Biomimetic Mineralization in External Layer of Decalcified Fish Scale
Abstract
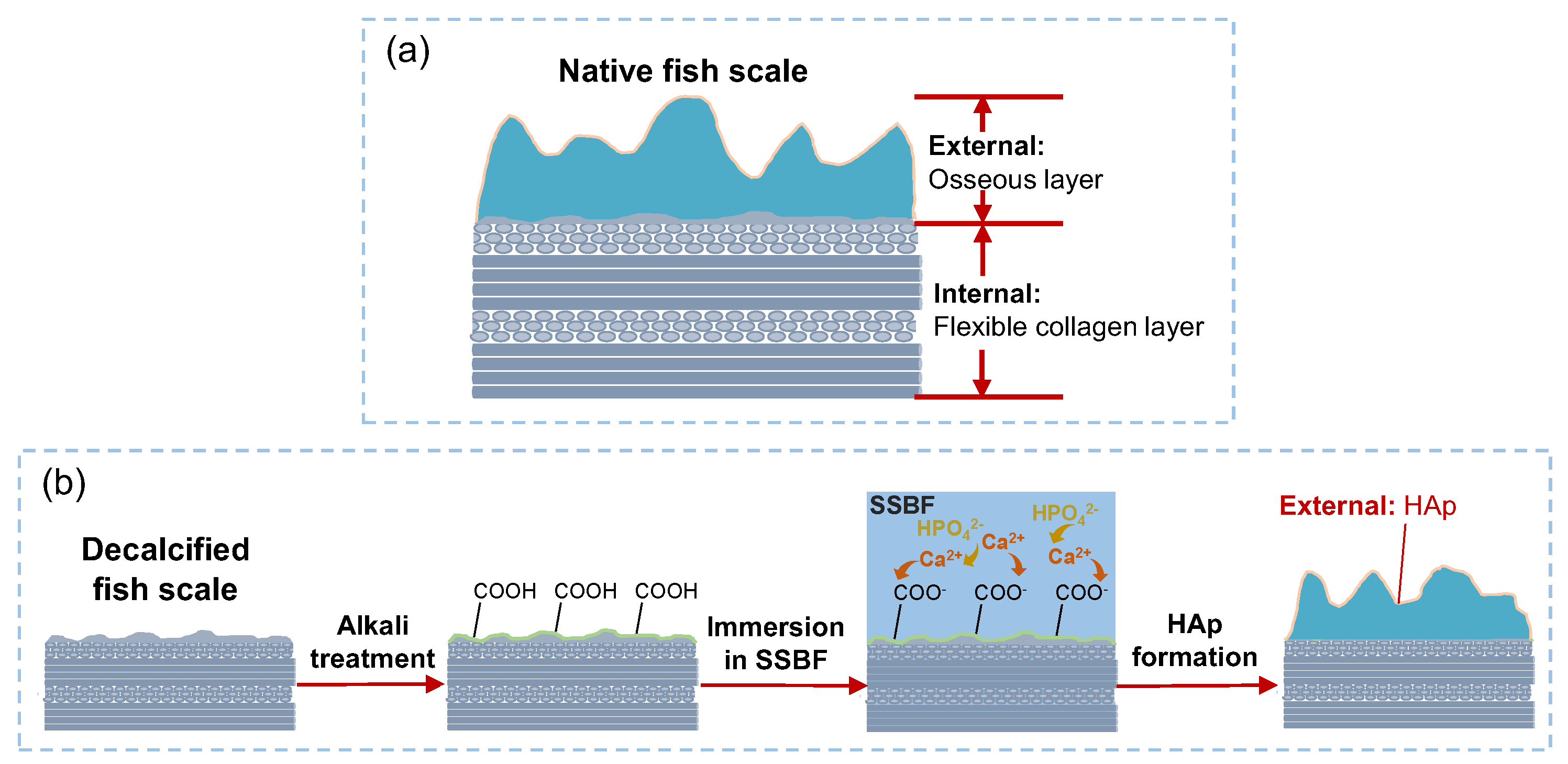
:1. Introduction
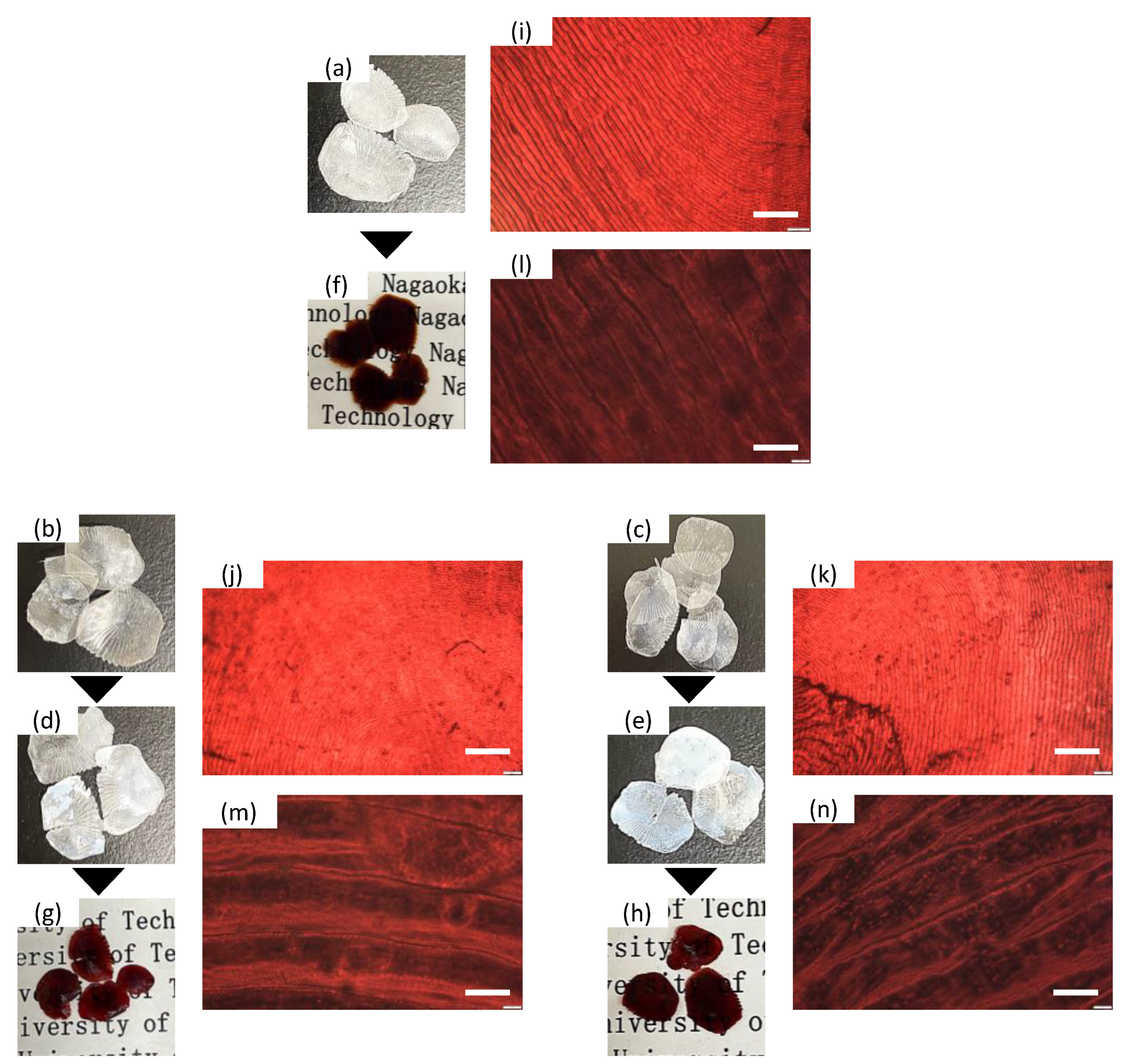
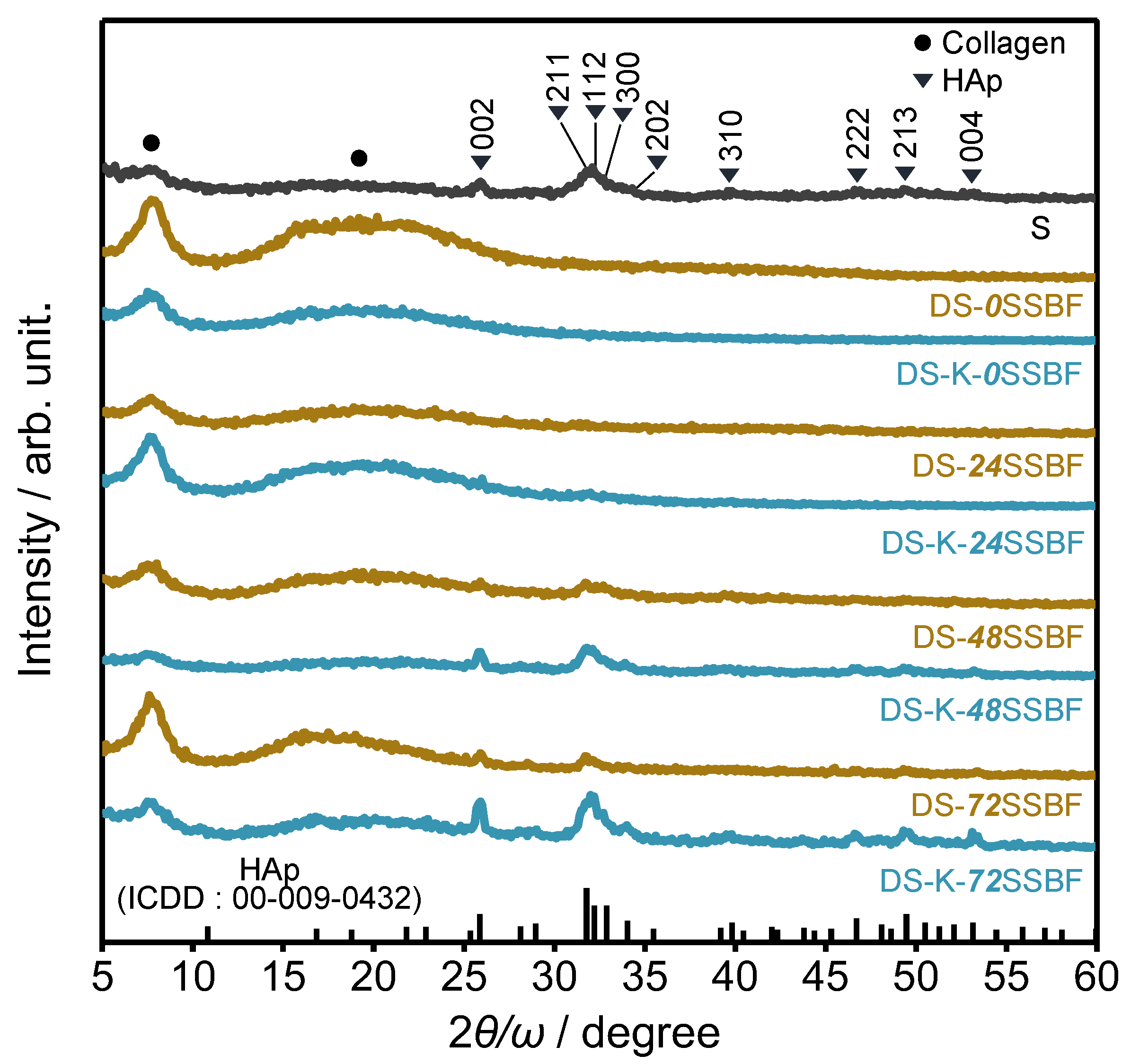
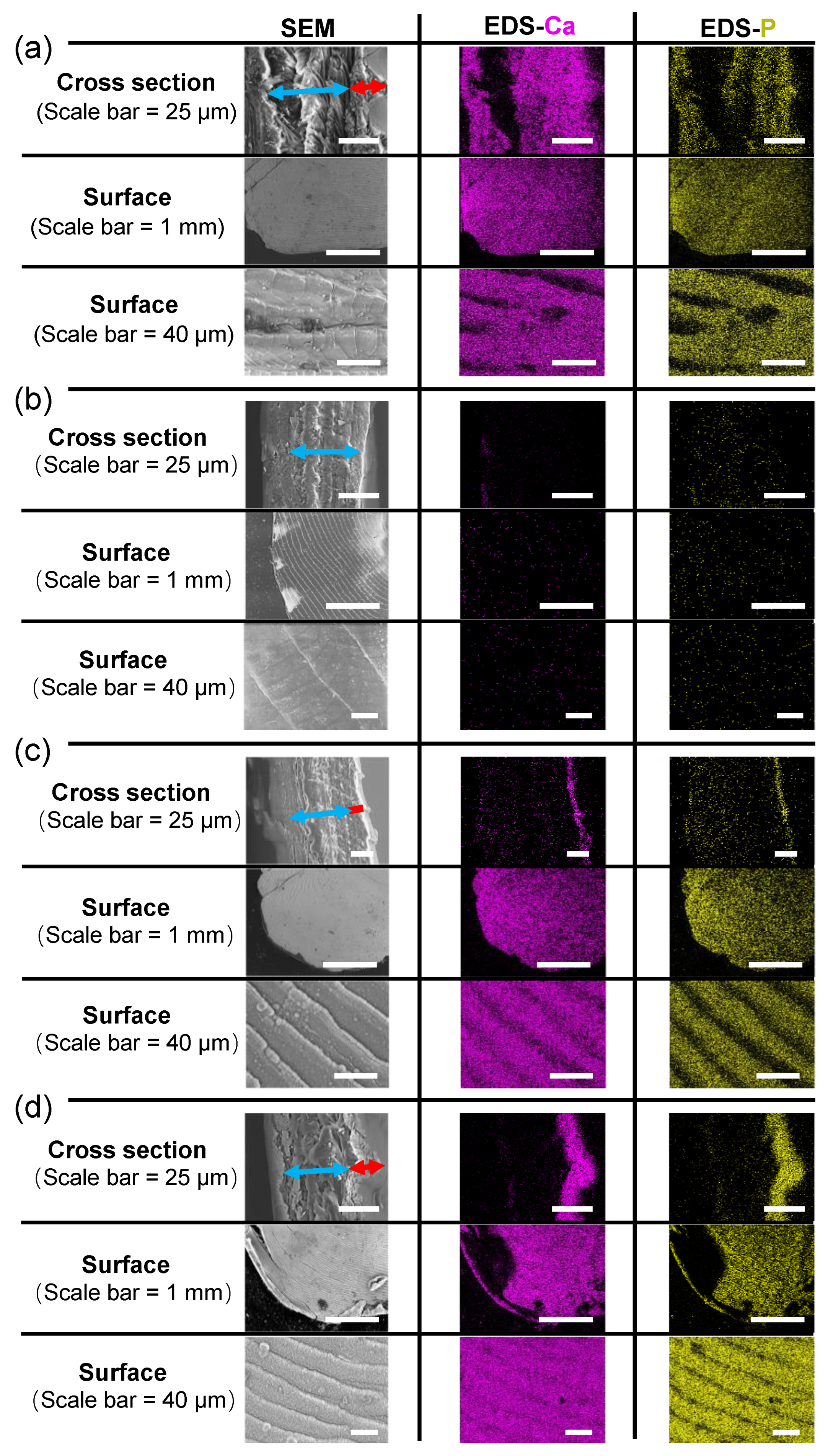
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Microstructure, Mechanical, and Biomimetic Properties of Fish Scales from Pagrus Major. J. Struct. Biol. 2003, 142, 327–333. [Google Scholar] [CrossRef]
- Chen, P.Y.; Schirer, J.; Simpson, A.; Nay, R.; Lin, Y.S.; Yang, W.; Lopez, M.I.; Li, J.; Olevsky, E.A.; Meyers, M.A. Predation versus Protection: Fish Teeth and Scales Evaluated by Nanoindentation. J. Mater. Res. 2012, 27, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Wang, Y.; Feng, Q.; Kienzle, A.; Müller, W.E.G. Hierarchical Structure and Cytocompatibility of Fish Scales from Carassius Auratus. Mater. Sci. Eng. C 2014, 43, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.R.; Zarei, F.; Sanjarani Vahed, N.; Masoudi, M. Scale Morphology and Phylogenetic Character Mapping of Scale-Surface Microstructures in Sixteen Aphanius Species (Teleostei: Aphaniidae). Micron 2019, 119, 39–53. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.G. Development and Application of Fish Scale Wastes as Versatile Natural Biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Mackey, M.; Zimmermann, E.A.; Chang, E.H.; Schaible, E.; Qin, Z.; Buehler, M.J.; Ritchie, R.O.; et al. Protective Role of Arapaima Gigas Fish Scales: Structure and Mechanical Behavior. Acta Biomater. 2014, 10, 3599–3614. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, A.; Mandal, N.; Mondal, B.; Mukhopadhyay, S.S.; Dey, A.; Singh, S. Physico-Chemical Characterization and Biological Response of Labeo Rohita-Derived Hydroxyapatite Scaffold. Bioprocess Biosyst. Eng. 2014, 37, 1233–1240. [Google Scholar] [CrossRef]
- Lin, Y.S.; Wei, C.T.; Olevsky, E.A.; Meyers, M.A. Mechanical Properties and the Laminate Structure of Arapaima Gigas Scales. J. Mech. Behav. Biomed. Mater. 2011, 4, 1145–1156. [Google Scholar] [CrossRef]
- Arola, D.; Murcia, S.; Stossel, M.; Pahuja, R.; Linley, T.; Devaraj, A.; Ramulu, M.; Ossa, E.A.; Wang, J. The Limiting Layer of Fish Scales: Structure and Properties. Acta Biomater. 2018, 67, 319–330. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Fish Scales as a Biocomposite of Collagen and Calcium Salts. Key Eng. Mater. 2014, 587, 185–190. