Pannetier-Lecoeur, M. In vivo magnetic recording of neuronal activity. Neuron 2017, 95, 1283–1291. [Google Scholar] [CrossRef]
- Proudfoot, M.; Woolrich, M.W.; Nobre, A.C.; Turner, M.R. Magnetoencephalography. Pract. Neurol. 2014, 14, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Avci, R.; Whittington, J.R.; Blossom, S.J.; Escalona-Vargas, D.; Siegel, E.R.; Preissl, H.T.; Eswaran, H. Studying the effect of maternal pregestational diabetes on fetal neurodevelopment using magnetoencephalography. Clin. EEG Neurosci. 2020, 51, 331–338. [Google Scholar] [CrossRef]
- Moser, J.; Schleger, F.; Weiss, M.; Sippel, K.; Semeia, L.; Preissl, H. Magnetoencephalographic signatures of conscious processing before birth. Dev. Cogn. Neurosci. 2021, 49, 100964. [Google Scholar] [CrossRef]
- Seager, W. Consciousness, information and panpsychism. J. Conscious. Stud. 1995, 2, 272–288. [Google Scholar]
- Anastassiou, C.A.; Perin, R.; Markram, H.; Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 2011, 14, 217–223. [Google Scholar] [CrossRef]
- Katz, B.; Schmitt, O.H. Electric interaction between two adjacent nerve fibres. J. Physiol. 1940, 97, 471. [Google Scholar] [CrossRef]
- Ruffini, G.; Salvador, R.; Tadayon, E.; Sanchez-Todo, R.; Pascual-Leone, A.; Santarnecchi, E. Realistic modeling of mesoscopic ephaptic coupling in the human brain. PLoS Comput. Biol. 2020, 16, e1007923. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-S.; Guo, C.; Chen, C.H.; Witter, L.; Osorno, T.; Regehr, W.G. Ephaptic Coupling Promotes Synchronous Firing of Cerebellar Purkinje Cells. Neuron 2018, 100, 564–578.e3. [Google Scholar] [CrossRef] [PubMed]
- Bokil, H.; Laaris, N.; Blinder, K.; Ennis, M.; Keller, A. Ephaptic interactions in the mammalian olfactory system. J. Neurosci. 2001, 21, RC173. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Shivacharan, R.S.; Wei, X.; Gonzalez-Reyes, L.E.; Durand, D.M. Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J. Physiol. 2019, 597, 249–269. [Google Scholar] [CrossRef]
- Anastassiou, C.A.; Koch, C. Ephaptic coupling to endogenous electric field activity: Why bother? Curr. Opin. Neurobiol. 2015, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Edelman, G.; Tononi, G. A Universe of Consciousness: How Matter Becomes Imagination; Basic Books: New York, NY, USA, 2000. [Google Scholar]
- Martinez-Banaclocha, M. Ephaptic Coupling of Cortical Neurons: Possible Contribution of Astroglial Magnetic Fields? Neuroscience 2018, 370, 37–45. [Google Scholar] [CrossRef]
- Stolz, J.F.; Chang, S.-B.R.; Kirschvink, J. Magnetotactic bacteria and single-domain magnetite in hemipelagic sediments. Nature 1986, 321, 849–851. [Google Scholar] [CrossRef]
- Kirschvink, J.; Padmanabha, S.; Boyce, C.; Oglesby, J. Measurement of the threshold sensitivity of honeybees to weak, extremely low-frequency magnetic fields. J. Exp. Biol. 1997, 200, 1363–1368. [Google Scholar] [CrossRef]
- Walcott, C.; Gould, J.L.; Kirschvink, J.L. Pigeons have magnets. Science 1979, 205, 1027–1029. [Google Scholar] [CrossRef]
- Kirschvink, J.L. Magnetite biomineralization and geomagnetic sensitivity in higher animals: An update and recommendations for future study. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1989, 10, 239–259. [Google Scholar] [CrossRef]
- Holland, R.A.; Kirschvink, J.L.; Doak, T.G.; Wikelski, M. Bats use magnetite to detect the earth’s magnetic field. PLoS ONE 2008, 3, e1676. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R. Human navigation and magnetoreception: The Manchester experiments do replicate. Anim. Behav. 1987, 35, 691–704. [Google Scholar] [CrossRef]
- Mouritsen, H. Long-distance navigation and magnetoreception in migratory animals. Nature 2018, 558, 50–59. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Gould, J.L. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 1981, 13, 181–201. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Woodford, B.J. Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA 1992, 89, 7683–7687. [Google Scholar] [CrossRef]
- Khan, S.; Cohen, D. Using the magnetoencephalogram to noninvasively measure magnetite in the living human brain. Hum. Brain Mapp. 2019, 40, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H.; Dudchenko, P.; Wood, E.; Shapiro, M.; Tanila, H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron 1999, 23, 209–226. [Google Scholar] [CrossRef]
