Renal Leukemic Infiltration Overlapping Acute Focal Bacterial Nephritis during Myelodysplastic Syndrome: An Autopsy Case Report
Abstract
:1. Introduction
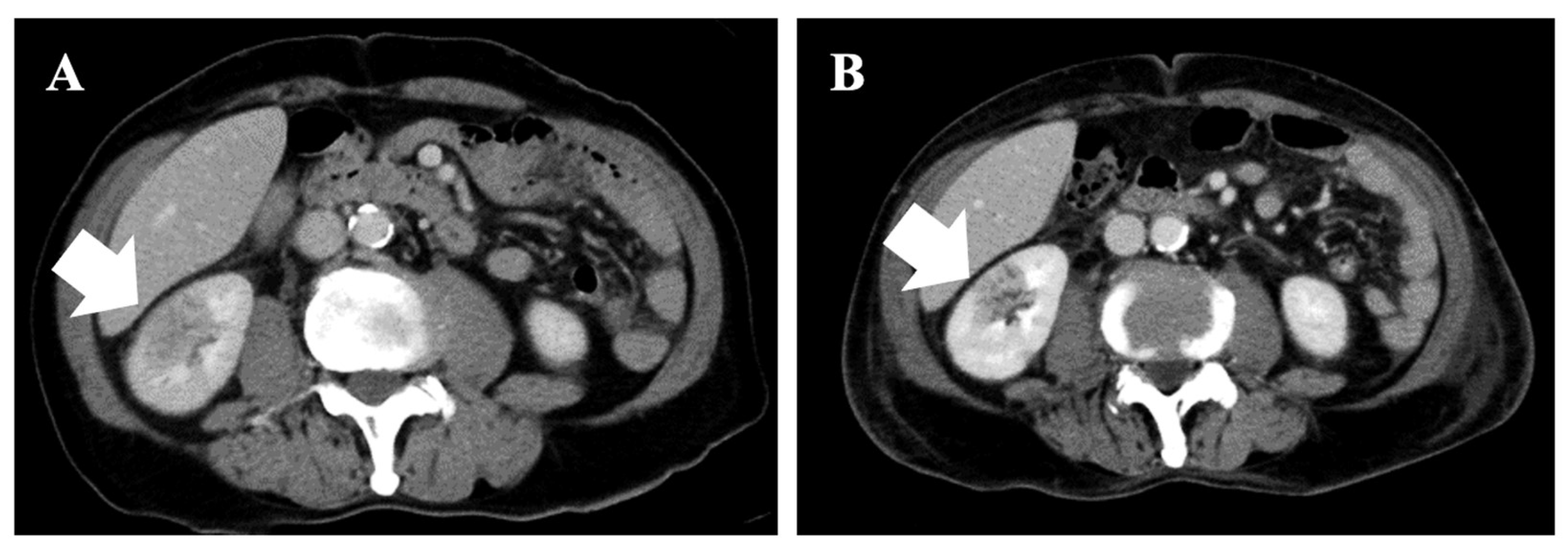
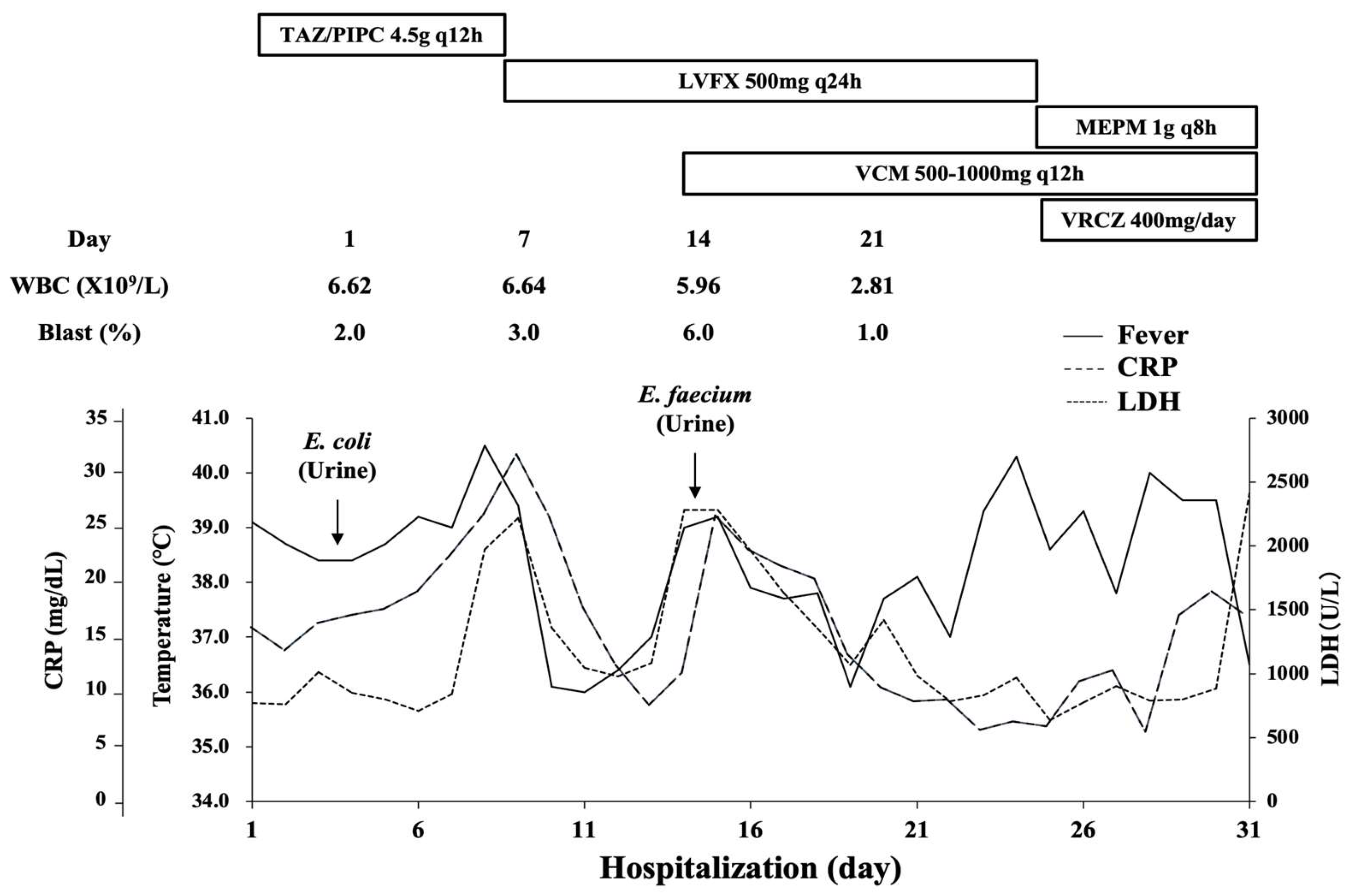
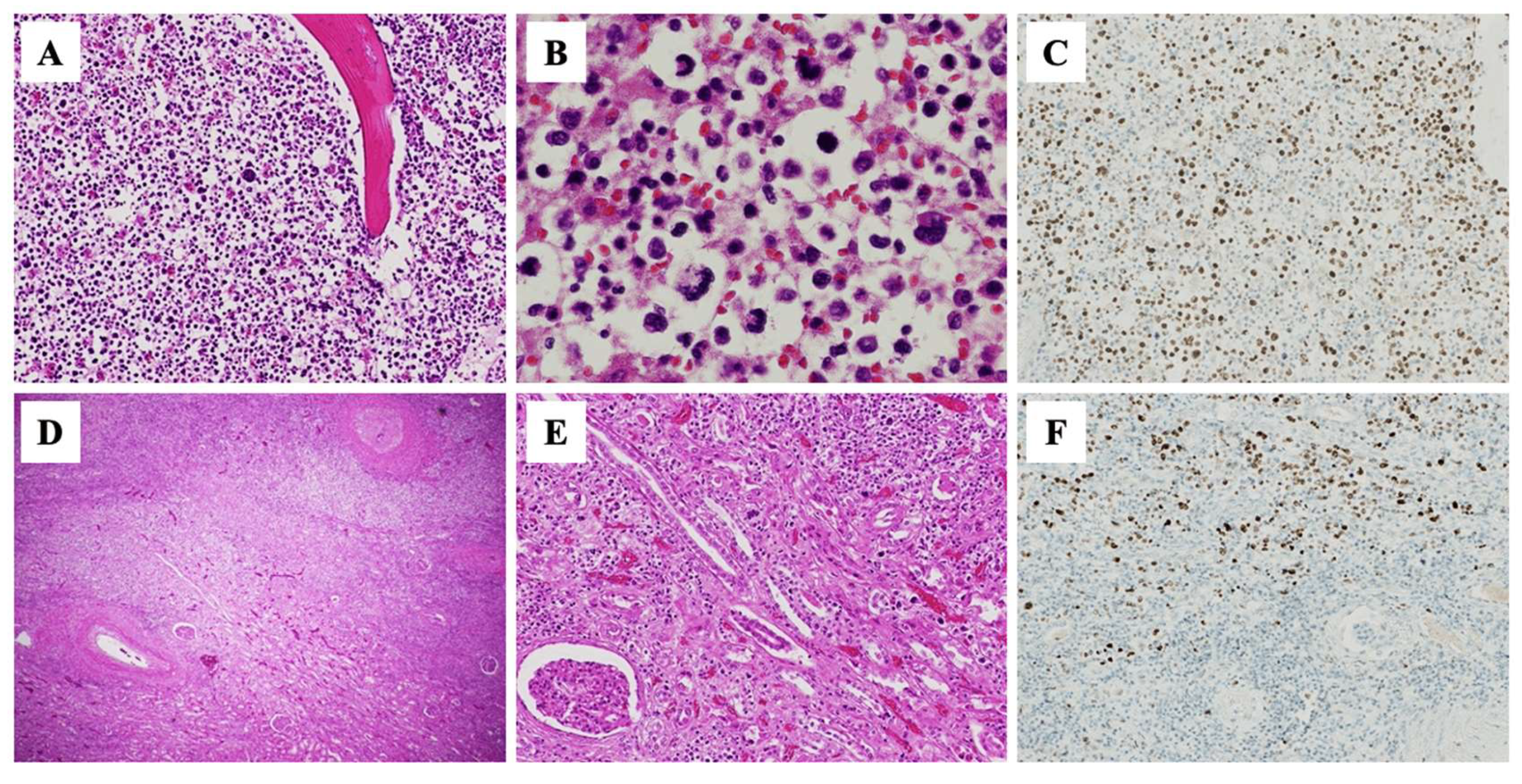
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef]
- Nachtkamp, K.; Stark, R.; Strupp, C.; Kundgen, A.; Giagounidis, A.; Aul, C.; Hildebrandt, B.; Haas, R.; Gattermann, N.; Germing, U. Causes of death in 2877 patients with myelodysplastic syndromes. Ann. Hematol. 2016, 95, 937–944. [Google Scholar] [CrossRef]
- Toma, A.; Fenaux, P.; Dreyfus, F.; Cordonnier, C. Infections in myelodysplastic syndromes. Haematologica 2012, 97, 1459–1470. [Google Scholar] [CrossRef]
- Rosenfield, A.T.; Glickman, M.G.; Taylor, K.J.; Grade, M.; Hodson, J. Acute focal bacterial nephritis (acute lobar nephrons). Radiology 1979, 132, 553–561. [Google Scholar] [CrossRef]
- Sieger, N.; Kyriazis, I.; Schaudinn, A.; Kallidonis, P.; Neuhaus, J.; Liatsikos, E.N.; Ganzer, R.; Stolzenburg, J.U. Acute focal bacterial nephritis is associated with invasive diagnostic procedures—A cohort of 138 cases extracted through a systematic review. BMC Infect. Dis. 2017, 17, 240. [Google Scholar] [CrossRef]
- Hilmes, M.A.; Dillman, J.R.; Mody, R.J.; Strouse, P.J. Pediatric renal leukemia: Spectrum of CT imaging findings. Pediatr. Radiol. 2008, 38, 424–430. [Google Scholar] [CrossRef]
