Maleimide-Functionalized Liposomes: Prolonged Retention and Enhanced Efficacy of Doxorubicin in Breast Cancer with Low Systemic Toxicity
Abstract
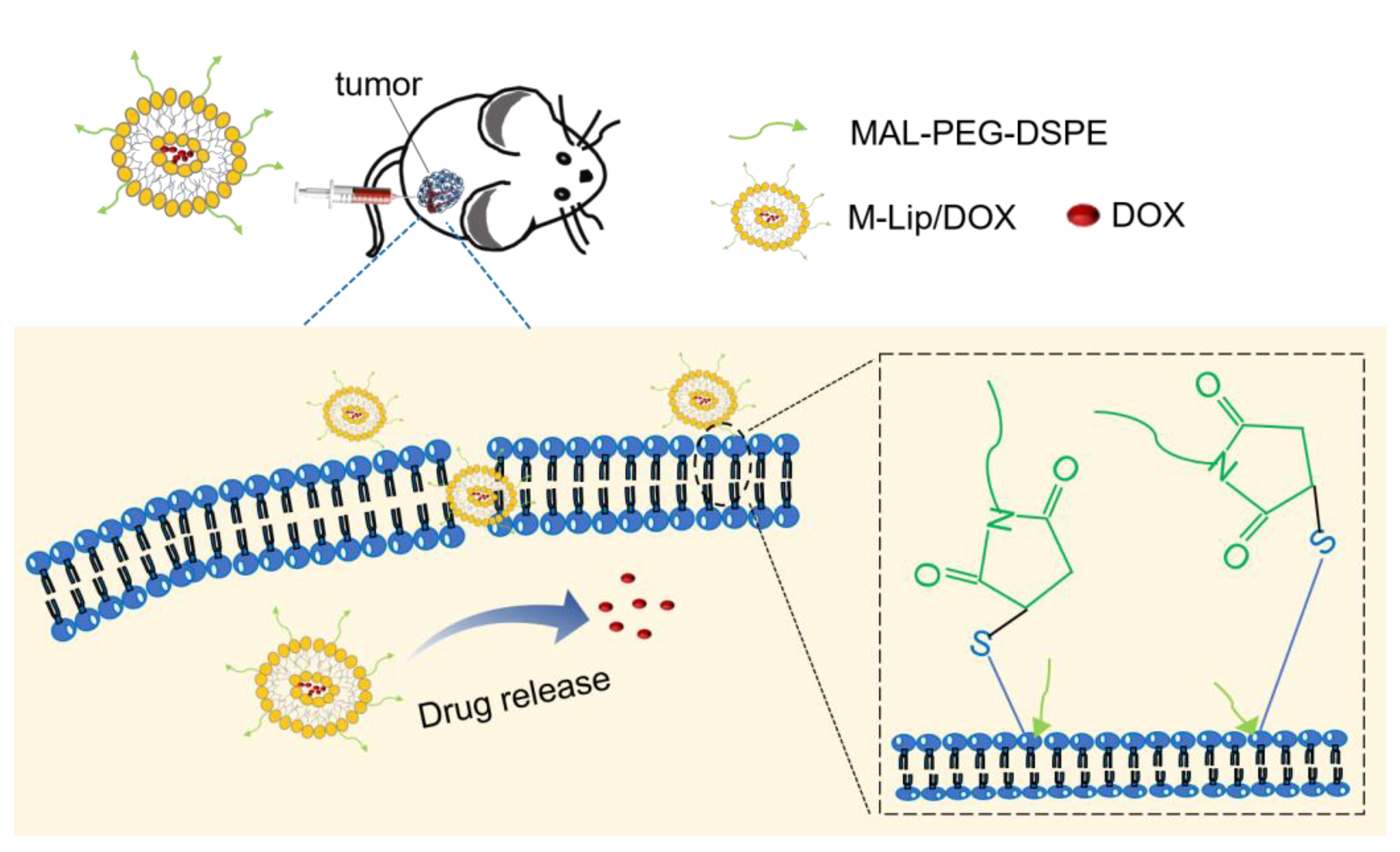
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Animals
2.3. Liposome Preparation
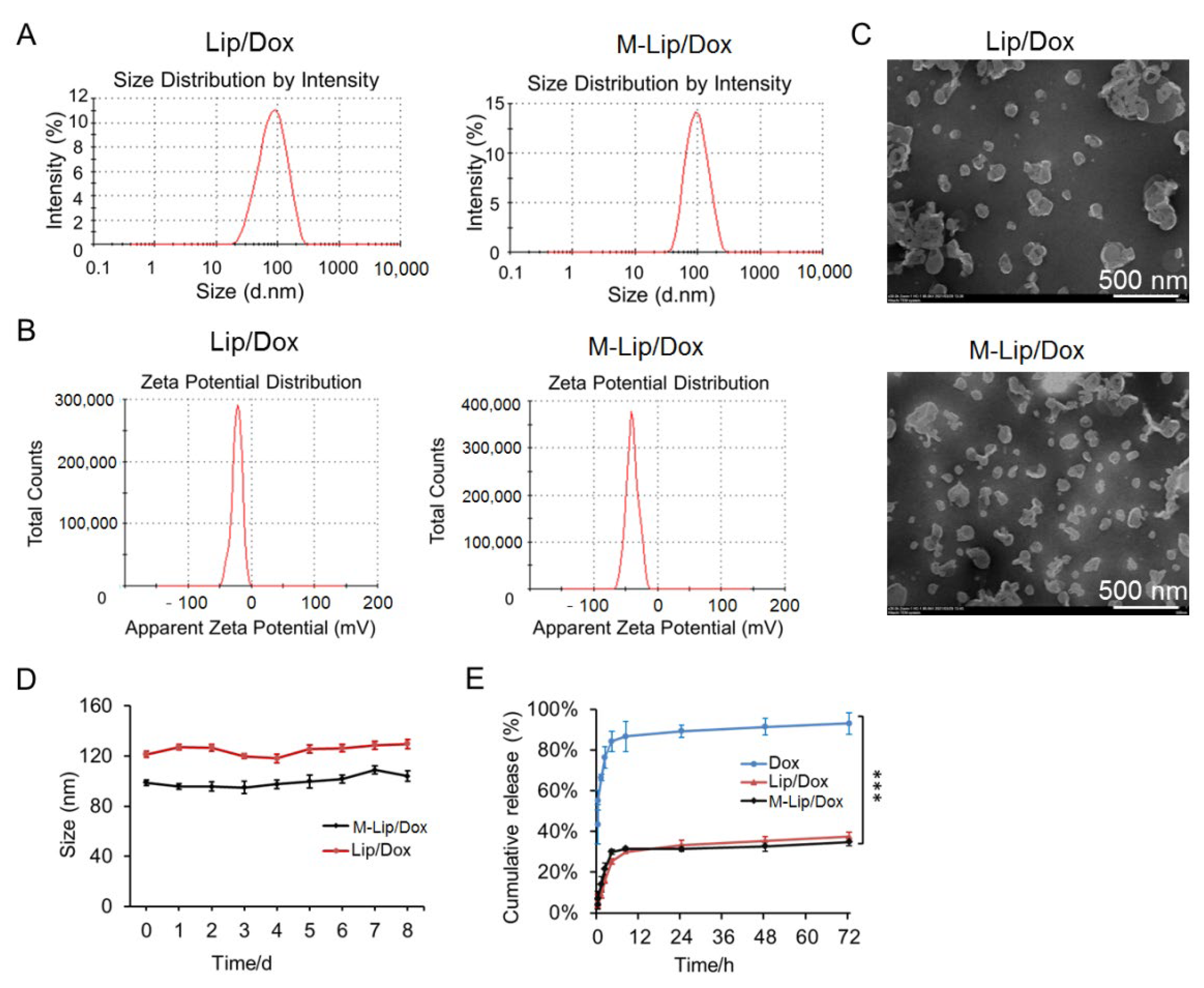
2.4. Liposome Characterization
2.5. Drug Loading and Encapsulation Efficiencies
2.6. In Vitro Drug Release
2.7. Liposome Uptake by Cancer Cells
2.8. Cytotoxicity of Blank Liposomes
2.9. In Vitro Antitumor Efficacy of Dox-Loaded Liposomes
2.10. Tumor-Bearing Mouse Model
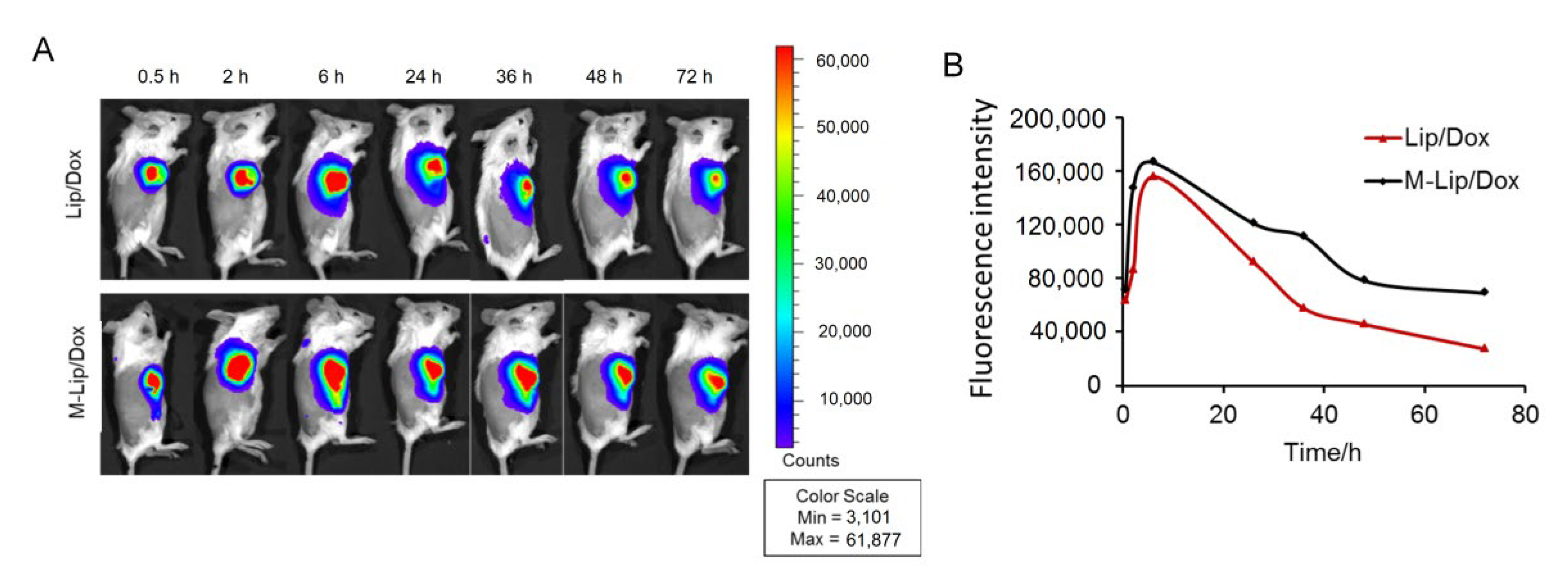
2.11. Near-Infrared Fluorescence Imaging
2.12. Antitumor Efficacy and Safety In Vivo
2.13. Statistical Analysis
3. Results and Discussion
3.1. Liposome Characterization
3.2. Uptake by Cells and Cytotoxicity
3.3. Liposome Retention in the Tumor
3.4. In Vivo Antitumor Efficacy and Safety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darre, T.; Tchaou, M.; Djiwa, T.; Tcharié, E.L.; Napo-Koura, G. Breast cancer: Knowledge, attitudes on risk factors and means of screening by medical students from lomé, togo. Adv. Breast Cancer Res. 2020, 9, 127–137. [Google Scholar] [CrossRef]
- Jiménez-Martínez, Y.; Griñán-Lisón, C.; Khaldy, H.; Martín, A.; Cambrils, A.; Ibáñez Grau, A.; Jiménez, G.; Marchal, J.; Boulaiz, H. LdrB Toxin with In Vitro and In Vivo Antitumor Activity as a Potential Tool for Cancer Gene Therapy. Cancers 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Wu, H.; Yang, R.; Xu, A.; Zhang, Q.; Dong, J.; Qian, C.; Sun, M. GSH depletion liposome adjuvant for augmenting the photothermal immunotherapy of breast