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological Materials: Structure and Mechanical Properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and Tooth Mineralization: Why Apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Yuasa, T.; Miyamoto, Y.; Ishikawa, K.; Takechi, M.; Momota, Y.; Tatehara, S.; Nagayama, M. Effects of Apatite Cements on Proliferation and Differentiation of Human Osteoblasts In Vitro. Biomaterials 2004, 25, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Tagaya, M. Simple Preparation of Hydroxyapatite Nanostructures Derived from Fish Scales. Mater. Lett. 2018, 222, 156–159. [Google Scholar] [CrossRef]
- Chai, Y.; Nishikawa, M.; Tagaya, M. Preparation of Gold/Hydroxyapatite Hybrids Using Natural Fish Scale Template and Their Effective Albumin Interactions. Adv. Powder Technol. 2018, 29, 1198–1203. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-structure Changes in Bioactive Glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Tanahashi, M.; Yao, T.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T.; Yamamuro, T. Apatite Coated on Organic Polymers by Biomimetic Process: Improvement in Its Adhesion to Substrate by NaOH Treatment. J. Appl. Biomater. 1994, 5, 339–347. [Google Scholar] [CrossRef]
- Gemelli, E.; Resende, C.X.; De Almeida Soares, G.D. Nucleation and Growth of Octacalcium Phosphate on Treated Titanium by Immersion in a Simplified Simulated Body Fluid. J. Mater. Sci. Mater. Med. 2010, 21, 2035–2047. [Google Scholar] [CrossRef]
