Pharmacological Efficacy of Probiotics in Respiratory Viral Infections: A Comprehensive Review
Abstract
:1. Introduction
2. Materials and Methods
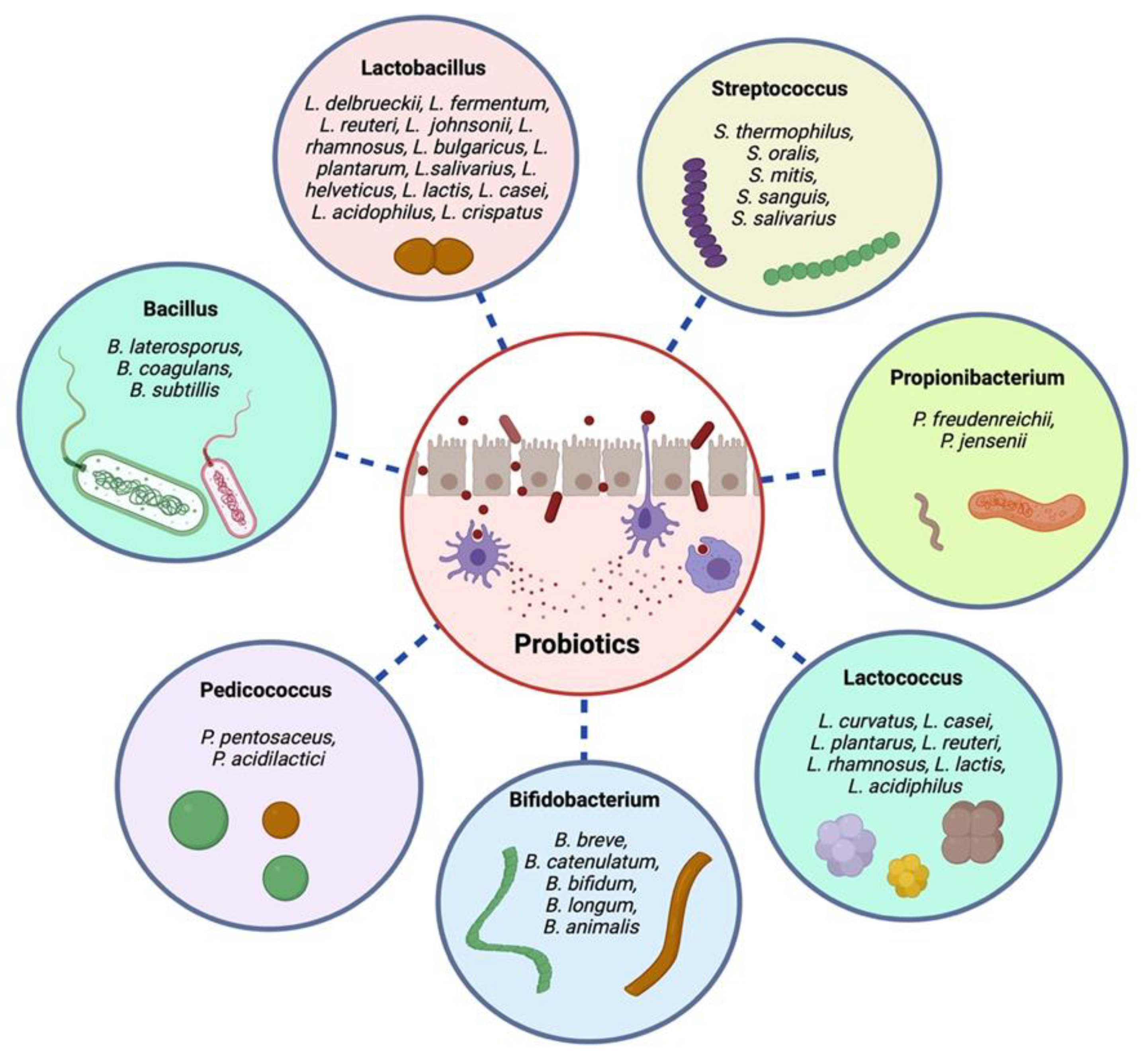
3. Probiotic Bacteria Strains
4. Probiotic Isolates and Their Health Advantages
5. Probiotics Effect on Viral Replication
5.1. Human Immunodeficiency Virus (HIV)
5.2. Herpes Simplex Virus (HSV)
5.3. Swine Influenza Virus
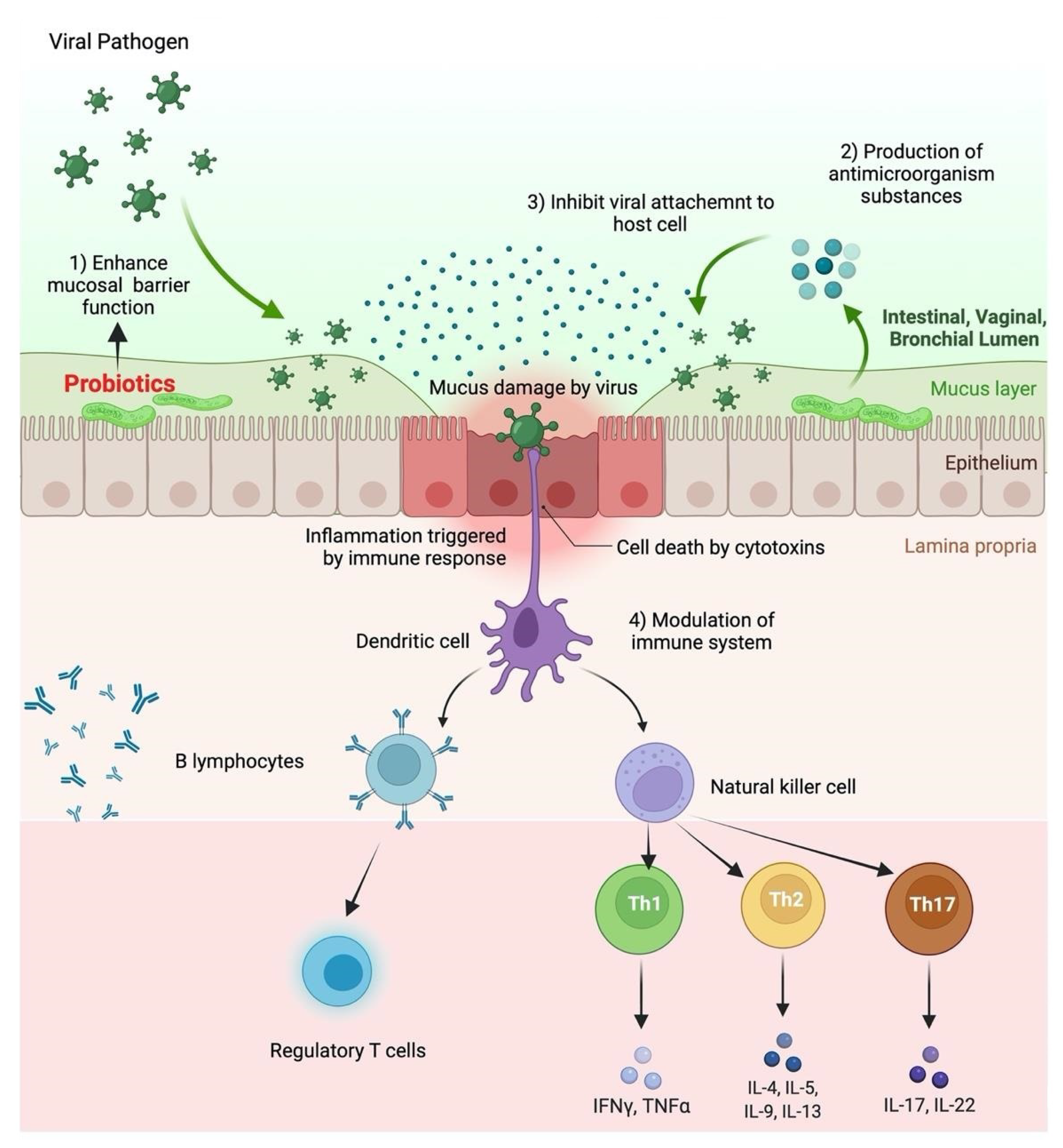
6. Immunomodulatory Effects of Probiotic
7. Role of Probiotics in Respiratory Tract Infections
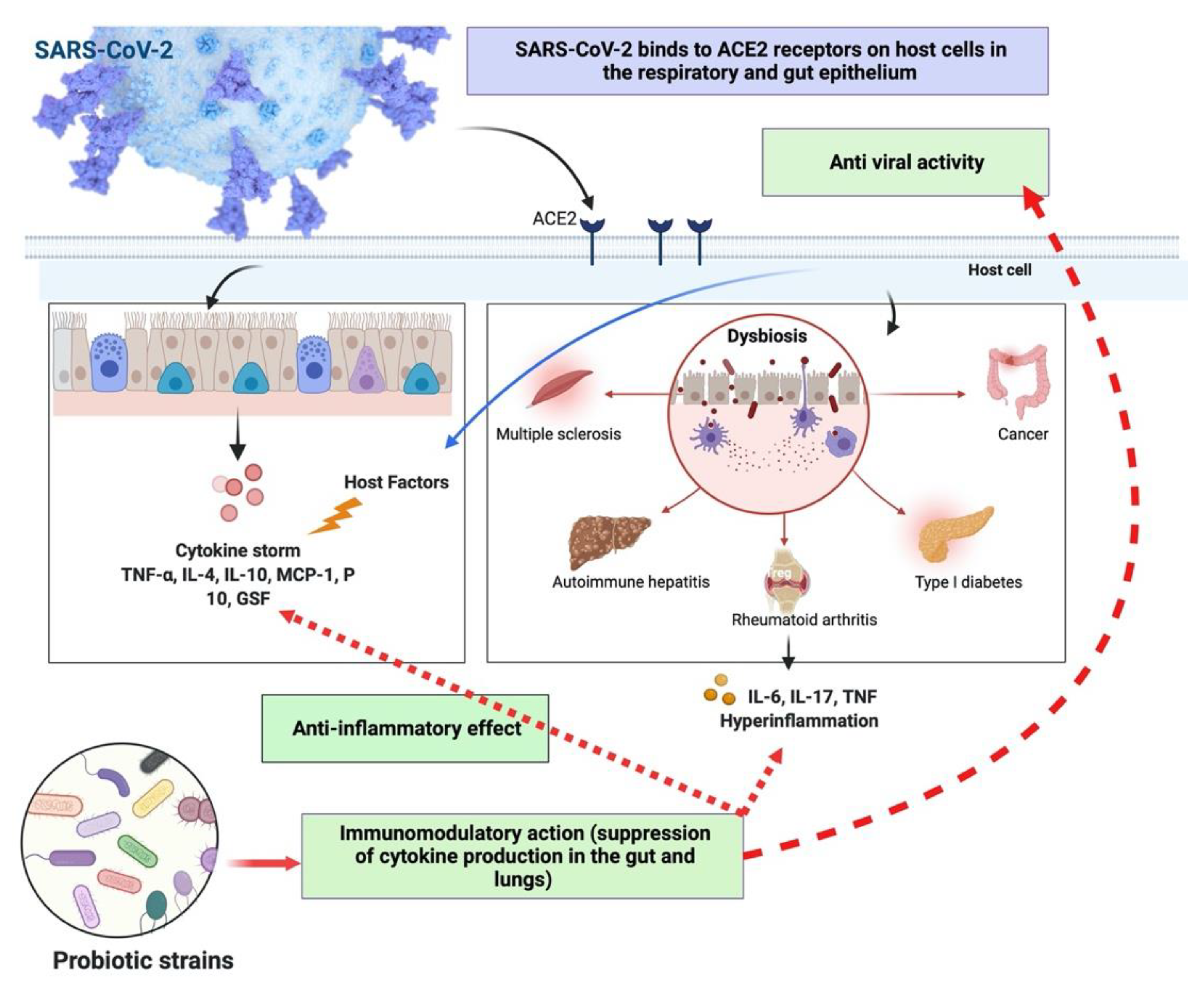
8. Role of Probiotics in SARS-CoV-2 Infection
9. Clinical Trial on Probiotics’ Role in Respiratory Viral Infections
10. Scope of Prebiotics or Probiotics and Vaccine Development to Prevent Viral Infections
11. Factors Affecting the Delivery of Probiotics
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, S.; Smatti, M.K.; Al Ansari, K.; Nasrallah, G.K.; Al Thani, A.A.; Yassine, H.M. Mixed Viral-Bacterial Infections and Their Effects on Gut Microbiota and Clinical Illnesses in Children. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, I.; Wahab, S.; Nisar, N.; Dera, A.A.; Alshahrani, M.Y.; Abullias, S.S.; Irfan, S.; Alam, M.M.; Srivastava, S. Evaluation of Antibacterial Properties of Matricaria Aurea on Clinical Isolates of Periodontitis Patients with Special Reference to Red Complex Bacteria. Saudi Pharm. J. 2020, 28, 1203–1209. [Google Scholar] [CrossRef]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment? Microb. Pathog. 2020, 148, 104452. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.-R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Zeng, W.; Shen, J.; Bo, T.; Peng, L.; Xu, H.; Ide Nasser, M.; Zhuang, Q.; Zhao, M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J. Immunol. Res. 2019, 2019, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Chemistry, J.S.-C. Medicinal; 2017, U. Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Available online: Ingentaconnect.com (accessed on 5 May 2022).