- Olton, D.S.; Becker, J.T.; Handelmann, G.E. Hippocampus, space, and memory. Behav. Brain Sci. 1979, 2, 313–322. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Ranck, J.B., Jr. Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res. 1980, 189, 49–66. [Google Scholar] [CrossRef]
- Alonso, A.; Llinás, R.R. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 1989, 342, 175–177. [Google Scholar] [CrossRef]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.J. Hysteresis properties of magnetite and their dependence on particle size: A test of pseudo-single-domain remanence models. J. Geophys. Res. Solid Earth 1986, 91, 9569–9584. [Google Scholar] [CrossRef]
- Cragg, B.G.; Temperley, H.N.V. Memory: The analogy with ferromagnetic hysteresis. Brain 1955, 78, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Fero, K.; Driever, W.; Burgess, H.A. Enlightening the brain: Linking deep brain photoreception with behavior and physiology. Bioessays 2013, 35, 775–779. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Li, Z.; Xiao, F.; Dai, J. Human high intelligence is involved in spectral redshift of biophotonic activities in the brain. Proc. Natl. Acad. Sci. USA 2016, 113, 8753–8758. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Sordillo, L.A. The Mystery of Chemotherapy Brain: Kynurenines, Tubulin and Biophoton Release. Anticancer Res. 2020, 40, 1189–1200. [Google Scholar] [CrossRef]
- Rahnama, M.; Tuszynski, J.A.; Bókkon, I.; Cifra, M.; Sardar, P.; Salari, V. Emission of mitochondrial biophotons and their effect on electrical activity of membrane via microtubules. J. Integr. Neurosci. 2011, 10, 65–88. [Google Scholar] [CrossRef]
- Van Wijk, R.; Schamhart, D.H.J. Regulatory aspects of low intensity photon emission. Experientia 1988, 44, 586–593. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Spatiotemporal Imaging of Glutamate-Induced Biophotonic Activities and Transmission in Neural Circuits. PLoS ONE 2014, 9, e85643. [Google Scholar] [CrossRef]
- Kumar, S.; Boone, K.; Tuszyński, J.; Barclay, P.; Simon, C. Possible existence of optical communication channels in the brain. Sci. Rep. 2016, 6, 36508. [Google Scholar] [CrossRef]
- Traill, R.R. The case that mammalian intelligence is based on sub-molecular memory coding and fibre-optic capabilities of myelinated nerve axons. Specul. Sci. Technol. 1988, 11, 173–181. [Google Scholar]
- Ji, Z.; Liu, W.; Krylyuk, S.; Fan, X.; Zhang, Z.; Pan, A.; Feng, L.; Davydov, A.; Agarwal, R. Photocurrent detection of the orbital angular momentum of light. Science 2020, 368, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiao, X.; Midya, B.; Liu, K.; Sun, J.; Wu, T.; Liu, W.; Agarwal, R.; Jornet, J.M.; Longhi, S.; et al. Tunable topological charge vortex microlaser. Science 2020, 368, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, S.; Snyder, S.H. Encephalopsin: A novel mammalian extraretinal opsin discretely localized in the brain. J. Neurosci. 1999, 19, 3681–3690. [Google Scholar] [CrossRef]
- Sugihara, T.; Nagata, T.; Mason, B.; Koyanagi, M.; Terakita, A. Absorption Characteristics of Vertebrate Non-Visual Opsin, Opn3. PLoS ONE 2016, 11, e0161215. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouares, K.; Beurrier, C.; Canepari, M.; Laverne, G.; Kuczewski, N. Opto nongenetics inhibition of neuronal firing. Eur. J. Neurosci. 2019, 49, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Nakane, Y.; Ikegami, K.; Ono, H.; Yamamoto, N.; Yoshida, S.; Hirunagi, K.; Yoshimura, T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA 2010, 107, 15264–15268. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Dai, J. Biophotons as neural communication signals demonstrated by in situ biophoton autography. Photochem. Photobiol. Sci. 2010, 9, 315–322. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Djamgoz, M.B.A. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J. Cell Sci. 2004, 117, 1631–1639. [Google Scholar] [CrossRef]