cancer. Sci. Adv. 2020, 6, eabc4373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Yue, Q.; Xu, H.; Zhong, X.; Sun, L.; Li, G.; Gong, Y.; Yang, N.; Wang, Z.; et al. Liquid exfoliation of TiN nanodots as novel sonosensitizers for photothermal-enhanced sonodynamic therapy against cancer. Nano Today 2021, 39, 101170. [Google Scholar]
- Zhong, X.; Wang, X.; Li, J.; Hu, J.; Cheng, L.; Yang, X. Ros-based dynamic therapy synergy with modulating tumor cell-microenvironment mediated by inorganic nanomedicine. Coord. Chem. Rev. 2021, 437, 213828. [Google Scholar]
- Zhao, N.; Woodle, M.C.; Mixson, A.-J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef]
- Radu, I.-C.; Zaharia, C.; Hudiță, A.; Tanasă, E.; Ginghină, O.; Marin, M.; Gălățeanu, B.; Costache, M. In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage. Polymers 2021, 13, 2047. [Google Scholar] [CrossRef]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1527. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.-D.; Urbanowicz, R.-A.; Tarr, A.-W.; Ball, J.-K. Hepatitis C Virus Vaccine: Challenges and Prospects. Vaccines 2020, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, L.; Wang, L.; Wang, L.; Zhang, J.; Chen, F.; Zhang, X.; Huang, M.; Li, S.; Ma, W.; et al. HER2-targeted antibody drug conjugates for ovarian cancer therapy. Eur. J. Pharm. Sci. 2016, 93, 274–286. [Google Scholar] [CrossRef]
- Luo, J.-W.; Zhang, T.; Zhang, Q.; Cao, X.; Zeng, X.; Fu, Y.; Zhang, Z.-R.; Gong, T. A novel injectable phospholipid gel co-loaded with doxorubicin and bromotetrandrine for resistant breast cancer treatment by intratumoral injection. Coll. Surf. B Biointerface 2016, 140, 538–547. [Google Scholar] [CrossRef]
