The Imbibition of Pea (Pisum sativum L.) Seeds in Silver Nitrate Reduces Seed Germination, Seedlings Development and Their Metabolic Profile
Abstract
:1. Introduction
2. Results
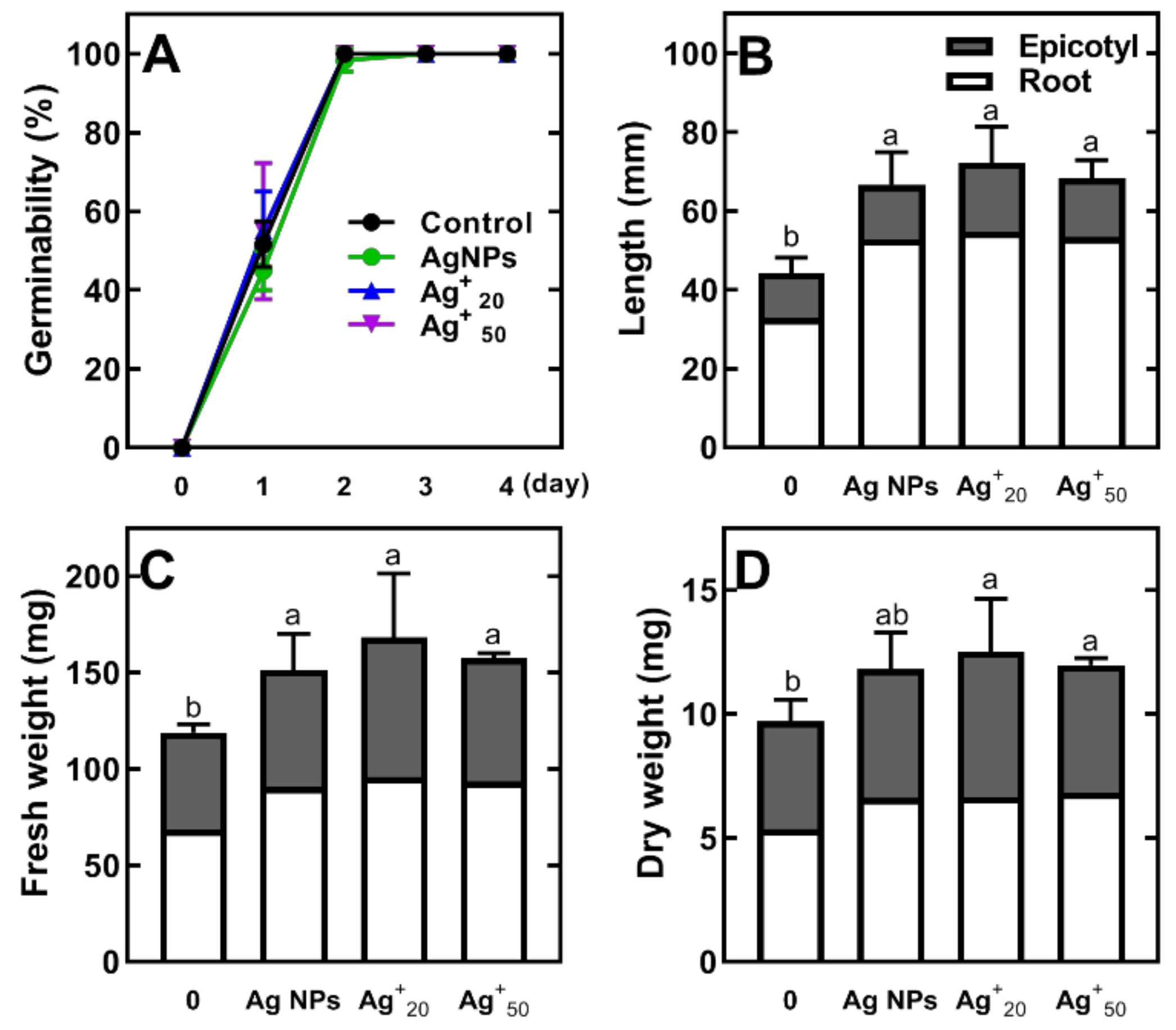
2.1. The Effect of Ag NPs and AgNO3 on Germination, Seedling Development and Metabolic Profile of Pea
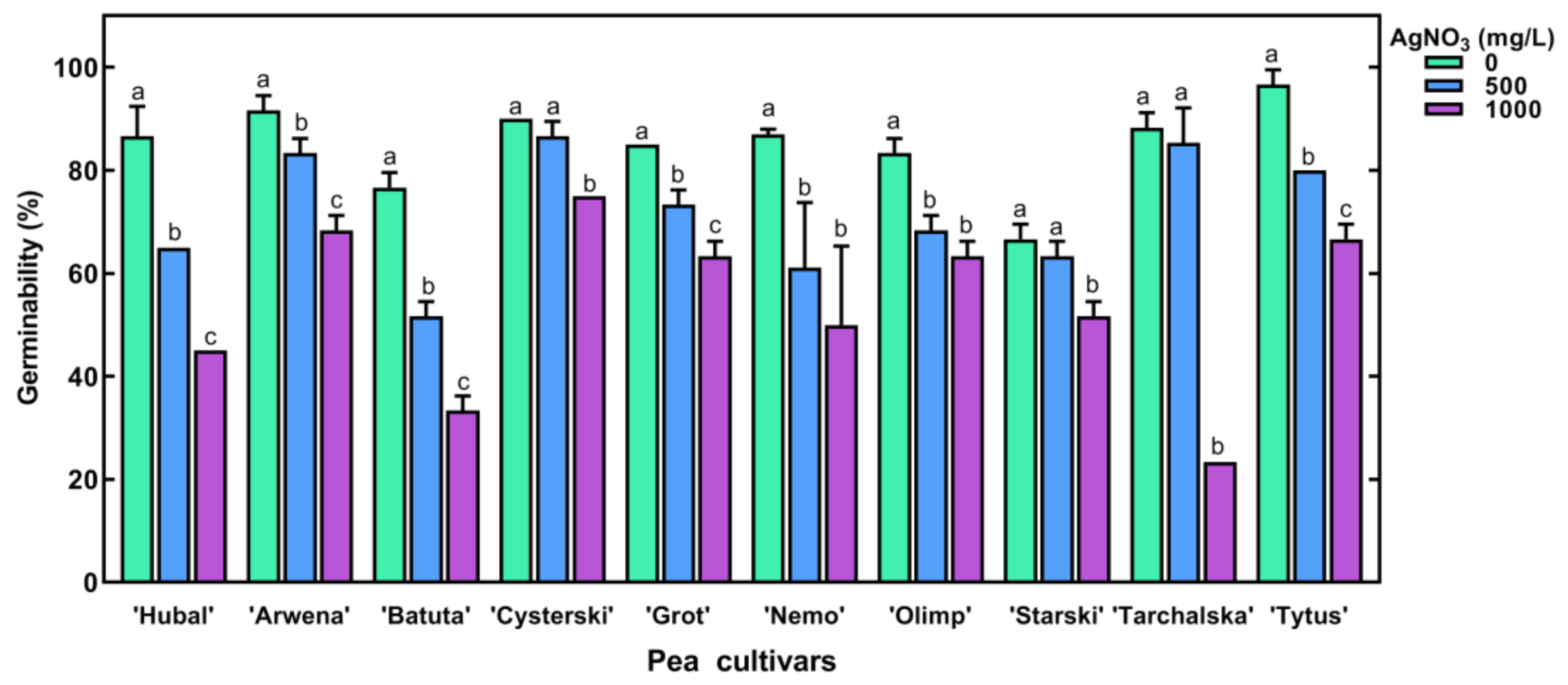
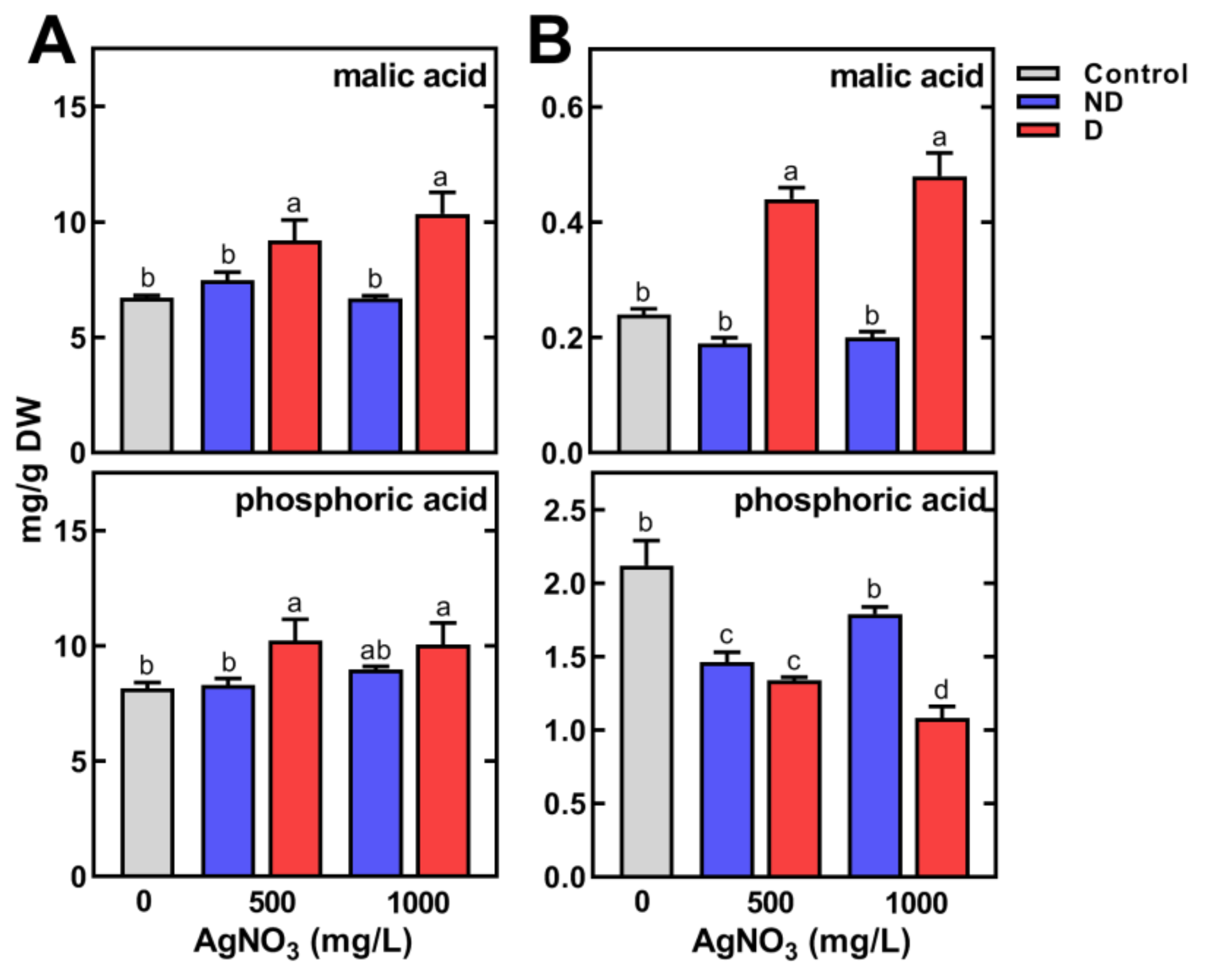