- Havelka, D.; Cifra, M. Calculation of the electromagnetic field around a microtubule. Acta Polytech. 2009, 49. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Liu, J.; Li, L.; Hu, X.; Sun, H.; Tian, J. Pulsed electromagnetic field enhances brain-derived neurotrophic factor expression through L-type voltage-gated calcium channel-and Erk-dependent signaling pathways in neonatal rat dorsal root ganglion neurons. Neurochem. Int. 2014, 75, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Cappa, S.F.; Geroldi, C.; Zanetti, O.; Rossini, P.M.; Miniussi, C. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch. Neurol. 2006, 63, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Ko, J.H.; Monchi, O. Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: The contribution of expectation. Neuroimage 2006, 31, 1666–1672. [Google Scholar] [CrossRef]
- Vucic, S.; Kiernan, M.C. Transcranial Magnetic Stimulation for the Assessment of Neurodegenerative Disease. Neurotherapeutics 2017, 14, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Pascual-Leone, A.; Valls-Sole, J.; Cohen, L.G.; Hallett, M. Focal transcranial magnetic stimulation and response bias in a forced-choice task. J. Neurol. Neurosurg. Psychiatry 1992, 55, 964–966. [Google Scholar] [CrossRef]
- McRobbie, D.W. Occupational exposure in MRI. Br. J. Radiol. 2012, 85, 293–312. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Cummings, J.L. Temporal lobe epilepsy: Its association with psychiatric impairment and appropriate dental management. Oral Surg. Oral Med. Oral Pathol. 1989, 68, 288–292. [Google Scholar] [CrossRef]
- Stephani, C.; Vaca, G.F.-B.; Maciunas, R.; Koubeissi, M.; Lüders, H.O. Functional neuroanatomy of the insular lobe. Anat. Embryol. 2011, 216, 137–149. [Google Scholar] [CrossRef]
- Veniero, D.; Vossen, A.; Gross, J.; Thut, G. Lasting EEG/MEG Aftereffects of Rhythmic Transcranial Brain Stimulation: Level of Control Over Oscillatory Network Activity. Front. Cell. Neurosci. 2015, 9, 477. [Google Scholar] [CrossRef]
- Baker-Price, L.A.; Persinger, M.A. Weak, but complex pulsed magnetic fields may reduce depression following traumatic brain injury. Percept. Mot. Ski. 1996, 83, 491–498. [Google Scholar] [CrossRef]
- Persinger, M.A.; Belanger-Chellew, G. Facilitation of Seizures in Limbic Epileptic Rats by Complex 1 Microtesla Magnetic Fields. Percept. Mot. Ski. 1999, 89, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Koren, S.A.; Persinger, M.A. Thermal analgesic effects from weak, complex magnetic fields and pharmacological interactions. Pharmacol. Biochem. Behav. 2004, 78, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Persinger, M.A. Experimental induction of the “sensed presence” in normal subjects and an exceptional subject. Percept. Mot. Ski. 1997, 85, 683–693. [Google Scholar]
- Persinger, M.A. Religious and Mystical Experiences as Artifacts of Temporal Lobe Function: A General Hypothesis. Percept. Mot. Ski. 1983, 57 (Suppl. S3), 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Persinger, M.A. Neuropsychological Bases of God Beliefs; Praeger: New York, NY, USA, 1987. [Google Scholar]
- Saroka, K.S.; Mulligan, B.P.; Murphy, T.R.; Persinger, M.A. Experimental Elicitation of an Out of Body Experience and Concomitant Cross-Hemispheric Electroencephalographic Coherence. NeuroQuantology 2010, 8, 466–477. [Google Scholar]
- Mulligan, B.P.; Persinger, M.A. Experimental simulation of the effects of sudden increases in geomagnetic activity upon quantitative measures of human brain activity: Validation of correlational studies. Neurosci. Lett. 2012, 516, 54–56. [Google Scholar] [CrossRef]
- Persinger, M.A. A potential multiple resonance mechanism by which weak magnetic fields affect molecules and medical problems: The example of melatonin and experimental “multiple sclerosis”. Med. Hypotheses 2006, 66, 811–815. [Google Scholar] [CrossRef]
- St-Pierre, L.S.; Persinger, M.A. Conspicuous Histomorphological Anomalies in the Hippocampal Formation of Rats Exposed Prenatally to a Complex Sequenced Magnetic Field within the Nanotesla Range. Percept. Mot. Ski. 2003, 97, 1307–1314. [Google Scholar] [CrossRef]