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J.; et al. Lactobacillus Plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasui, H.; Kiyoshima, J.; Hori, T. Reduction of Influenza Virus Titer and Protection against Influenza Virus Infection in Infant Mice Fed Lactobacillus Casei Shirota. Am. Soc. Microbiol. 2004, 11, 675–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, H.; Tsunemine, S.; Isa, Y.; Shimakawa, M.; Yamamura, H. Oral Administration of Bifidobacterium Bifidum G9-1 Suppresses Total and Antigen Specific Immunoglobulin E Production in Mice. Biol. Pharm. Bull. 2005, 28, 1462–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, M.; Immunology, H.G.-I. Can Immunoregulatory Lactic Acid Bacteria Be Used as Dietary Supplements to Limit Allergies? Int. Arch. Allergy Immunol. 2001, 125, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Kirjavainen, P.V.; Salminen, S. Probiotics: A Role in the Treatment of Intestinal Infection and Inflammation? Gut 2002, 50, iii54–iii59. [Google Scholar] [CrossRef] [PubMed]
- Hajavi, J.; Esmaeili, S.-A.; Varasteh, A.-R.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The Immunomodulatory Role of Probiotics in Allergy Therapy. Wiley Online Libr. 2018, 234, 2386–2398. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The Influence of Probiotics on Vaccine Responses—A Systematic Review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ali, M.S.; Alsayegh, A.R.A.; Ahmad, S.; Alam, N.; Wahab, S.; Ali, M.S.; Athar, M.T. A Current Novel Perspective Approach for Coronavirus Disease-2019 Pandemic Outbreak. J. Adv. Pharm. Technol. Res. 2021, 12, 311–320. [Google Scholar]
- Hung, Y.P.; Lee, C.C.; Lee, J.C.; Tsai, P.J.; Ko, W.C. Gut Dysbiosis during COVID-19 and Potential Effect of Probiotics. Microorganisms 2021, 9, 1605. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Ghanavati, R.; Afifirad, R.; Darb Emamie, A.; Kakanj, M.; Talebi, M. The Effect of Probiotics on Respiratory Tract Infection with Special Emphasis on COVID-19: Systemic Review 2010–20. Int. J. Infect. Dis. 2021, 105, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Shanahan, F. Basic Aspects and Pharmacology of Probiotics: An Overview of Pharmacokinetics, Mechanisms of Action and Side-Effects. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 725–740. [Google Scholar] [CrossRef]
- Khaled, J.M.A. Probiotics, Prebiotics, and COVID-19 Infection: A Review Article. Saudi J. Biol. Sci. 2021, 28, 865–869. [Google Scholar] [CrossRef]
- Mishra, S.; Rath, S.; Mohanty, N. Probiotics—A Complete Oral Healthcare Package. J. Integr. Med. 2020, 18, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Samot, J.; Badet, C. Antibacterial Activity of Probiotic Candidates for Oral Health. Anaerobe 2013, 19, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. The Oral Microbiome: Its Role in Health and in Oral and Systemic Infections. Clin. Microbiol. Newsl. 2013, 35, 163–169. [Google Scholar] [CrossRef]
- Russell, D.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Metabolic Activities and Probiotic Potential of Bifidobacteria. Int. J. Food Microbiol. 2011, 149, 88–105. [Google Scholar] [CrossRef]
- Khalid, M.; Alqarni, M.H.; Wahab, S.; Annadurai, S.; Alamri, M.A.; Foudah, A.I.; Aljarba, T.M.; Akhtar, J.; Badruddeen; Ahmad, S. Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias Mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study. J. Clin. Med. 2022, 11, 3732. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Kong, L.; Xu, N.; Lei, H. Promotive Effects of Sesamin on Proliferation and Adhesion of Intestinal Probiotics and Its Mechanism of Action. Food Chem. Toxicol. 2021, 149, 112049. [Google Scholar] [CrossRef]
- Hamasalim, H.J. The Impact of Some Widely Probiotic (Iraqi Probiotic) on Health and Performance. J. Biosci. Med. 2015, 3, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma Lucidum: A Potential Pleiotropic Approach of Ganoderic Acids in Health Reinforcement and Factors Influencing Their Production. Fungal. Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Hirano, J.; Yoshida, T.; Sugiyama, T.; Koide, N.; Mori, I.; Yokochi, T. The Effect of Lactobacillus Rhamnosus on Enterohemorrhagic Escherichia Coli Infection of Human Intestinal Cells in Vitro. Microbiol. Immunol. 2003, 47, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Brassart, D.; Servin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune Modulation of Blood Leukocytes in Humans by Lactic Acid Bacteria: Criteria for Strain Selection. Am. J. Clin. Nutr. 1997, 66, 515S–520S. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Zafar, A.; Ahmad, R.; Ahmad, W.; Sarafroz, M.; Khalid, M.; Ghoneim, M.M.; Alshehri, S.; Wahab, S.; Ahmad, S.; et al. Quality Control Standardization, Contaminant Detection and In Vitro Antioxidant Activity of Prunus Domestica Linn. Fruit. Plants 2022, 11, 706. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A. Listeria Monocytogens—Amended Understanding of Its Pathogenesis with a Complete Picture of Its Membrane Vesicles, Quorum Sensing, Biofilm and Invasion. Microb. Pathog. 2020, 149, 104575. [Google Scholar] [CrossRef]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative Studies of Inhibitory and Antioxidant Activities, and Organic Acids Compositions of Postbiotics Produced by Probiotic Lactiplantibacillus Plantarum Strains Isolated From Malaysian Foods. Front. Vet. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Review Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef]
- Ahmad, W.; Yusuf, M.; Ahmad, A.; Hassan, Y.A.; Amir, M.; Wahab, S. Development and Validation of Ultra Performance Liquid Chromatography (UPLC) Method for the Quantitative Estimation of Caffeine in Non-Alcoholic Soft and Energy Drinks. J. AOAC Int. 2022, 105, 1146–1152. [Google Scholar] [CrossRef]
- Wu, X.; Vallance, B.A.; Boyer, L.; Bergstrom, K.S.B.; Walker, J.; Madsen, K.; O’Kusky, J.R.; Buchan, A.M.; Jacobson, K. Saccharomyces Boulardii Ameliorates Citrobacter Rodentium -Induced Colitis through Actions on Bacterial Virulence Factors. Am. J. Physiol. Liver Physiol. 2008, 294, G295–G306. [Google Scholar] [CrossRef] [Green Version]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum Sensing System: Target to Control the Spread of Bacterial Infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef]
