The Neural Control Mechanisms of Gekkonid Adhesion Locomotion: The Effect of Spinal Cord Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedures
2.2. Performance Tests
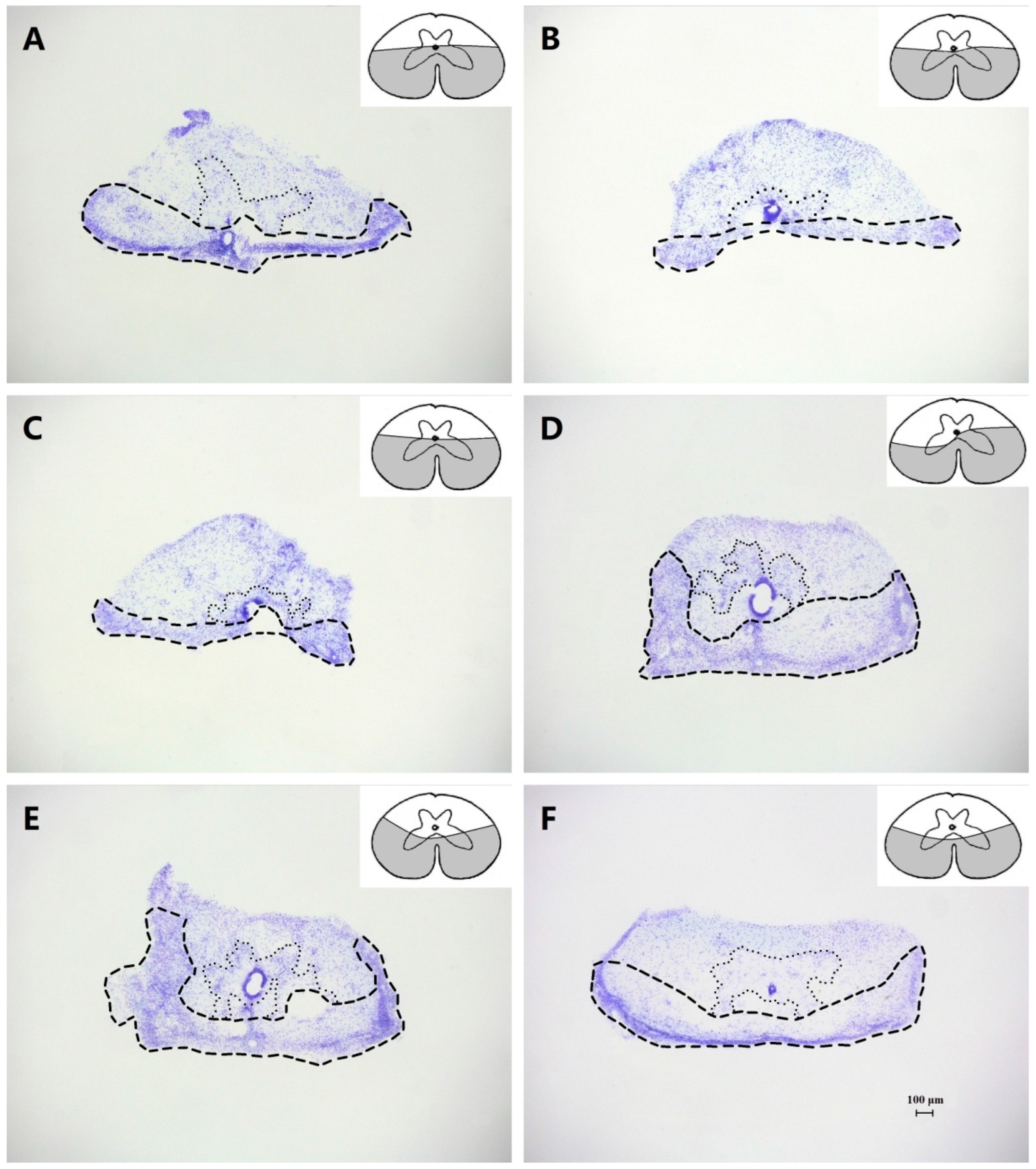
2.3. Histological Procedures
3. Results
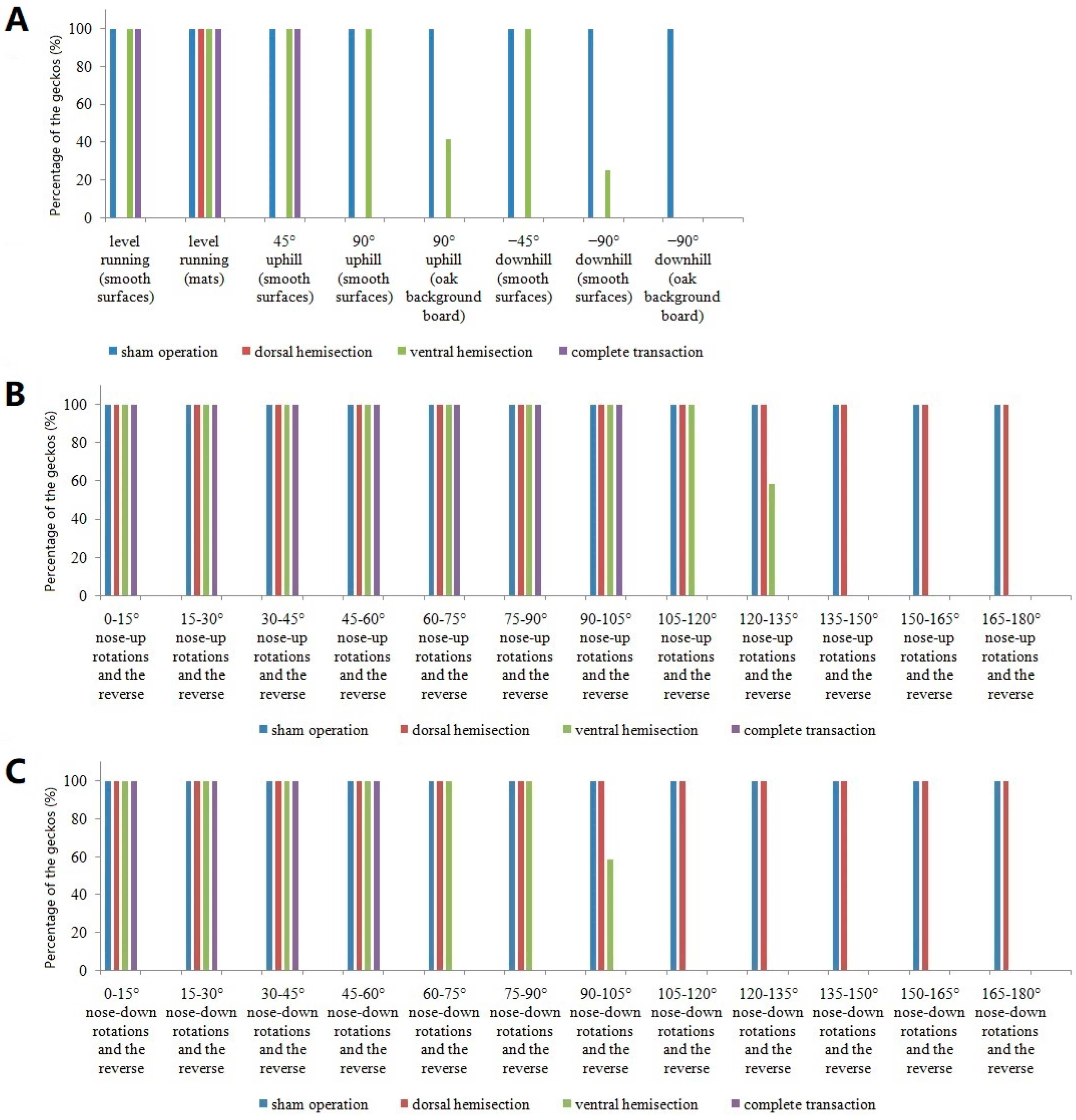
3.1. Locomotor Performance in Intact Geckos
3.2. Effects of Complete Transection
3.3. Effects of Dorsal Hemisection
3.4. Effects of Ventral Hemisection
4. Discussion
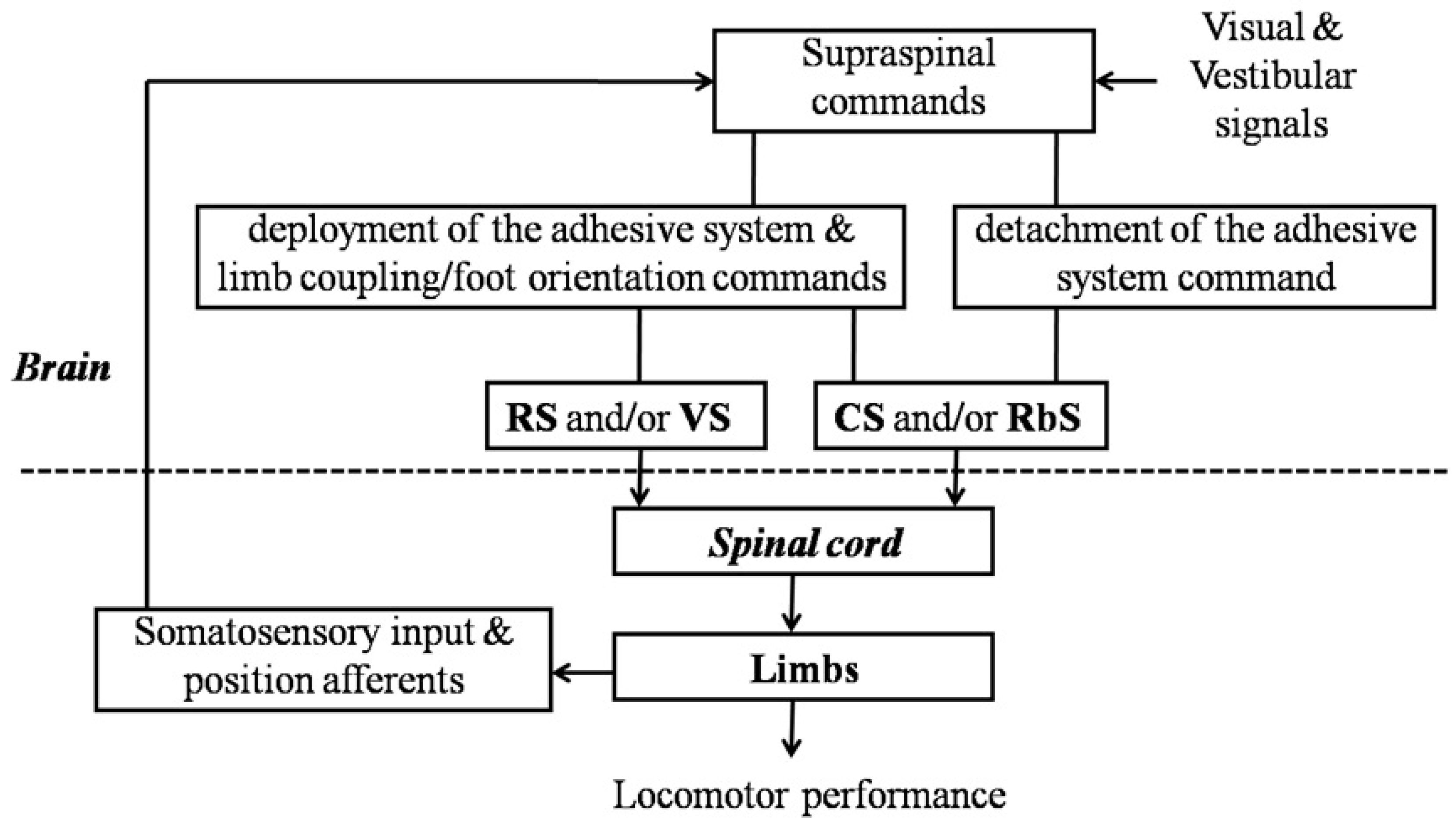
4.1. Maintenance of Locomotor Stability
4.2. Neural Control of the Deployment and the Detachment of the Adhesive System
4.3. Correlation Betweensupraspinal Structure and Adhesion Locomotor Performance
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Dai, Z.; Li, W.; Ji, A.; Wang, W. How do the substrate reaction forces acting on a gecko’s limbs respond to inclines? Sci. Nat. 2015, 102, 72015. [Google Scholar] [CrossRef] [PubMed]
- Birn-Jeffery, A.V.; Higham, T.E. Geckos decouple fore- and hind limb kinematics in response to changes in incline. Front. Zool. 2016, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Birn-Jeffery, A.V.; Higham, T.E. Data from: Geckos significantly alter foot orientation to facilitate adhesion during downhill locomotion. Biol. Lett. 2014, 10, 20140456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossignol, S.; Drew, T.; Brustein, E.; Jiang, W. Chapter 31 Locomotor Performance and Adaptation after Partial or Complete Spinal Cord Lesions in the Cat. Prog. Brain Res. 1999, 123, 349–365. [Google Scholar] [CrossRef]
- Rossignol, S.; Bouyer, L.; Barthélemy, D.; Langlet, C.; Leblond, H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res. Rev. 2002, 40, 257–266. [Google Scholar] [CrossRef]
- Lyalka, V.F.; Zelenin, P.V.; Karayannidou, A.; Orlovsky, G.N.; Grillner, S.; Deliagina, T.G. Impairment and Recovery of Postural Control in Rabbits With Spinal Cord Lesions. J. Neurophysiol. 2005, 94, 3677–3690. [Google Scholar] [CrossRef]