- Whissell, P.D.; Tsang, E.W.; Mulligan, B.P.; Persinger, M.A. Prenatal Exposures to LTP-Patterned Magnetic Fields: Quantitative Effects on Specific Limbic Structures and Acquisition of Contextually Conditioned Fear. Int. J. Neurosci. 2009, 119, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Dmochowski, J.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Proceedings #18. Transcranial Infrared Brain Stimulation Modulates EEG Alpha Power. Brain Stimul. 2017, 10, e67–e69. [Google Scholar]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef]
- Kuo, J.-R.; Lin, S.-S.; Liu, J.; Chen, S.-H.; Chio, C.-C.; Wang, J.-J.; Liu, J.-M. Deep brain light stimulation effects on glutamate and dopamine concentration. Biomed. Opt. Express 2015, 6, 23–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flyktman, A.; Mänttäri, S.; Nissilä, J.; Timonen, M.; Saarela, S. Transcranial light affects plasma monoamine levels and expression of brain encephalopsin in the mouse. J. Exp. Biol. 2015, 218, 1521–1526. [Google Scholar] [PubMed]
- Kubota, Y.; Kamatani, D.; Tsukano, H.; Ohshima, S.; Takahashi, K.; Hishida, R.; Shibuki, K. Transcranial photo-inactivation of neural activities in the mouse auditory cortex. Neurosci. Res. 2008, 60, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lothet, E.H.; Shaw, K.M.; Lu, H.; Zhuo, J.; Wang, Y.; Gu, S.; Stolz, D.B.; Jansen, E.D.; Horn, C.C.; Chiel, H.J.; et al. Selective inhibition of small-diameter axons using infrared light. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.M. Nuclear georeactor generation of the earth’s geomagnetic field. Curr. Sci. 2007, 93, 1485–1487. [Google Scholar]
- Appelt, S.; Kühn, H.; Häsing, F.W.; Blümich, B. Chemical analysis by ultrahigh-resolution nuclear magnetic resonance in the Earth’s magnetic field. Nat. Phys. 2006, 2, 105–109. [Google Scholar] [CrossRef]
- Valet, J.-P. Time variations in geomagnetic intensity. Rev. Geophys. 2003, 41. [Google Scholar] [CrossRef]
- Samson, J.C.; Jacobs, J.A.; Rostoker, G. Latitude-dependent characteristics of long-period geomagnetic micropulsations. J. Geophys. Res. 1971, 76, 3675–3683. [Google Scholar] [CrossRef]
- Harrison, R.G. Fair weather atmospheric electricity. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2011; Volume 301, p. 012001. [Google Scholar]
- Skiles, D.D. The geomagnetic field its nature, history, and biological relevance. In Magnetite Biomineralization and Magnetoreception in Organisms; Springer: Boston, MA, USA, 1985; pp. 43–102. [Google Scholar]
- Friedman, H.; Becker, R.O.; Bachman, C.H. Geomagnetic parameters and psychiatric hospital admissions. Nature 1963, 200, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Res. Treat. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, M.; Mitra, S. Correlation between convulsive seizure and geomagnetic activity. Neurosci. Lett. 1981, 24, 187–191. [Google Scholar] [CrossRef]
- Chae, K.S.; Oh, I.T.; Lee, S.H.; Kim, S.C. Blue light-dependent human magnetoreception in geomagnetic food orientation. PLoS ONE 2019, 14, e0211826. [Google Scholar]
- Christian, H.J.; Blakeslee, R.J.; Boccippio, D.J.; Boeck, W.L.; Buechler, D.E.; Driscoll, K.T.; Stewart, M.F. Global frequency and distribution of lightning as observed from space by the Optical Transient Detector. J. Geophys. Res. Atmos. 2003, 108, ACL-4. [Google Scholar] [CrossRef]
- Balser, M.; Wagner, C.A. Observations of Earth–ionosphere cavity resonances. Nature 1960, 188, 638–641. [Google Scholar] [CrossRef]
- Colgin, L.L. Theta–gamma coupling in the entorhinal–hippocampal system. Curr. Opin. Neurobiol. 2015, 31, 45–50. [Google Scholar] [CrossRef]
- König, H.; Ankermüller, F. Über den Einfluß besonders niederfrequenter elektrischer Vorgänge in der Atmosphäre auf den Menschen. Naturwissenschaften 1960, 47, 486–490. [Google Scholar] [CrossRef]
- Wang, C.X.; Hilburn, I.A.; Wu, D.A.; Mizuhara, Y.; Cousté, C.P.; Abrahams, J.N.; Kirschvink, J.L. Transduction of the geomagnetic field as evidenced from alpha-band activity in the human brain. eNeuro 2019. [Google Scholar] [CrossRef]
- Saroka, K.S.; Vares, D.E.; Persinger, M.A. Similar Spectral Power Densities within the Schumann Resonance and a Large Population of Quantitative Electroencephalographic Profiles: Supportive Evidence for Koenig and Pobachenko. PLoS ONE 2016, 11, e0146595. [Google Scholar] [CrossRef]