- Resende, C.X.; Dille, J.; Platt, G.M.; Bastos, I.N.; Soares, G.A. Characterization of Coating Produced on Titanium Surface by a Designed Solution Containing Calcium and Phosphate Ions. Mater. Chem. Phys. 2008, 109, 429–435. [Google Scholar] [CrossRef]
- Taguchi, T.; Xu, L.; Kobayashi, H.; Taniguchi, A.; Kataoka, K.; Tanaka, J. Encapsulation of Chondrocytes in Injectable Alkali-Treated Collagen Gels Prepared Using Poly(Ethylene Glycol)-Based 4-Armed Star Polymer. Biomaterials 2005, 26, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Darvish, D.M. Collagen Fibril Formation in Vitro: From Origin to Opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of Alkaline Pretreatments and Acid Extraction Conditions on the Acid-Soluble Collagen from Grass Carp (Ctenopharyngodon Idella) Skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.H.; Kenten, R.H. The Effect of Alkalis on Collagen. Biochem. J. 1948, 43, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Tanahashi, M.; Matsuda, T. Surface Functional Group Dependence on Apatite Formation on Self-Assembled Monolayers in a Simulated Body Fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Zhou, Y.; Chai, Y.; Miyata, M.; Tagaya, M. Preparation of Citric Acid-Modified Poly(Vinyl Alcohol) Films for Effectively Precipitating Calcium Phosphate Particles. Cryst. Eng. Com. 2022. [Google Scholar] [CrossRef]
