FT-IR Method Limitations for β-Glucan Analysis
Abstract
1. Introduction
2. Results
2.1. Polysaccharide Extraction
2.2. Scanning Electron Microscopy
2.3. Fourier-Transform Infrared Spectroscopy
2.4. Nuclear Magnetic Resonance Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Yeast Growth
4.2. Extraction of Cell Wall Polysaccharides
4.3. Scanning Electron Microscopy
4.4. Fourier-Transform Infrared Spectroscopy
4.5. 13C Solid-State Nuclear Magnetic Resonance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Cui, S.; Liu, H. A New Isolation Method of β-d-Glucans from Spent Yeast Saccharomyces Cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Novak, M.; Vetvicka, V. β-Glucans, History, and the Present: Immunomodulatory Aspects and Mechanisms of Action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.; Choi, J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. β(1-3)-D-Glucan Affects Adipogenesis, Wound Healing and Inflammation. Orient. Pharm. Exp. Med. 2011, 11, 169–175. [Google Scholar] [CrossRef]
- Lee, J.-N.; Lee, D.-Y.; Ji, I.-H.; Kim, G.-E.; Kim, H.N.; Sohn, J.; Kim, S.; Kim, C.-W. Purification of Soluble β-Glucan with Immune-Enhancing Activity from the Cell Wall of Yeast. Biosci. Biotechnol. Biochem. 2001, 65, 837–841. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Waszkiewicz-Robak, B. Spent Brewer’s Yeast and Beta-Glucans Isolated from Them as Diet Components Modifying Blood Lipid Metabolism Disturbed by an Atherogenic Diet. In Lipid Metabolism; Valenzuela Baez, R., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0944-0. [Google Scholar]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Shen, C.-C.; Gao, F.-L.; Wei, H.; Ren, D.-F.; Lu, J. Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera Cordifolia. Molecules 2017, 22, 1276. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic Studies on the Temperature-Dependent Molecular Arrangements in Hybrid Chitosan/1,3-β-D-Glucan Polymeric Matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Lowman, D.W.; West, L.J.; Bearden, D.W.; Wempe, M.F.; Power, T.D.; Ensley, H.E.; Haynes, K.; Williams, D.L.; Kruppa, M.D. New Insights into the Structure of (1→3,1→6)-β-D-Glucan Side Chains in the Candida Glabrata Cell Wall. PLoS ONE 2011, 6, e27614. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.J.; Masson, A.J.; Patterson, J.C. The Structure of a P-(1-*3)-D-Glucan from Yeast Cell Walls. Biochem. J. 1973, 135, 12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules 2019, 25, 57. [Google Scholar] [CrossRef]