- Rehermann, M.I.; Marichal, N.; Russo, R.E.; Trujillo-Cenóz, O. Neural reconnection in the transected spinal cord of the freshwater turtle Trachemys dorbignyi. J. Comp. Neurol. 2009, 515, 197–214. [Google Scholar] [CrossRef]
- Srivastava, V.K. Functional recovery following reptilean (Calotus calotus) spinal cord transection. Indian J. Physiol. Pharmacol. 1992, 36, 193–196. [Google Scholar]
- Alibardi, L. Observations on the recovering lumbar spinal cord of lizards show multiple origins of the cells forming the bridge region including immune cells. J. Morphol. 2019, 281, 95–109. [Google Scholar] [CrossRef]
- Alibardi, L. Observations on Lumbar Spinal Cord Recovery after Lesion in Lizards Indicates Regeneration of a Cellular and Fibrous Bridge Reconnecting the Injured Cord. J. Dev. Biol. 2014, 2, 210–229. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Maheshwari, V.; Tyagi, S.P.; Ali, S. Histological changes in reptilean spinal cord transection: Correlation with functional recovery. Indian J. Physiol. Pharmacol. 1994, 38, 189–192. [Google Scholar] [PubMed]
- Brinkman, D. The hind limb step cycle of Iguana and primitive reptiles. J. Zool. 1981, 193, 91–103. [Google Scholar] [CrossRef]
- Russell, A.P. A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia: Gekkonidae). J. Zool. 1975, 176, 437–476. [Google Scholar] [CrossRef]
- Zaaf, A.; Herrel, A.; Aerts, P.; De Vree, F. Morphology and morphometrics of the appendicular musculature in geckoes with different locomotor habits (Lepidosauria). Zoomorphology 1999, 119, 9–22. [Google Scholar] [CrossRef]
- Pesika, N.S.; Tian, Y.; Zhao, B.; Rosenberg, K.; Zeng, H.; McGuiggan, P.; Autumn, K.; Israelachvili, J.N. Peel-Zone Model of Tape Peeling Based on the Gecko Adhesive System. J. Adhes. 2007, 83, 383–401. [Google Scholar] [CrossRef]