- Kimura, Y.; Kiyota, K.; Koga, H.; Suenobu, S.; Ihara, K. Renal lesions mimicking acute focal bacterial nephritis in pediatric leukemia. Pediatr. Int. 2022, 64, e14838. [Google Scholar] [CrossRef]
- Xiao, J.C.; Walz-Mattmuller, R.; Ruck, P.; Horny, H.P.; Kaiserling, E. Renal involvement in myeloproliferative and lymphoproliferative disorders. A study of autopsy cases. Gen. Diagn. Pathol. 1997, 142, 147–153. [Google Scholar]
- Saha, A.; Dandekar, S.; Milla, S.; Roman, E.; Bhatla, T. Bilateral parotid gland enlargement and palpable nephromegaly in infant acute lymphoblastic leukemia: Case report and review of the literature. J. Pediatr. Hematol. Oncol. 2014, 36, 246–248. [Google Scholar] [CrossRef]
- Sabui, T.K.; Sardar, S.; Sumanta, L.; Roy, A. Bilateral nephromegaly and arthritis: A rare presentation of acute lymphoblastic leukemia. Open J. Pediatr. 2013, 3, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Aratani, S.; Aburakawa, S.; Ryotokuji, T.; Marumo, A.; Sakai, Y.; Inokuchi, K.; Tsuruoka, S. Primary tumor infiltration and severe acute kidney injury in patients with acute myeloblastic leukemia. J. Nippon Med. Sch. 2020, 87, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Ooi, K.Y.; Gujadhur, A.; Pham, A.; Lukito, P.; Flanc, R.; Menahem, S. Infiltrative acute myeloid leukemia as a cause of acute kidney injury. Clin. Kidney J. 2013, 6, 448–449. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Della Porta, M.G.; Malcovati, L. The genetic basis of myelodysplasia and its clinical relevance. Blood 2013, 122, 4021–4034. [Google Scholar] [CrossRef] [Green Version]