- To, H.T.A.; Chhetri, V.; Settachaimongkon, S.; Prakitchaiwattana, C. Stress Tolerance-Bacillus with a Wide Spectrum Bacteriocin as an Alternative Approach for Food Bio-Protective Culture Production. Food Control. 2022, 133, 108598. [Google Scholar] [CrossRef]
- Medellin-Peña, M.J.; Wang, H.; Johnson, R.; Anand, S.; Griffiths, M.W. Probiotics Affect Virulence-Related Gene Expression in Escherichia Coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4259–4267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, O.; Usmani, S.; Gupta, A.; Jafri, A.; Ullah, M.F.; Wahab, S.; Arshad, M.; Kumar, S. Bioactive Extracts of Ziziphus Mauritiana Induces Apoptosis in A549 Human Lung Epithelial Carcinoma Cells through the Generation of Reactive Oxygen Species. Curr. Cancer Ther. Rev. 2021, 18, 57–68. [Google Scholar] [CrossRef]
- Gómez-Llorente, C.; Muñoz, S.; Gil, A. Role of Toll-like Receptors in the Development of Immunotolerance Mediated by Probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, B.K.; Claes, I.J.J.; Lebeer, S. Functional Mechanisms of Probiotics. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, P.; Wahab, S.; Dera, A.; Chandramoorthy, H.; Irfan, S.; Patel, A.; Abullias, S.; Zaman, G.; Ahmad, I. Anti-Cancer Activity of Ethanolic Leaf Extract of Salvia Officinalis against Oral Squamous Carcinoma Cells in Vitro via Caspase Mediated Mitochondrial Apoptosis. Pharmacogn. Mag. 2020, 16, 554. [Google Scholar] [CrossRef]
- Waserman, S.; Shah, A.; Cruikshank, H.; Avilla, E. Recognition and Management of Food Allergy and Anaphylaxis in the School and Community Setting. Immunol. Allergy Clin. North Am. 2022, 42, 91–103. [Google Scholar] [CrossRef]
- Gowri, R.S.; Meenambigai, P.; Prabhavathi, P.; Raja Rajeswari, P.; Yesudoss, L.A. Probiotics and Its Effects on Human Health-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Abatenh, E.; Gizaw, B.; Tsegay, Z.; Tefera, G.; Aynalem, E. Health Benefits of Probiotics. J. Food Sci. Technol. 2018; undefined. [Google Scholar]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud. Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ismail, I.H.; Balloch, A.; Mui, M.; Hoe, E.; Lamb, K.; Tang, M.L.K. Maternal Supplementation with LGG Reduces Vaccine-Specific Immune Responses in Infants at High-Risk of Developing Allergic Disease. Front. Immunol. 2013, 4, 381. [Google Scholar] [CrossRef] [Green Version]
- Mikov, M.; Stojancevic, M.; Bojic, G. Probiotics as a Promising Treatment for Inflammatory Bowel Disease. Hosp. Pharmacol. Int. Multidiscip. J. 2014, 1, 52–60. [Google Scholar] [CrossRef]
- Toumi, R.; Samer, A.; Soufli, I.; Rafa, H.; Touil-Boukoffa, C. Role of Probiotics and Their Metabolites in Inflammatory Bowel Diseases (IBDs). Gastroenterol. Insights 2021, 12, 56–66. [Google Scholar]
- Jadhav, V.; Bhagare, A.; Wahab, S.; Lokhande, D.; Vaidya, C.; Dhayagude, A.; Khalid, M.; Aher, J.; Mezni, A.; Dutta, M. Green Synthesized Calcium Oxide Nanoparticles (CaO NPs) Using Leaves Aqueous Extract of Moringa Oleifera and Evaluation of Their Antibacterial Activities. J. Nanomater. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma Lucidum: A Rational Pharmacological Approach to Surmount Cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current Trends and Future Perspectives of Nanomedicine for the Management of Colon Cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Hussain, A.; Farooqui, A.H.A.; Parwez Ahmad, M. Authentication and Quality Evaluation of an Important Ayurvedic Drug Averrhoa Carambola Linn Leaves. Asian J. Pharm. Clin. Res. 2013, 6, 52–56. [Google Scholar]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.K.; et al. Cancer-Preventing Attributes of Probiotics: An Update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K.; Bella, V. Probiotics and Prebiotics in the Prevention and Management of Human Cancers (Colon Cancer, Stomach Cancer, Breast Cancer, and Cervix Cancer). In Probiotics in the Prevention and Management of Human Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 187–212. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An Updated Knowledge of Black Seed (Nigella Sativa Linn.): Review of Phytochemical Constituents and Pharmacological Properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Sanders, M.E.; Klaenhammer, T.R. Invited Review. The Scientific Basis of Lactobacillus Acidophilus NCFM Functionality as a Probiotic. J. Dairy Sci. 2001, 84, 319–331. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, C.L.; Esteves, E.A.; Prates, R.P.; Moreno, L.G.; Santos, C.S. Probiotics in the Prevention and Management of Cardiovascular Diseases with Focus on Dyslipidemia. In Probiotics in the Prevention and Management of Human Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 337–351. [Google Scholar]
- Wahab, S.; Ahmad, I.; Irfan, S.; Ahmad, M.F.; Usmani, S.; Shoaib, A.; Ahmad, W. Hydrogel: An Encouraging Nanocarrier System for the Delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 17, 797–807. [Google Scholar] [CrossRef]
- Madsen, K. Probiotics and the Immune Response. J. Clin. Gastroenterol. 2006, 40, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Abdolalipour, E.; Mahooti, M.; Salehzadeh, A.; Torabi, A.; Mohebbi, S.R.; Gorji, A.; Ghaemi, A. Evaluation of the Antitumor Immune Responses of Probiotic Bifidobacterium Bifidum in Human Papillomavirus-Induced Tumor Model. Microb. Pathog. 2020, 145, 104207. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Velásquez, A.; Domínguez-Cañedo, L.; Melgar-Lalanne, G. Growth Kinetic Model, Antioxidant and Hypoglycemic Effects at Different Temperatures of Potential Probiotic Lactobacillus Spp. Rev. Mex. Ing. Quim. 2021, 20, 37–49. [Google Scholar] [CrossRef]
- Parle, M.; Malik, J. Curd: A Sedative with a Bonus Bowl of Useful Side Effects. Int. Res. J. Pharm. 2014, 5, 131–135. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic Lactobacillus Spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of Probiotics to Combat Viral Infections with Emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef]