- Autumn, K.; Dittmore, A.; Santos, D.; Spenko, M.; Cutkosky, M. Frictional adhesion: A new angle on gecko attachment. J. Exp. Biol. 2006, 209, 3569–3579. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.-T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Tang, Y.; Dai, Z. Subdivisions of the mesencephalon and isthmus in the lizard Gekko gecko as revealed by ChAT immunohistochemistry. Anat. Rec. 2021, 304, 2014–2031. [Google Scholar] [CrossRef]
- Martin, J.H.; Ghez, C. Red nucleus and motor cortex: Parallel motor systems for the initiation and control of skilled movement. Behav. Brain Res. 1988, 28, 217–223. [Google Scholar] [CrossRef]
- Sarrafizadeh, R.; Keifer, J.; Houk, J. Somatosensory and movement-related properties of red nucleus: A single unit study in the turtle. Exp. Brain Res. 1996, 108, 1–17. [Google Scholar] [CrossRef]
- ten Donkelaar, H.J. Evolution of Motor Systems: Corticospinal, Reticulospinal, Rubrospinal and Vestibulospinal Systems. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1248–1254. [Google Scholar]
- Lavoie, S.; Drew, T. Discharge Characteristics of Neurons in the Red Nucleus During Voluntary Gait Modifications: A Comparison with the Motor Cortex. J. Neurophysiol. 2002, 88, 1791–1814. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, A.; Dai, Z. A trajectory planning method for sprawling robot inspired by a trotting animal. J. Mech. Sci. Technol. 2017, 31, 327–334. [Google Scholar] [CrossRef]
- Russell, A.P.; Higham, T.E. A new angle on clinging in geckos: Incline, not substrate, triggers the deployment of the adhesive system. Proc. R. Soc. B Biol. Sci. 2009, 276, 3705–3709. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.J.; Higham, T.E. Data from: Passively stuck: Death does not affect gecko adhesion strength. Biol. Lett. 2014, 10, 20140701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drew, T.; Rossignol, S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J. Neurophysiol. 1990, 64, 767–781. [Google Scholar] [CrossRef]