- Nakamura, K.; Oaki, Y.; Imai, H. Multistep Crystal Growth of Oriented Fluorapatite Nanorod Arrays for Fabrication of Enamel-like Architectures on a Polymer Sheet. Cryst. Eng. Com. 2017, 19, 669–674. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, A.; Li, Z.; He, S.; Shao, L. Preparation and Characterisation of Collagen from Freshwater Fish Scales. Food Nutr. Sci. 2011, 2, 818–823. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chai, Y.; Mikami, K.; Tagaya, M. Biomimetic Mineralization in External Layer of Decalcified Fish Scale. Biomimetics 2022, 7, 97. https://doi.org/10.3390/biomimetics7030097
Zhou Y, Chai Y, Mikami K, Tagaya M. Biomimetic Mineralization in External Layer of Decalcified Fish Scale. Biomimetics. 2022; 7(3):97. https://doi.org/10.3390/biomimetics7030097
Chicago/Turabian StyleZhou, Yanni, Yadong Chai, Kurisu Mikami, and Motohiro Tagaya. 2022. "Biomimetic Mineralization in External Layer of Decalcified Fish Scale" Biomimetics 7, no. 3: 97. https://doi.org/10.3390/biomimetics7030097
APA StyleZhou, Y., Chai, Y., Mikami, K., & Tagaya, M. (2022). Biomimetic Mineralization in External Layer of Decalcified Fish Scale. Biomimetics, 7(3), 97. https://doi.org/10.3390/biomimetics7030097