- Ramasamy, T.; Haidar, Z.-S.; Tran, T.-H.; Choi, J.-Y.; Jeong, J.-H.; Shin, B.-S.; Choi, H.-G.; Yong, C.-S.; Kim, J.-O. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014, 10, 5116–5127. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, H.-S.; Kang, N.-W.; Lee, S.-Y.; Kim, D.-H.; Kim, S.; Yoon, I.-S.; Cho, H.-J.; Kim, D.-D. Blood component ridable and CD44 receptor targetable nanoparticles based on a maleimide-functionalized chondroitin sulfate derivative. Carbohydr. Polym. 2020, 230, 115568. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.-K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-M.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.-E.; He, E.; Zou, L.; Peng, Q. The Protein Corona and its Effects on Nanoparticle-Based Drug Delivery Systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef]
- Torres, A.-G.; Gait, M.-J. Exploiting cell surface thiols to enhance cellular uptake. Trends Biotechnol. 2012, 30, 185–190. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Schroeder, A.; Bianco-Peled, H. Alginate modified with maleimide-terminated PEG as drug carriers with enhanced mucoadhesion. Carbohydr. Polym. 2017, 175, 337–346. [Google Scholar] [CrossRef]
- Kaldybekov, D.-B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.-V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-M.; Tang, Q.; Wang, Y.; Li, M.; Wang, Y.-N.; Zhu, H.; Geng, F.; Wu, D.; Peng, L.-X.; Zhao, G.; et al. Injectable thermosensitive lipo-hydrogels loaded with ropivacaine for prolonging local anesthesia. Int. J. Pharm. 2022, 611, 121291. [Google Scholar] [CrossRef]