- Germing, U.; Hildebrandt, B.; Pfeilstocker, M.; Nosslinger, T.; Valent, P.; Fonatsch, C.; Lubbert, M.; Haase, D.; Steidl, C.; Krieger, O.; et al. Refinement of the international prognostic scoring system (IPSS) by including LDH as an additional prognostic variable to improve risk assessment in patients with primary myelodysplastic syndromes (MDS). Leukemia 2005, 19, 2223–2231. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
| Complete Blood Count | Blood Chemistry | Urinalysis | ||||||
|---|---|---|---|---|---|---|---|---|
| White Blood Cells | 6.6 × 109 | /L | Total Protein | 7.5 | g/dL | Color | Yellow | |
| Blast | 2.0 | % | Albumin | 3.5 | g/dL | pH | 5.5 | |
| Metamyelocytes | 1.0 | % | Aspartate transaminase | 34 | IU/L | Specific gravity | 1.043 | |
| Segmented neutrophils | 36.0 | % | Alanine transaminase | 15 | IU/L | Protein | − | |
| Rod-shaped neutrophils | 13.0 | % | Lactate dehydrogenase | 768 | IU/L | Bilirubin | − | |
| Eosinophils | 2.0 | % | Total bilirubin | 1.1 | mg/dL | Urobilinogen | +− | |
| Basophils | 0.0 | % | Direct bilirubin | 0.2 | mg/dL | Occult blood | 3+ | |
| Lymphocytes | 24.0 | % | Creatinine | 1.52 | mg/dL | Glucose | − | |
| Monocytes | 21.0 | % | Blood urea nitrogen | 38.2 | mg/dL | Ketone | − | |
| Atypical lymphocytes | 1.0 | % | Alkaline phosphatase | 199 | IU/L | Nitrite | − | |
| Erythroblasts | 6 | /100 | γ-Glutamyl transpeptidase | 22 | IU/L | White Blood Cells/HPF | 30–49 | /HPF |