- Mori, S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog. Neurobiol. 1987, 28, 161–195. [Google Scholar] [CrossRef]
- Duysens, J.; Clarac, F.; Cruse, H. Load-Regulating Mechanisms in Gait and Posture: Comparative Aspects. Physiol. Rev. 2000, 80, 83–133. [Google Scholar] [CrossRef]
- Muir, G.D.; Whishaw, I.Q. Red nucleus lesions impair overground locomotion in rats: A kinetic analysis. Eur. J. Neurosci. 2000, 12, 1113–1122. [Google Scholar] [CrossRef]
- Van Kan, P.L.; McCurdy, M.L. Role of Primate Magnocellular Red Nucleus Neurons in Controlling Hand Preshaping during Reaching to Grasp. J. Neurophysiol. 2001, 85, 1461–1478. [Google Scholar] [CrossRef]
- Van Kan, P.L.; McCurdy, M.L. Contribution of Primate Magnocellular Red Nucleus to Timing of Hand Preshaping during Reaching to Grasp. J. Neurophysiol. 2002, 87, 1473–1487. [Google Scholar] [CrossRef]
- Wang, W.-B.; Guo, C.; Sun, J.-R.; Dai, Z.-D. Locomotion Elicited by Electrical Stimulation in the Midbrain of the Lizard Gekko gecko. Intell. Unmanned Syst. Theory Appl. 2009, 192, 145–153. [Google Scholar]
- Miller, L.E.; Gibson, A.R. Red Nucleus. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 55–62. [Google Scholar]



| Inclines | Fore Limbs | Hind Limbs | ||
|---|---|---|---|---|
| Locomotor Performance/Foot Orientation | Locomotor Function | Locomotor Performance/Foot Orientation | Locomotor Function | |
| level | extend anterolaterally | brake | extend laterally | driver/propulsion |
| 45°/90° uphill | extend forward and upward | propulsion | extend laterally and anterolaterally | driver |
| −45°/−90° downhill | extend anteriorly and anterolaterally | propulsion | rotate posteriorly and posterolaterally | brake and/or stabilizer |
| inverted (180°) surface | extend anterolaterally | propulsion | extend posterolaterally | brake and stabilizer |
| Inclines | Complete Transaction (n = 8) | Dorsal Hemisection (n = 12) | Ventral Hemisection (n = 12) |
|---|---|---|---|