- Minhas, P.-S.; Liu, L.; Moon, P.-K.; Joshi, A.-U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.-A.; Coronado, M.; et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 2019, 20, 50–63. [Google Scholar] [CrossRef]
- Li, H.-M.; Xu, Y.-L.; Tong, Y.-N.; Yin, D.; Zhou, T.-T.; He, J.-M.; Liu, S.; Zhu, Y.-X. Sucrose Acetate Isobutyrate as an In Situ Forming Implant for Sustained Release of Local Anesthetics. Curr. Drug Deliv. 2019, 16, 331–340. [Google Scholar] [CrossRef]
- Paschall, A.-V.; Liu, K. An Orthotopic Mouse Model of Spontaneous Breast Cancer Metastasis. J. Vis. Exp. 2016, 114, 54040. [Google Scholar] [CrossRef]
- Zaleskis, G.; Garberytė, S.; Pavliukevičienė, B.; Valinčius, G.; Characiejus, D.; Mauricas, M.; Kraśko, J.-A.; Žilionytė, K.; Žvirblė, M.; Pašukonienė, V. Doxorubicin uptake in ascitic lymphoma model: Resistance or curability is governed by tumor cell density and prolonged drug retention. J. Cancer 2020, 11, 6497–6506. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, M.; Yuan, M.; Liu, Z.; Yuan, W. Developments in human growth hormone preparations: Sustained-release, prolonged half-life, novel injection devices, and alternative delivery routes. Int. J. Nanomed. 2014, 9, 3527–3538. [Google Scholar]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef]
- Charbe, N.-B.; Amnerkar, N.-D.; Ramesh, B.; Tambuwala, M.-M.; Bakshi, H.-A.; Aljabali, A.; Khadse, S.-C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef]
- Li, T.; Takeoka, S. A novel application of maleimide for advanced drug delivery: In Vitro and In Vivo evaluation of maleimide-modified pH-sensitive liposomes. Int. J. Nanomed. 2013, 8, 3855–3866. [Google Scholar]