- Rouleau, N.; Lehman, B.; Persinger, M.A. Focal attenuation of specific electroencephalographic power over the right parahippocampal region during transcerebral copper screening in living subjects and hemispheric asymmetric voltages in fixed brain tissue. Brain Res. 2016, 1644, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Tsang, E.W.; Koren, S.A.; Persinger, M.A. Power increases within the gamma range over the frontal and occipital regions during acute exposures to cerebrally counterclockwise rotating magnetic fields with specific derivatives of change. Int. J. Neurosci. 2004, 114, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L. Toward a physics of the neocortex. In Neocortical Dynamics and Human FEG Rhythms; Nunez, P.L., Ed.; Oxford University Press: New York, NY, USA, 1995; pp. 68–132. [Google Scholar]
- Burch, J.B.; Reif, J.S.; Yost, M.G. Geomagnetic activity and human melatonin metabolite excretion. Neurosci. Lett. 2008, 438, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J. Relationship between isolated sleep paralysis and geomagnetic influences: A case study. Percept. Mot. Ski. 1995, 80, 1263–1273. [Google Scholar] [CrossRef]
- Lipnicki, D.M. An association between geomagnetic activity and dream bizarreness. Med. Hypotheses 2009, 73, 115–117. [Google Scholar] [CrossRef]
- Hekmatmanesh, A.; Banaei, M.; Haghighi, K.S.; Najafi, A. Bedroom design orientation and sleep electroencephalography signals. Acta Med. Int. 2019, 6, 33–37. [Google Scholar] [CrossRef]
- Ruhenstroth-Bauer, G.; Rüther, E.; Reinertshofer, T. Dependence of a sleeping parameter from the N-S or E-W sleeping direction. Z. Für Nat. C J. Biosci. 1987, 42, 1140–1142. [Google Scholar] [CrossRef]
- Conesa, J. Isolated sleep paralysis, vivid dreams and geomagnetic influences: II. Percept. Mot. Ski. 1997, 85, 579–584. [Google Scholar] [CrossRef]
- De Gennaro, L.; Ferrara, M. Sleep spindles: An overview. Sleep Med. Rev. 2003, 7, 423–440. [Google Scholar] [CrossRef]
- Persinger, M.A. (Ed.) ELF and VLF Electromagnetic Field Effects; Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Rouleau, N.; Persinger, M.A. Differential responsiveness of the right parahippocampal region to electrical stimulation in fixed human brains: Implications for historical surgical stimulation studies? Epilepsy Behav. 2016, 60, 181–186. [Google Scholar] [CrossRef]
- Rouleau, N.; Persinger, M.A. Neural Tissues Filter Electromagnetic Fields: Investigating Regional Processing of Induced Current in Ex vivo Brain Specimens. Biol. Med. 2017, 9. [Google Scholar] [CrossRef]
- Alvarado, C.S.; Krippner, S. Nineteenth century pioneers in the study of dissociation: William James and psychical research. J. Conscious. Stud. 2010, 17, 19–43. [Google Scholar]
- Hawkins, S.L. William James, Gustav Fechner, and Early Psychophysics. Front. Physiol. 2011, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.K. An emerging new model for consciousness: The consciousness field model. In Neuroscience, Consciousness and Spirituality; Springer: Dordrecht, The Netherland, 2011; pp. 279–288. [Google Scholar]
- Rouleau, N.; Murugan, N.J.; Kaplan, D.L. Toward Studying Cognition in a Dish. Trends Cogn. Sci. 2021, 25, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.L.; Nieland, T.J.F.; Dingle, Y.-T.L.; Kaplan, D.L. Innovations in 3D Tissue Models of Human Brain Physiology and Diseases. Adv. Funct. Mater. 2020, 30, 1909146. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric scaffolds as smart mate-rials for neural tissue engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Mantler, A.; Logan, A.C. Natural environments and mental health. Adv. Integr. Med. 2015, 2, 5–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouleau, N.; Cimino, N. A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness. NeuroSci 2022, 3, 440-456. https://doi.org/10.3390/neurosci3030032
Rouleau N, Cimino N. A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness. NeuroSci. 2022; 3(3):440-456. https://doi.org/10.3390/neurosci3030032
Chicago/Turabian StyleRouleau, Nicolas, and Nicholas Cimino. 2022. "A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness" NeuroSci 3, no. 3: 440-456. https://doi.org/10.3390/neurosci3030032
APA StyleRouleau, N., & Cimino, N. (2022). A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness. NeuroSci, 3(3), 440-456. https://doi.org/10.3390/neurosci3030032