- Byrtusová, D.; Shapaval, V.; Holub, J.; Šimanský, S.; Rapta, M.; Szotkowski, M.; Kohler, A.; Márová, I. Revealing the Potential of Lipid and β-Glucans Coproduction in Basidiomycetes Yeast. Microorganisms 2020, 8, 1034. [Google Scholar] [CrossRef]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latgé, J.-P. Cell Wall β-(1,6)-Glucan of Saccharomyces Cerevisiae: Structural characterization and in situ synthesis. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the Efficiency of Different Disruption Methods on Yeast Cell Wall Preparation for β-Glucan Isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef]
- Kath, F.; Kulicke, W.-M. Mild Enzymatic Isolation of Mannan and Glucan from YeastSaccharomyces Cerevisiae. Angew. Makromol. Chem. 1999, 268, 59–68. [Google Scholar] [CrossRef]
- Soares, E.V.; Soares, H.M.V.M. Bioremediation of Industrial Effluents Containing Heavy Metals Using Brewing Cells of Saccharomyces Cerevisiae as a Green Technology: A Review. Environ. Sci. Pollut. Res. 2012, 19, 1066–1083. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Application of Different Methods for the Extraction of Yeast β-Glucan. E-J. Sci. Technol. 2016, 15. [Google Scholar]
- Xing, Y.; Chen, C.; Sun, W.; Zhang, B.; Sang, Y.; Xiu, Z.; Dong, Y. An Environment-Friendly Approach to Isolate and Purify Glucan from Spent Cells of Recombinant Pichia Pastoris and the Bioactivity Characterization of the Purified Glucan. Eng. Life Sci. 2018, 18, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Zechner-Krpan, V.; Petravi, V.; Gospodari, I.; Sajli, L. Characterization of B-Glucans Isolated from Brewer’s Yeast and Dried by Different Methods. Food Technol. Biotechnol. 2010, 48, 189–197. [Google Scholar]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhang, J.; Yan, M.; Li, D.; Zhou, S.; Feng, J.; Liu, Y. Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis Latifolia. Polymers 2022, 14, 549. [Google Scholar] [CrossRef]