- Martín, V.; Maldonado, A.; Fernández, L.; Rodríguez, J.M.; Connor, R.I. Inhibition of Human Immunodeficiency Virus Type 1 by Lactic Acid Bacteria from Human Breastmilk. Breastfeed. Med. 2010, 5, 153–158. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M.T. An Antibacterial and Antiviral Peptide Produced by Enterococcus Mundtii ST4V Isolated from Soya Beans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Farías, M.E.; Takeda, E.; Sesma, F.; de Ruiz Holgado, A.P.; de Torres, R.A.; Coto, C.E. Antiviral Activity of Enterocin CRL35 against Herpesviruses. Int. J. Antimicrob. Agents 1999, 12, 293–299. [Google Scholar] [CrossRef]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of Herpes Simplex Virus Type 2 by Vaginal Lactobacilli. J. Physiol. Pharmacol. 2009, 60, 19–26. [Google Scholar] [PubMed]
- Mastromarino, P.; Cacciotti, F.; Masci, A.; Mosca, L. Antiviral Activity of Lactobacillus Brevis towards Herpes Simplex Virus Type 2: Role of Cell Wall Associated Components. Anaerobe 2011, 17, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Botić, T.; Klingberg, T.D.; Weingartl, H.; Cencič, A. A Novel Eukaryotic Cell Culture Model to Study Antiviral Activity of Potential Probiotic Bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, W.; Burwinkel, M.; Twardziok, S.; Wrede, P.; Palissa, C.; Esch, B.; Schmidt, M.F.G. Inhibitory Influence of Enterococcus Faecium on the Propagation of Swine Influenza A Virus in Vitro. PLoS ONE 2013, 8, 53043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Serkedjieva, J.; Danova, S.; Ivanova, I. Antiinfluenza Virus Activity of a Bacteriocin Produced by Lactobacillus delbrueckii. Appl. Biochem. Biotechnol. 2000, 88, 285–298. [Google Scholar] [CrossRef]
- Alshahrani, M.Y.; Alfaifi, M.; Al Shahrani, M.; Alshahrani, A.S.; Alkhathami, A.G.; Dera, A.A.; Ahmad, I.; Wahab, S.; Beg, M.M.A.; Hakamy, A.; et al. Increased MRNA Expression of Key Cytokines among Suspected Cases of Pneumocystis Jirovecii Infection. BMC Infect. Dis. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lalani, I.; Bhol, K.; Ahmed, A.R. Interleukin-10: Biology, Role in Inflammation and Autoimmunity. Ann. Allergy, Asthma Immunol. 1997, 79, 469–484. [Google Scholar] [CrossRef]
- Alsayari, A.; Muhsinah, A.B.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological Efficacy of Ginseng against Respiratory Tract Infections. Molecules 2021, 26, 4095. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Practice, J.S.-G. Probiotics, Nuclear Receptor Signaling, and Anti-Inflammatory Pathways. Gastroenterol. Res. Pract. 2011, 2011, 16. [Google Scholar] [CrossRef] [Green Version]
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza Glabra in the Treatment of Respiratory Tract Infections. Mini Reviews Med. Chem. 2021, 21, 1476–1494. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.A.; Chingwaru, W.; Gradisnik, L.; Tsakalidou, E.; Cencic, A. Lactic Acid Bacteria Efficiently Protect Human and Animal Intestinal Epithelial and Immune Cells from Enteric Virus Infection. Int. J. Food Microbiol. 2010, 141, S91–S97. [Google Scholar] [CrossRef]
- Cha, M.K.; Lee, D.K.; An, H.M.; Lee, S.W.; Shin, S.H.; Kwon, J.H.; Kim, K.J.; Ha, N.J. Antiviral Activity of Bifidobacterium Adolescentis SPM1005-A on Human Papillomavirus Type 16. BMC Med. 2012, 10, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirichokchatchawan, W.; Temeeyasen, G.; Nilubol, D.; Prapasarakul, N. Protective Effects of Cell-Free Supernatant and Live Lactic Acid Bacteria Isolated from Thai Pigs Against a Pandemic Strain of Porcine Epidemic Diarrhea Virus. Probiotics Antimicrob. Proteins 2018, 10, 383–390. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.S.; Liu, Q.; Jiang, Y.L.; Yang, W.T.; Huang, H.B.; Shi, C.W.; Yang, G.L.; Wang, C.F. Surface-Displayed Porcine IFN-Λ3 in Lactobacillus Plantarum Inhibits Porcine Enteric Coronavirus Infection of Porcine Intestinal Epithelial Cells. J. Microbiol. Biotechnol. 2020, 30, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Wang, L.; Zheng, D.Z.; Chen, S.; Shi, W.; Qiao, X.Y.; Jiang, Y.P.; Tang, L.J.; Xu, Y.G.; Li, Y.J. Oral Immunization with a Lactobacillus Casei-Based Anti-Porcine Epidemic Diarrhoea Virus (PEDV) Vaccine Expressing Microfold Cell-Targeting Peptide Co1 Fused with the COE Antigen of PEDV. J. Appl. Microbiol. 2018, 124, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral Recombinant Lactobacillus Vaccine Targeting the Intestinal Microfold Cells and Dendritic Cells for Delivering the Core Neutralizing Epitope of Porcine Epidemic Diarrhea Virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Küskü-Kiraz, Z.; Genc, S.; Bekpınar, S.; Ünlücerci, Y.; Çevik, A.; Olgaç, V.; Gürdöl, F.; Uysal, M. Effects of Betaine Supplementation on Nitric Oxide Metabolism, Atherosclerotic Parameters, and Fatty Liver in Guinea Pigs Fed a High Cholesterol plus Methionine Diet. Nutrition 2018, 45, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.; Keeling, S.; Bradley, S.; Johnson-Green, P.; Green-Johnson, J.M. Interactions in the Mucosal Microenvironment: Vasoactive Intestinal Peptide Modulates the down-Regulatory Action of Lactobacillus Rhamnosus on LPS-Induced Interleukin-8 Production by Intestinal Epithelial Cells. Microb. Ecol. Health Dis. 2007, 19, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.S.; Cross, M.L.; Rutherfurd, K.J.; Gopal, P.K. Dietary Probiotic Supplementation to Enhance Cellular Immunity in the Elderly. Br. J. Biomed. Sci. 2001, 58, 94–96. [Google Scholar] [PubMed]
- Kankaanpää, P.; Sütas, Y.; Salminen, S.; Isolauri, E. Homogenates Derived from Probiotic Bacteria Provide Down-Regulatory Signals for Peripheral Blood Mononuclear Cells. Food Chem. 2003, 83, 269–277. [Google Scholar] [CrossRef]
- Bodera, P.; Chcialowski, A. Immunomodulatory Effect of Probiotic Bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, V.; Goddeeris, B.; Cox, E. The Role of Enterocytes in the Intestinal Barrier Function and Antigen Uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef]
- Biswas, G.; Korenaga, H.; Nagamine, R.; Kawahara, S.; Takeda, S.; Kikuchi, Y.; Dashnyam, B.; Yoshida, T.; Kono, T.; Sakai, M. Elevated Cytokine Responses to Vibrio Harveyi Infection in the Japanese Pufferfish (Takifugu Rubripes) Treated with Lactobacillus Paracasei Spp. Paracasei (06TCa22) Isolated from the Mongolian Dairy Product. Fish Shellfish Immunol. 2013, 35, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.E.; Wadsworth, S.; Secombes, C.J. Cytokine Expression in the Intestine of Rainbow Trout (Oncorhynchus Mykiss) during Infection with Aeromonas Salmonicida. Fish Shellfish Immunol. 2007, 23, 747–759. [Google Scholar] [CrossRef]