- Oussoren, C.; Velinova, M.; Scherphof, G.; van der Want, J.-J.; van Rooijen, N.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. IV. Fate of liposomes in regional lymph nodes. Biochim. Biophys. Acta 1998, 1370, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, U.; Irfan Bukhari, N.; Ovais, M.; Abass, N.; Hussain, K.; Raza, A. Advances in nano-delivery systems for doxorubicin: An updated insight. J. Drug Target. 2018, 26, 296–310. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Yin, D.; Liu, T.; Gou, R.; Fu, J.; Tang, Q.; Wang, Y.; Zou, L.; Li, H. Maleimide-Functionalized Liposomes: Prolonged Retention and Enhanced Efficacy of Doxorubicin in Breast Cancer with Low Systemic Toxicity. Molecules 2022, 27, 4632. https://doi.org/10.3390/molecules27144632
Tang C, Yin D, Liu T, Gou R, Fu J, Tang Q, Wang Y, Zou L, Li H. Maleimide-Functionalized Liposomes: Prolonged Retention and Enhanced Efficacy of Doxorubicin in Breast Cancer with Low Systemic Toxicity. Molecules. 2022; 27(14):4632. https://doi.org/10.3390/molecules27144632
Chicago/Turabian StyleTang, Chuane, Dan Yin, Tianya Liu, Rui Gou, Jiao Fu, Qi Tang, Yao Wang, Liang Zou, and Hanmei Li. 2022. "Maleimide-Functionalized Liposomes: Prolonged Retention and Enhanced Efficacy of Doxorubicin in Breast Cancer with Low Systemic Toxicity" Molecules 27, no. 14: 4632. https://doi.org/10.3390/molecules27144632
APA StyleTang, C., Yin, D., Liu, T., Gou, R., Fu, J., Tang, Q., Wang, Y., Zou, L., & Li, H. (2022). Maleimide-Functionalized Liposomes: Prolonged Retention and Enhanced Efficacy of Doxorubicin in Breast Cancer with Low Systemic Toxicity. Molecules, 27(14), 4632. https://doi.org/10.3390/molecules27144632