- Manabe, N.; Yamaguchi, Y. 3D Structural Insights into β-Glucans and Their Binding Proteins. Int. J. Mol. Sci. 2021, 22, 1578. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Plata, M.R.; Koch, C.; Wechselberger, P.; Herwig, C.; Lendl, B. Determination of Carbohydrates Present in Saccharomyces Cerevisiae Using Mid-Infrared Spectroscopy and Partial Least Squares Regression. Anal. Bioanal. Chem. 2013, 405, 8241–8250. [Google Scholar] [CrossRef]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from Spent Brewer’s Yeast: Preparation, Analysis and Use as a Potential Immunostimulant in Shrimp Feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Surface Chemical and Morphological Analysis of Chitosan/1,3-β-d-Glucan Polysaccharide Films Cross-Linked at 90 °C. Int. J. Mol. Sci. 2022, 23, 5953. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novak, M. Structural Analysis of Glucans. Ann. Transl. Med. 2014, 2, 14. [Google Scholar] [CrossRef]
- Fusté, N.P.; Guasch, M.; Guillen, P.; Anerillas, C.; Cemeli, T.; Pedraza, N.; Ferrezuelo, F.; Encinas, M.; Moralejo, M.; Garí, E. Barley β-Glucan Accelerates Wound Healing by Favoring Migration versus Proliferation of Human Dermal Fibroblasts. Carbohydr. Polym. 2019, 210, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Climova, A.; Ibrahim, M.N.G.; Salamahina, A.; Savin, A.M.; Dukhinova, M.S.; Barakova, N.V.; Krivoshapkina, E.F. Application of Extracted β-Glucan from Oat for β-Carotene Encapsulation. J. Food Sci. Technol. 2021, 58, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR Spectroscopy for Analysis of the Quality of Honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef]
- Klähn, M.; Schlitter, J.; Gerwert, K. Theoretical IR Spectroscopy Based on QM/MM Calculations Provides Changes in Charge Distribution, Bond Lengths, and Bond Angles of the GTP Ligand Induced by the Ras-Protein. Biophys. J. 2005, 88, 3829–3844. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-Glucans, Their Preparation, Physico-Chemical Characterization and Immunomodulatory Activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- da Nascimento Santos, M.S.; de Magalhães, J.M.; Castro, L.; de Sousa Pinheiro, T.; Sabry, D.; Nobre, L.; Lima, J.; Baseia, I.; Leite, E. Effect of Glucans from Caripia Montagnei Mushroom on TNBS-Induced Colitis. Int. J. Mol. Sci. 2014, 15, 2368–2385. [Google Scholar] [CrossRef]