- Wahab, S.; Hussain, A. Cytokines as Targets for Immunomodulation. Int. J. Pharm. Pharm. Sci. 2013, 5, 60–64. [Google Scholar]
- Galdeano, C.M.; de Moreno de LeBlanc, A.; Vinderola, G.; Bonet, M.E.B.; Perdigón, G. Proposed Model: Mechanisms of Immunomodulation Induced by Probiotic Bacteria. Clin. Vaccine Immunol. 2007, 14, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the Treatment of Chronic Inflammatory Diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double Blind, Placebo Controlled Trial of Two Probiotic Strains in Interleukin 10 Knockout Mice and Mechanistic Link with Cytokine Balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef]
- Peña, J.A.; Rogers, A.B.; Ge, Z.; Ng, V.; Li, S.Y.; Fox, J.G.; Versalovic, J. Probiotic Lactobacillus Spp. Diminish Helicobacter Hepaticus -Induced Inflammatory Bowel Disease in Interleukin-10-Deficient Mice. Infect. Immun. 2005, 73, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Karamese, M.; Aydin, H.; Sengul, E.; Gelen, V.; Sevim, C.; Ustek, D.; Karakus, E. The Immunostimulatory Effect of Lactic Acid Bacteria in a Rat Model. Iran. J. Immunol. 2016, 13, 220–228. [Google Scholar]
- Borruel, N. Increased Mucosal Tumour Necrosis Factor Alpha Production in Crohn’s Disease Can Be Downregulated Ex Vivo by Probiotic Bacteria. Gut 2002, 51, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Reséndiz-Albor, A.A.; Reina-Garfias, H.; Rojas-Hernández, S.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Miliar-García, A.; Campos-Rodríguez, R. Regionalization of PIgR Expression in the Mucosa of Mouse Small Intestine. Immunol. Lett. 2010, 128, 59–67. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152. [Google Scholar] [CrossRef]
- Ahmad, M.D.F.; Wahab, S.; Ali Ahmad, F.; Intakhab Alam, M.; Ather, H.; Siddiqua, A.; Amir Ashraf, S.; Abu Shaphe, M.; Idreesh Khan, M.; Ali Beg, R. A Novel Perspective Approach to Explore Pros and Cons of Face Mask in Prevention the Spread of SARS-CoV-2 and Other Pathogens. Saudi Pharm. J. 2021, 29, 121–133. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma Lucidum: A Potential Source to Surmount Viral Infections through β-Glucans Immunomodulatory and Triterpenoids Antiviral Properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef]
- Philley, J.V.; Kannan, A.; Olusola, P.; Mcgaha, P.; Singh, K.P.; Samten, B.; Griffith, D.E.; Dasgupta, S. Microbiome Diversity in Sputum of Nontuberculous Mycobacteria Infected Women with a History of Breast Cancer. Cell. Physiol. Biochem. 2019, 52, 263–279. [Google Scholar] [CrossRef]
- Tunney, M.M.; Einarsson, G.G.; Wei, L.; Drain, M.; Klem, E.R.; Cardwell, C.; Ennis, M.; Boucher, R.C.; Wolfgang, M.C.; Elborn, J.S. Lung Microbiota and Bacterial Abundance in Patients with Bronchiectasis When Clinically Stable and during Exacerbation. Am. J. Respir. Crit. Care Med. 2013, 187, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Hani, U.; Yasmin Begum, M.; Wahab, S.; Siddiqua, A.; Osmani, R.A.M.; Rahmathulla, M. A Comprehensive Review of Current Perspectives on Novel Drug Delivery Systems and Approaches for Lung Cancer Management. J. Pharm. Innov. 2021, 1–24. [Google Scholar]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Rampelli, S.; Soverini, M.; D’Amico, F.; Barone, M.; Tavella, T.; Monti, D.; Capri, M.; Astolfi, A.; Brigidi, P.; Biagi, E.; et al. Shotgun Metagenomics of Gut Microbiota in Humans with up to Extreme Longevity and the Increasing Role of Xenobiotic Degradation. mSystems 2020, 5, e00124-20. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhya, I.; Segal, J.P.; Carding, S.R.; Hart, A.L.; Hold, G.L. The Gut Virome: The ‘Missing Link’ between Gut Bacteria and Host Immunity? Therap. Adv. Gastroenterol. 2019, 12, 175628481983662. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [Green Version]
- Taverniti, V.; Guglielmetti, S. The Immunomodulatory Properties of Probiotic Microorganisms beyond Their Viability (Ghost Probiotics: Proposal of Paraprobiotic Concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, I.; Usmani, S.; Ahmad, M.P. Efficacy of Dexamethasone for the Treatment of COVID-19 Infection: A Perspective Review. Curr. Drug Deliv. 2021, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Long COVID: Let Patients Help Define Long-Lasting COVID Symptoms. Nature 2020, 586, 170. [CrossRef] [PubMed]
- Baradaran Ghavami, S.; Pourhamzeh, M.; Farmani, M.; Raftar, S.K.A.; Shahrokh, S.; Shpichka, A.; Asadzadeh Aghdaei, H.; Hakemi-Vala, M.; Hossein-khannazer, N.; Timashev, P.; et al. Cross-Talk between Immune System and Microbiota in COVID-19. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.T.H.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The Gut Microbiota Plays a Protective Role in the Host Defence against Pneumococcal Pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.-L.L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar] [CrossRef] [Green Version]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-Intestinal Cross-Talk in Mucosal Inflammatory Disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Yazar, A.; Atis, S.; Konca, K.; Pata, C.; Akbay, E.; Calikoglu, M.; Hafta, A. Respiratory Symptoms and Pulmonary Functional Changes in Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2001, 96, 1511–1516. [Google Scholar] [CrossRef]
- Morais, A.H.A.; Passos, T.S.; Maciel, B.L.L.; da Silva-Maia, J.K. Can Probiotics and Diet Promote Beneficial Immune Modulation and Purine Control in Coronavirus Infection? Nutrients 2020, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, X.; Tang, L.; Jiang, Y.; Ma, G.; Li, Y. A Phase Trial of the Oral Lactobacillus Casei Vaccine Polarizes Th2 Cell Immunity against Transmissible Gastroenteritis Coronavirus Infection. Appl. Microbiol. Biotechnol. 2016, 100, 7457–7469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An Outbreak of Severe Kawasaki-like Disease at the Italian Epicentre of the SARS-CoV-2 Epidemic: An Observational Cohort Study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Anwar, F.; Altayb, H.N.; Al-Abbasi, F.A.; Al-Malki, A.L.; Kamal, M.A.; Kumar, V. Antiviral Effects of Probiotic Metabolites on COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 4175–4184. [Google Scholar] [CrossRef]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug Targets 2020, 21, 777–784. [Google Scholar] [CrossRef]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci 2019, 23, 8645–8657. [Google Scholar]