| Red blood cells | 1.39 × 1012 | L | Creatine kinase | 500 | mg/dL | Gram-negative rods | 2+ | |
| Hemoglobin | 5.4 | g/dL | Sodium | 136 | mEq/L | Coagulation System | ||
| Hematocrit | 25.0 | % | Potassium | 4.3 | mEq/L | APTT | 25.0 | Sec |
| Mean corpuscular volume | 112.2 | fL | Chloride | 100 | mEq/L | PT-INR | 1.46 | |
| Reticulocytes | 29 × 109 | L | C-reactive protein | 13.97 | mg/dL | Fibrinogen | 244 | mg/dL |
| Platelets | 24 × 109 | L | Ferritin | 1665 | ng/mL | D-dimer | 80.04 | μg/dL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, K.; Tamura, S.; Maruyama, A.; Naitoh, T.; Teramoto, K.; Mikasa, Y.; Tanaka, M.; Murata, S.; Kato, S. Renal Leukemic Infiltration Overlapping Acute Focal Bacterial Nephritis during Myelodysplastic Syndrome: An Autopsy Case Report. Medicina 2022, 58, 1060. https://doi.org/10.3390/medicina58081060
Murakami K, Tamura S, Maruyama A, Naitoh T, Teramoto K, Mikasa Y, Tanaka M, Murata S, Kato S. Renal Leukemic Infiltration Overlapping Acute Focal Bacterial Nephritis during Myelodysplastic Syndrome: An Autopsy Case Report. Medicina. 2022; 58(8):1060. https://doi.org/10.3390/medicina58081060
Chicago/Turabian StyleMurakami, Keishu, Shinobu Tamura, Anna Maruyama, Tomomi Naitoh, Kan Teramoto, Yurina Mikasa, Masaoh Tanaka, Shinichi Murata, and Seiya Kato. 2022. "Renal Leukemic Infiltration Overlapping Acute Focal Bacterial Nephritis during Myelodysplastic Syndrome: An Autopsy Case Report" Medicina 58, no. 8: 1060. https://doi.org/10.3390/medicina58081060