- Piotrowska, M.; Masek, A. Saccharomyces Cerevisiae Cell Wall Components as Tools for Ochratoxin A Decontamination. Toxins 2015, 7, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR Spectroscopic Analysis of Saccharomyces Cerevisiae Cell Walls: Study of an Anomalous Strain Exhibiting a Pink-Colored Cell Phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.; Tkalčec, Z.; Mešić, A.; Kohler, A. Characterizing Aeroallergens by Infrared Spectroscopy of Fungal Spores and Pollen. PLoS ONE 2015, 10, e0124240. [Google Scholar] [CrossRef] [PubMed]
- Ami, D.; Mereghetti, P.; Maria, S. Multivariate Analysis for Fourier Transform Infrared Spectra of Complex Biological Systems and Processes. In Multivariate Analysis in Management, Engineering and the Sciences; Freitas, L., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0921-1. [Google Scholar]
- Lozano, M.; Rodríguez-Ulibarri, P.; Echeverría, J.C.; Beruete, M.; Sorolla, M.; Beriain, M.J. Mid-Infrared Spectroscopy (MIR) for Simultaneous Determination of Fat and Protein Content in Meat of Several Animal Species. Food Anal. Methods 2017, 10, 3462–3470. [Google Scholar] [CrossRef]
- Stagner, W.C.; Gaddam, S.; Parmar, R.; Ghanta, A.K. Chapter Five—Sucrose Octaacetate. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2019; Volume 44, pp. 267–291. ISBN 978-0-12-817165-3. [Google Scholar]
- Jantaramanant, P.; Sermwittayawong, D.; Noipha, K.; Wititsuwannakul, R. β-Glucan-Containing Polysaccharide Extract from the Grey Oyster Mushroom [Pleurotus Sajor-Caju (Fr.) Sing.] Stimulates Glucose Uptake by the L6 Myotubes. Int. Food Res. J. 2014, 21, 6. [Google Scholar]
- Shi, F.; Shi, J.; Li, Y. Mechanochemical Phosphorylation and Solubilisation of B-D-Glucan from Yeast Saccharomyces Cerevisiae and Its Biological Activities. PLoS ONE 2014, 9, e103494. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Q.; Wei, X.; Feng, W.; Chen, Y.; Huang, P.; Wang, Z. Application of Fourier Transform Infrared Spectroscopy with Chemometrics on Postmortem Interval Estimation Based on Pericardial Fluids. Sci. Rep. 2017, 7, 18013. [Google Scholar] [CrossRef]