- Fu, Y.H.; Wen, J.B.; Wang, G.L.; Wen, P.; Gong, M.; Han, M.; Li, X. Effect of Enteral Nutrition on Cytokine Production and Plasma Endotoxin in Patients with Severe Acute Pancreatitis. World Chinese J. Dig. 2015, 23, 1174–1179. [Google Scholar] [CrossRef]
- Kageyama, Y.; Nishizaki, Y.; Aida, K.; Yayama, K.; Ebisui, T.; Akiyama, T.; Nakamura, T. Lactobacillus Plantarum Induces Innate Cytokine Responses That Potentially Provide a Protective Benefit against COVID-19: A Single-arm, Double-blind, Prospective Trial Combined with an in Vitro Cytokine Response Assay. Exp. Ther. Med. 2021, 23, 20. [Google Scholar] [CrossRef]
- Martino, H.; Tonucci, L.; Santos, K.; Oliveira, L.; Ribeiro, S. Effects of Probiotics on Glycemic Control and Inflammation in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-controlled Study. FASEB J. 2015, 29, 922.6. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. [Management of COVID-19: The Zhejiang Experience]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [CrossRef]
- Maruyama, M.; Abe, R.; Shimono, T.; Iwabuchi, N.; Abe, F.; Xiao, J.-Z. The Effects of Non-Viable Lactobacillus on Immune Function in the Elderly: A Randomised, Double-Blind, Placebo-Controlled Study. Int. J. Food Sci. Nutr. 2016, 67, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Tarnow, I.; Eskesen, D.; Morberg, C.M.; Michelsen, B.; Bügel, S.; Dragsted, L.O.; Rijkers, G.T.; Calder, P.C. Effect of Lactobacillus Paracasei Subsp. Paracasei, L. Casei 431 on Immune Response to Influenza Vaccination and Upper Respiratory Tract Infections in Healthy Adult Volunteers: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Am. J. Clin. Nutr. 2015, 101, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Van Puyenbroeck, K.; Hens, N.; Coenen, S.; Michiels, B.; Beunckens, C.; Molenberghs, G.; Van Royen, P.; Verhoeven, V. Efficacy of Daily Intake of Lactobacillus Casei Shirota on Respiratory Symptoms and Influenza Vaccination Immune Response: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Elderly Nursing Home Residents. Am. J. Clin. Nutr. 2012, 95, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A Probiotic Fermented Dairy Drink Improves Antibody Response to Influenza Vaccination in the Elderly in Two Randomised Controlled Trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.B. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium Longum-Infantis and Bifidobacterium Breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Kukkonen, K.; Nieminen, T.; Poussa, T.; Savilahti, E.; Kuitunen, M. Effect of Probiotics on Vaccine Antibody Responses in Infancy—A Randomized Placebo-Controlled Double-Blind Trial. Pediatr. Allergy Immunol. 2006, 17, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Méndez, M.; Pérez, M.; Farran, A.; Fuentes, M.C.; Cuñé, J. Lactobacillus Plantarum CECT7315 and CECT7316 Stimulate Immunoglobulin Production after Influenza Vaccination in Elderly. Nutr. Hosp. 2012, 27, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the Immune Benefits of Two Probiotic Strains Bifidobacterium Animalis Ssp. Lactis, BB-12® and Lactobacillus paracasei Ssp. Paracasei, L. Casei 431® in an Influenza Vaccination Model: A Randomised, Double-Blind, Placebo-Controlled Study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akatsu, H.; Arakawa, K.; Yamamoto, T.; Kanematsu, T.; Matsukawa, N.; Ohara, H.; Maruyama, M. Lactobacillus in Jelly Enhances the Effect of Influenza Vaccination in Elderly Individuals. J. Am. Geriatr. Soc. 2013, 61, 1828–1830. [Google Scholar] [CrossRef]
- Van Landingham, C.B.; Keast, D.R.; Longnecker, M.P. Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004. Nutrients 2021, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.C.-Y.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in Understanding and Applying Prebiotics in Research and Practice—An ISAPP Conference Paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef]
- de Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.F.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A Specific Mixture of Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides Induces a Beneficial Immunoglobulin Profile in Infants at High Risk for Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Schouten, B.; Van Esch, B.C.A.M.; Hofman, G.A.; Boon, L.; Knippels, L.M.J.; Willemsen, L.E.M.; Garssen, J. Oligosaccharide-Induced Whey-Specific CD25+ Regulatory T-Cells Are Involved in the Suppression of Cow Milk Allergy in Mice. J. Nutr. 2010, 140, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, A.P.; Haarman, M.; VanGinkel, J.W.H.; Knol, J.; Garssen, J.; Stahl, B.; Boehm, G.; M’Rabet, L. Dietary Supplementation of Neutral and Acidic Oligosaccharides Enhances Th1-Dependent Vaccination Responses in Mice. Pediatr. Allergy Immunol. 2007, 18, 304–312. [Google Scholar] [CrossRef]
- van’t Land, B.; Schijf, M.; van Esch, B.C.A.M.; van Bergenhenegouwen, J.; Bastiaans, J.; Schouten, B.; Boon, L.; Garssen, J. Regulatory T-Cells Have a Prominent Role in the Immune Modulated Vaccine Response by Specific Oligosaccharides. Vaccine 2010, 28, 5711–5717. [Google Scholar] [CrossRef]
- Gill, H.; Prasad, J. Probiotics, Immunomodulation, and Health Benefits. Adv. Exp. Med. Biol. 2008, 606, 423–454. [Google Scholar] [PubMed]
- Davidson, L.E.; Fiorino, A.-M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an Immune Adjuvant for Live-Attenuated Influenza Vaccine in Healthy Adults: A Randomized Double-Blind Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Torp, A.M.; Bahl, M.I.; Boisen, A.; Licht, T.R. Optimizing Oral Delivery of next Generation Probiotics. Trends Food Sci. Technol. 2022, 119, 101–109. [Google Scholar] [CrossRef]