| level locomotion (smooth surfaces) | Yes (paw drag with weak alternating movements) | No (paw drag with digital non-hyperextension) | Yes (paw drag with digital hyperextension) |
| level locomotion (mats) | Yes (paw drag with weak alternating movements) | Yes (paw drag) | Yes (paw drag) |
| 45° uphill (smooth surfaces) | Yes | No | Yes |
| 90° uphill (smooth surfaces) | No | No | Yes |
| 90° uphill (oak background board) | No | No | Poor |
| −45° downhill (smooth surfaces) | No | No | Yes |
| −90° downhill (smooth surfaces) | No | No | Poor |
| −90° downhill (oak background board) | No | No | No |
| periodic trapezoid tilting | maintain attachment during the 0–105° nose-up rotations and the reverse in turn a, as well as the 0–60° nose-down rotations and the reverse in turn b | Yes (maintain attachment in turn a, as well as in turn b) | maintain attachment during the 0–120° (n = 5) and the 0–135° (n = 7) nose-up rotations and the reverse in turn a, as well as the 0–90° (n = 5) and the 0–105° (n = 7) nose-down rotations and the reverse in turn b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, W.; Dai, Z. The Neural Control Mechanisms of Gekkonid Adhesion Locomotion: The Effect of Spinal Cord Lesions. Biomimetics 2022, 7, 98. https://doi.org/10.3390/biomimetics7030098
Wang X, Wang W, Dai Z. The Neural Control Mechanisms of Gekkonid Adhesion Locomotion: The Effect of Spinal Cord Lesions. Biomimetics. 2022; 7(3):98. https://doi.org/10.3390/biomimetics7030098
Chicago/Turabian StyleWang, Xiaoqing, Wenbo Wang, and Zhendong Dai. 2022. "The Neural Control Mechanisms of Gekkonid Adhesion Locomotion: The Effect of Spinal Cord Lesions" Biomimetics 7, no. 3: 98. https://doi.org/10.3390/biomimetics7030098
APA StyleWang, X., Wang, W., & Dai, Z. (2022). The Neural Control Mechanisms of Gekkonid Adhesion Locomotion: The Effect of Spinal Cord Lesions. Biomimetics, 7(3), 98. https://doi.org/10.3390/biomimetics7030098