- Poerio, A.; Girardet, T.; Petit, C.; Fleutot, S.; Jehl, J.-P.; Arab-Tehrany, E.; Mano, J.F.; Cleymand, F. Comparison of the Physicochemical Properties of Chitin Extracted from Cicada Orni Sloughs Harvested in Three Different Years and Characterization of the Resulting Chitosan. Appl. Sci. 2021, 11, 11278. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Sivamaruthi, B.S.; Sirilun, S.; Peerajan, S.; Kesika, P.; Chaiyasut, K.; Chaiyasut, C. Extraction of β-Glucan from Saccharomyces Cerevisiae: Comparison of Different Extraction Methods and in Vivo Assessment of Immunomodulatory Effect in Mice. Food Sci. Technol. 2016, 37, 124–130. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; MacNaughtan, W.; Kakouri, A.; Nesseris, K.; Israilides, C. Quality and Sensory Properties of Reduced Fat Yoghurt Made with Addition of β-Glucans. Food Nutr. Sci. 2018, 09, 390–402. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, X.; Guo, H.; Meng, Y.; Wang, Y.; Zhou, Y.; Sun, L. Structural Analysis and Macrophage Activation of a Novel Β-glucan Isolated from Cantharellus Cibarius. FNS 2021, 47, 50. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Kirui, A.; Muszyński, A.; Widanage, M.C.D.; Chen, A.; Azadi, P.; Wang, P.; Mentink-Vigier, F.; Wang, T. Molecular Architecture of Fungal Cell Walls Revealed by Solid-State NMR. Nat. Commun. 2018, 9, 2747. [Google Scholar] [CrossRef] [PubMed]
- Ehren, H.L.; Appels, F.V.W.; Houben, K.; Renault, M.A.M.; Wösten, H.A.B.; Baldus, M. Characterization of the Cell Wall of a Mushroom Forming Fungus at Atomic Resolution Using Solid-State NMR Spectroscopy. Cell Surf. 2020, 6, 100046. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Fernando, L.D.; Fang, W.; Dickwella Widanage, M.C.; Wei, P.; Jin, C.; Fontaine, T.; Latgé, J.-P.; Wang, T. A Molecular Vision of Fungal Cell Wall Organization by Functional Genomics and Solid-State NMR. Nat. Commun. 2021, 12, 6346. [Google Scholar] [CrossRef]
- Fričová, O.; Koval’aková, M. Solid-State 13 C CP MAS NMR Spectroscopy as a Tool for Detection of (1 → 3, 1 → 6)-β -D-Glucan in Products Prepared from Pleurotus Ostreatus. ISRN Anal. Chem. 2013, 2013, 248164. [Google Scholar] [CrossRef][Green Version]
- Kremmyda, A.; MacNaughtan, W.; Arapoglou, D.; Eliopoulos, C.; Metafa, M.; Harding, S.E.; Israilides, C. The Detection, Purity and Structural Properties of Partially Soluble Mushroom and Cereal β-D-Glucans: A Solid-State NMR Study. Carbohydr. Polym. 2021, 266, 118103. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Koczoń, P.; Błażejak, S.; Kozera, J.; Kieliszek, M. Valorization of Deproteinated Potato Juice Water into β-Glucan Preparation of C. Utilis Origin: Comparative Study of Preparations Obtained by Two Isolation Methods. Waste Biomass Valor. 2020, 11, 3257–3271. [Google Scholar] [CrossRef]