- Derrien, M.; van Hylckama Vlieg, J.E.T. Fate, Activity, and Impact of Ingested Bacteria within the Human Gut Microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Municiphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foditsch, C.; Santos, T.M.A.; Teixeira, A.G.V.; Pereira, R.V.V.; Dias, J.M.; Gaeta, N.; Bicalho, R.C. Isolation and Characterization of Faecalibacterium Prausnitzii from Calves and Piglets. PLoS ONE 2014, 9, e116465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, M.F.; Laursen, R.P.; Larnkjær, A.; Michaelsen, K.F.; Bahl, M.I.; Licht, T.R. Administration of Two Probiotic Strains during Early Childhood Does Not Affect the Endogenous Gut Microbiota Composition despite Probiotic Proliferation. BMC Microbiol. 2017, 17, 175. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Minelli, E.B.; Benini, A. Relationship between Number of Bacteria and Their Probiotic Effects. Microb. Ecol. Health Dis. 2008, 20, 180–183. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Brand | Product Benefits | Formulation | Strains |
|---|---|---|---|
| Boldfit | Immune support, digestive balance, weight loss, gut health | Each capsule has 30 billion CFU | L. acidophilus and B. lactis |
| Carbamide Forte Probiotics Supplement | Metabolism management | Each capsule has 30 billion CFU | L. casei, L. plantarum, L. reuteri, L. salivarius, L. paracasei, B. bifidum, B. berve, B. lactis, S. boulardii, S. thermophilus, and many more |
| HealthKart | Boost immunity by stimulating the activity of immune cells | Each capsule has 30 billion CFU | 14 critical strains such as L. plantarum, L. fermentum, L. reuteri, B. lactis, B. bifidum, B. boulardii, L. casei, L. acidophilus, S. thermophilus, B. berve, L. rhamnosus, B. lactis, L. paracasei, and L.salivarius |
| Inlife | Digestive support and energy management | Each capsule has 2.75 billion CFU | Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, and Saccharomyces boulardii |
| Jarrow | Improve digestion, metabolism, absorption of nutrients, and immunity | Each capsule contains only about 5 billion CFU | L. rhamnosus, L. helveticus, L. plantarum, L. lactis B. berve, Pediococcus acidilactici, and B. longum |
| Mountainor | Enhances immunity and digestive health | Each capsule contains a total of 50 billion CFU with 16 carefully selected probiotic strains, which are most beneficial for gut health | It contains most strains from the L, B, and S category |
| Neuherbs Daily Probiotics | Stomach health support | Each capsule contains 20 billion CFUs | Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, and Saccharomyces boulardii |
| Now Foods | Digestive health support | Each capsule contains 25 billion CFU | The probiotic supplement contains all the necessary and imperative L, S, and B category acid strains |
| Swisse | Boosts immunity, healthy digestion, intestinal balance, growth of good bacteria, bloating, and flatulence | Each tablet contains 35 billion CFU | B. lactis and L. acidophilus |
| TrueBasics | Immune support | Each capsule contains 30 billion CFU | Lactic acids and L. plantarum |
| Wow | Immune support | Each capsule contains 20 billion CFU | L. plantarum, L. casei, L. gasseri, B. bereve, B. infantis, L. fermentum, L. paracesi, L. acidophilus, B. bifidum, L. rhamnosus, L. salivarius, S. thermophilus, L. reuteri, and B. lactis |
| Participants | Interventions | Comparison | Outcomes | Study Design | Reference |
|---|---|---|---|---|---|
| 18 | L. plantarum, Bifidobacterium longum, and Lactococcus lactis ssp. | L. plantarum, Bifidobacterium longum, and Lactococcus lactis ssp. | As an anti-COVID-19 probiotic, L. plantarum should be consumed daily | A single-arm, double-blind, prospective trial | [144] |
| 20 infants | Bifidobacteria (B. longum/B. infantis and B. breve) | Bifidobacteria and placebo | Antipoliovirus reaction could be improved by intestinal Bifidobacteria | RPC trial | [151] |
| Infants (6 months of age) | Probiotic strains | Probiotics and placebo | Probiotics may boost the immune system’s response to Hib vaccination | RDPC, allergy-prevention trial | [152] |
| 60 | Lactobacillus plantarum CECT 7315/7316 | Lactobacillus plantarum CECT 7315/7316 and placebo | It possesses immunostimulant properties and can improve influenza vaccine effectiveness in the elderly | RDPC, human trial | [153] |
| 211 | Bifidobacterium animalis ssp. lactis (BB-12®) & Lactobacillus paracasei ssp. paracasei (L. casei 431®) | BB-12® (capsule) or L. casei 431® and placebo | Immune function may be improved by using BB-12® or L. casei 431® | RDPC, parallel-group study | [154] |
| 42 | Lactobacillus paracasei | Lactobacillus paracasei or placebo | Immune markers showed no significant changes | RDPC | [147] |
| 1104 healthy adults | L. paracasei and L. casei 431 | L. paracasei and L. casei 431 or placebo | L. casei 431 has no significant effect on influenza vaccination but lessens the period of URSs | RDPC, parallel-group study | [148] |
| 15 adults | Lactobacillus in Jelly | Lactobacillus and placebo | Lactobacillus in Jelly improves the influenza vaccine effectiveness in the elderly | RPC trial | [155] |
| 737 healthy people aged ≥ 65 | L. casei Shirota | L. casei Shirota and placebo | It reduces respiratory symptoms and boosts the immune response to the influenza vaccine | RDBPC trial | [149] |
| 308 elderly | L. casei DN-114 001 | L. casei and placebo | Boost specific antibody responses to influenza vaccination | Two RDMC studies | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, S.; Almaghaslah, D.; Mahmood, S.E.; Ahmad, M.F.; Alsayegh, A.A.; Abu Haddash, Y.M.; Rahman, M.A.; Ahamd, I.; Ahmad, W.; Khalid, M.; et al. Pharmacological Efficacy of Probiotics in Respiratory Viral Infections: A Comprehensive Review. J. Pers. Med. 2022, 12, 1292. https://doi.org/10.3390/jpm12081292
Wahab S, Almaghaslah D, Mahmood SE, Ahmad MF, Alsayegh AA, Abu Haddash YM, Rahman MA, Ahamd I, Ahmad W, Khalid M, et al. Pharmacological Efficacy of Probiotics in Respiratory Viral Infections: A Comprehensive Review. Journal of Personalized Medicine. 2022; 12(8):1292. https://doi.org/10.3390/jpm12081292
Chicago/Turabian StyleWahab, Shadma, Dalia Almaghaslah, Syed Esam Mahmood, Md Faruque Ahmad, Abdulrahman A. Alsayegh, Yahya M. Abu Haddash, Mohammad Akhlaquer Rahman, Irfan Ahamd, Wasim Ahmad, Mohammad Khalid, and et al. 2022. "Pharmacological Efficacy of Probiotics in Respiratory Viral Infections: A Comprehensive Review" Journal of Personalized Medicine 12, no. 8: 1292. https://doi.org/10.3390/jpm12081292