- Nybacka, L. FTIR Spectroscopy of Glucose. Master’s Thesis, Uppsala Universitet, Uppsala, Sweden, 2016. [Google Scholar]
- Gregory, G.L.; López-Vidal, E.M.; Buchard, A. Polymers from Sugars: Cyclic Monomer Synthesis, Ring-Opening Polymerisation, Material Properties and Applications. Chem. Commun. 2017, 53, 2198–2217. [Google Scholar] [CrossRef]

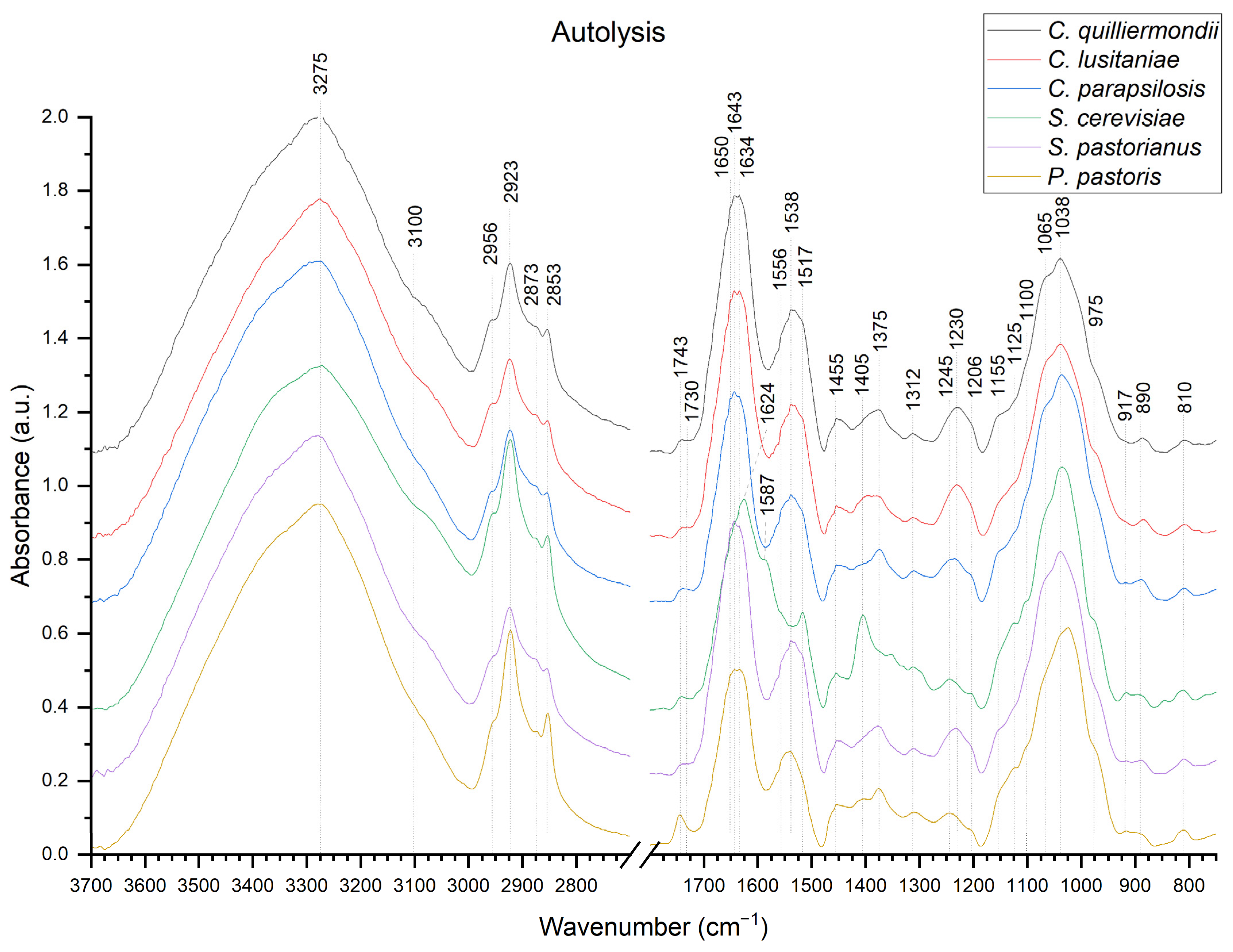

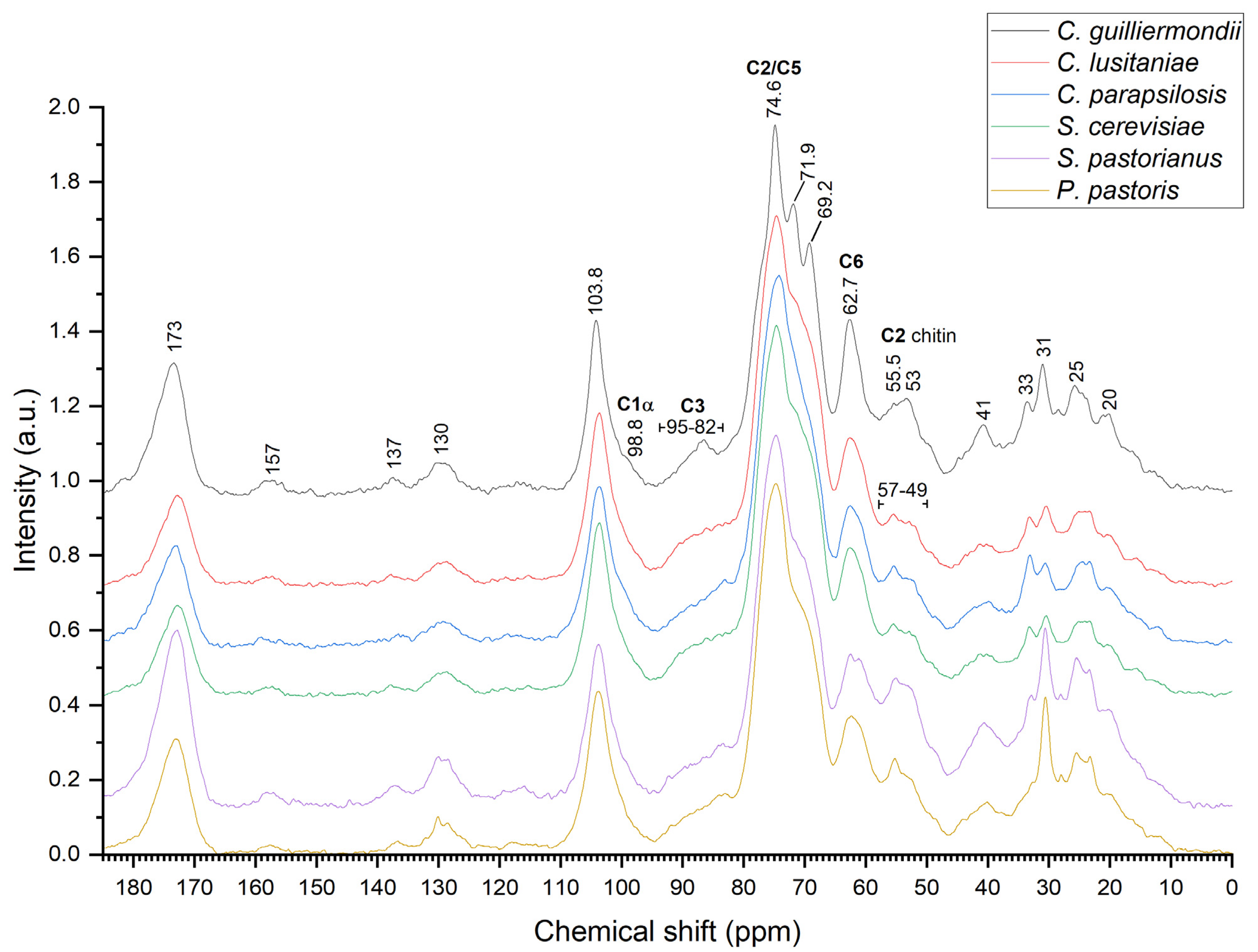
| Yeast | Autolysis | Autoclaving | Ultrasonication | Organic Solvent | β-Glucan Conc. Relative to Cell Wall Mass |
|---|---|---|---|---|---|
| C. guilliermondii | 13.34 ± 2.06 | 8.30 ± 1.96 | 6.70 ± 0.94 | 5.26 ± 0.52 | 40.22 ± 2.42 |
| C. lusitaniae | 14.91 ± 2.09 | 9.13 ± 0.84 | 7.20 ± 0.82 | 5.54 ± 0.73 | 37.26 ± 1.99 |
| C. parapsilosis | 11.39 ± 0.98 | 7.68 ± 0.99 | 6.64 ± 0.66 | 5.69 ± 0.61 | 49.92 ± 1.41 |
| P. pastoris | 13.05 ± 0.97 | 7.90 ± 0.55 | 6.07 ± 0.21 | 4.99 ± 0.45 | 38.49 ± 1.70 |
| S. cerevisiae | 12.81 ± 0.76 | 9.71 ± 0.60 | 8.32 ± 0.70 | 6.87 ± 0.65 | 53.55 ± 2.52 |
| S. pastorianus | 15.41 ± 3.32 | 11.71 ± 2.43 | 10.23 ± 1.90 | 8.83 ± 1.63 | 57.78 ± 3.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikmurzin, R.; Bandzevičiūtė, R.; Maršalka, A.; Maneikis, A.; Kalėdienė, L. FT-IR Method Limitations for β-Glucan Analysis. Molecules 2022, 27, 4616. https://doi.org/10.3390/molecules27144616
Bikmurzin R, Bandzevičiūtė R, Maršalka A, Maneikis A, Kalėdienė L. FT-IR Method Limitations for β-Glucan Analysis. Molecules. 2022; 27(14):4616. https://doi.org/10.3390/molecules27144616
Chicago/Turabian StyleBikmurzin, Ruslan, Rimantė Bandzevičiūtė, Arūnas Maršalka, Andrius Maneikis, and Lilija Kalėdienė. 2022. "FT-IR Method Limitations for β-Glucan Analysis" Molecules 27, no. 14: 4616. https://doi.org/10.3390/molecules27144616
APA StyleBikmurzin, R., Bandzevičiūtė, R., Maršalka, A., Maneikis, A., & Kalėdienė, L. (2022). FT-IR Method Limitations for β-Glucan Analysis. Molecules, 27(14), 4616. https://doi.org/10.3390/molecules27144616