2.2. The Effect of Short-Term Seed Imbibition in AgNO3 Solutions on Germination and Seedling Development of Pea
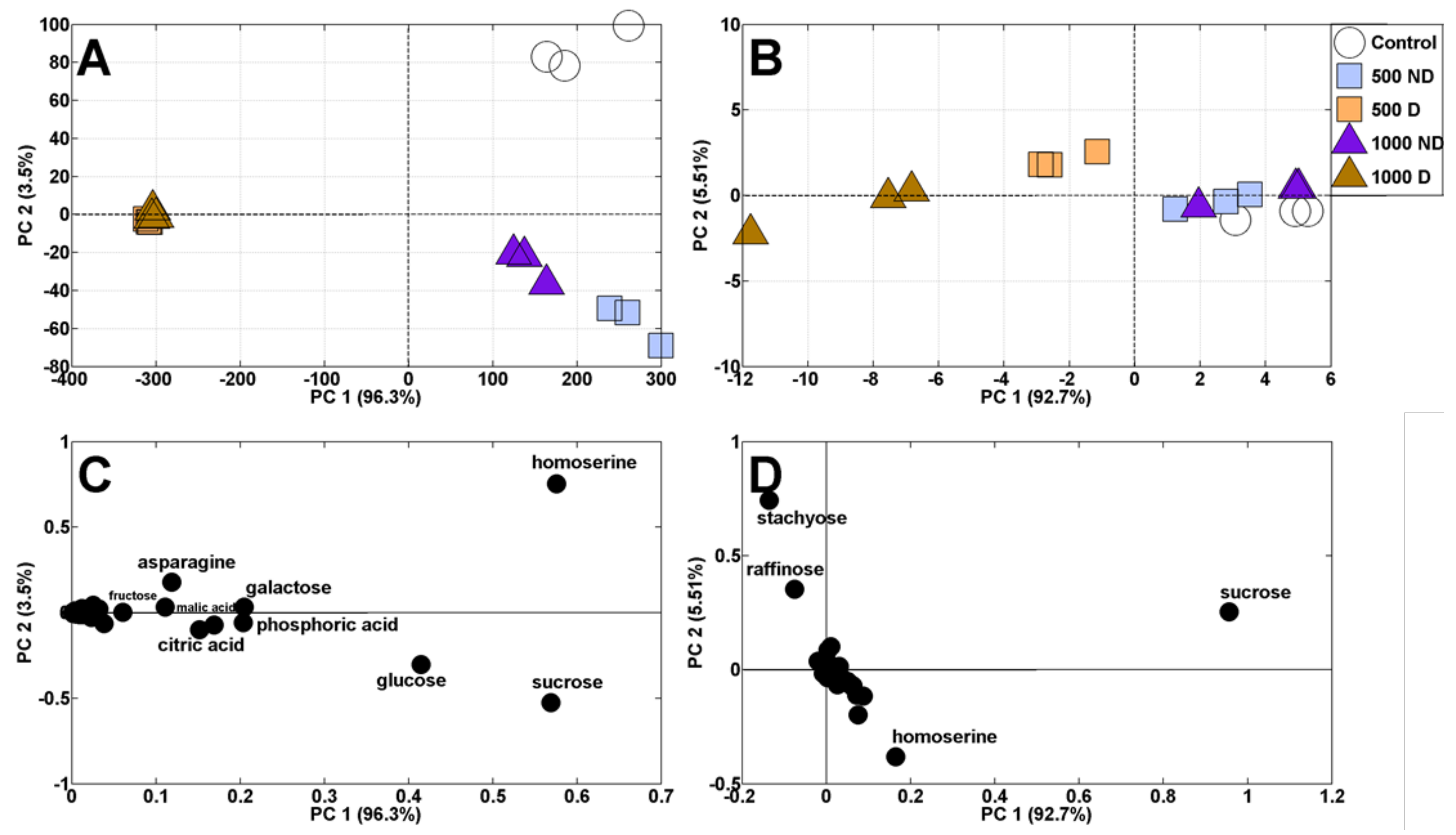
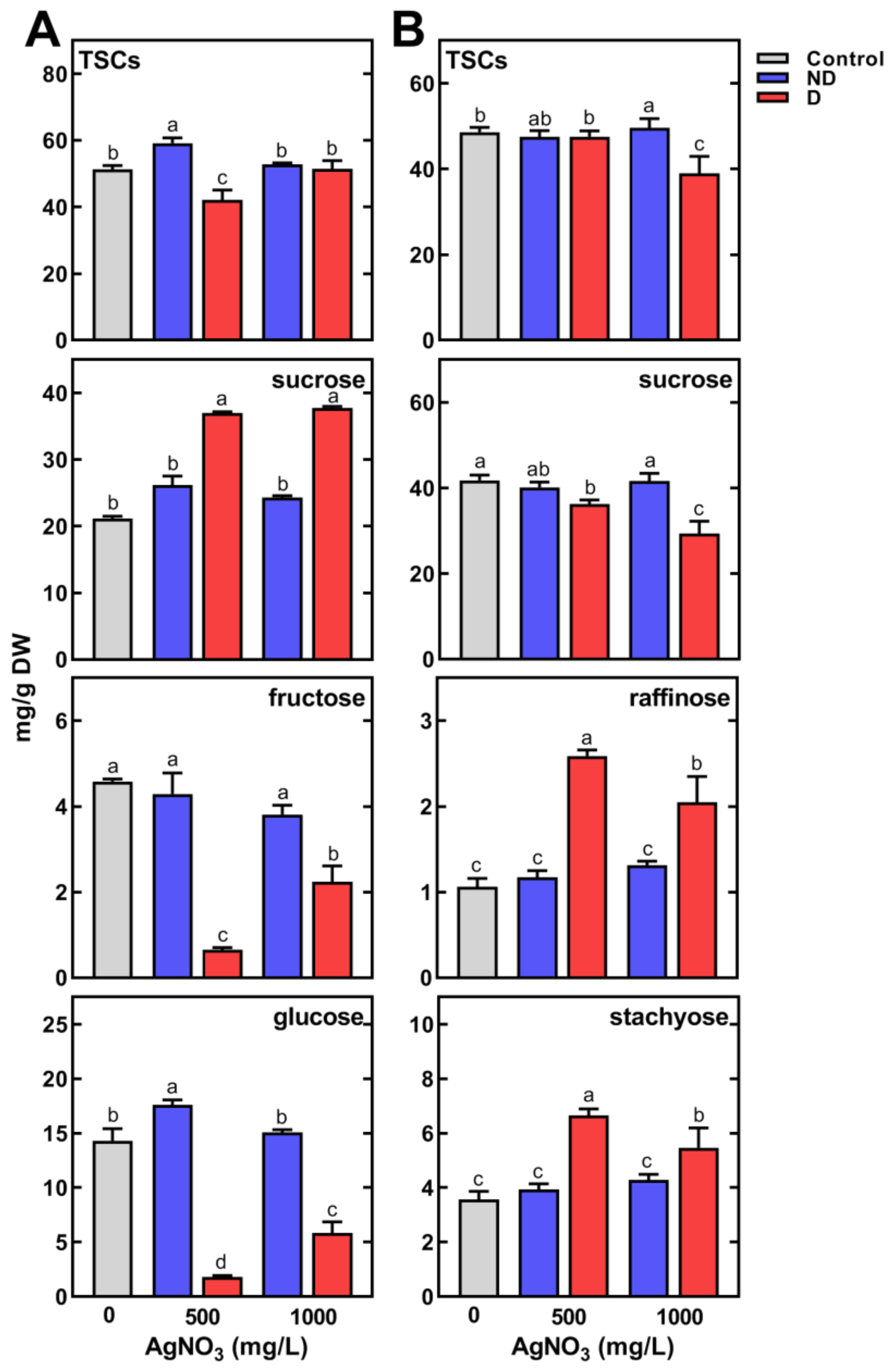
2.3. Changes in Metabolic Profiles of Pea Seedlings
2.3.1. Principal Component Analysis
2.3.2. Changes in the Concentration of Polar Metabolites
3. Discussion
3.1. Pea Response to Ag NPs and Ag+ Ions
3.2. Pea Response to AgNO3
3.2.1. Profile of Polar Metabolites in Control Pea Seedlings
3.2.2. Effect of AgNO3 on Metabolic Profiles of Pea Seedlings
4. Materials and Methods
4.1. The Effect of Ag NPs and AgNO3 on Seed Germination and Seedling Development of Pea
4.2. The Effect of Short-Term Seeds’ Imbibition with AgNO3 on Germination and Seedling Development of Pea
4.3. Polar Metabolite Profiling
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [Green Version]
- Hicks, A.L.; Temizel-Sekeryan, S. Understanding the potential environmental benefits of nanosilver enabled consumer products. NanoImpact 2019, 16, 100183. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food. Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Kraas, M.; Schlich, K.; Knopf, B.; Wege, F.; Kägi, R.; Terytze, K.; Hund-Rinke, K. Long-term effects of sulfidized silver nanoparticles in sewage sludge on soil microflora. Environ. Toxicol. Chem. 2017, 36, 3305–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühr, S.; Schneider, S.; Meisterjahn, B.; Schlich, K.; Hund-Rinke, K.; Schlechtriem, C. Silver nanoparticles in sewage treatment plant effluents: Chronic effects and accumulation of silver in the freshwater amphipod Hyalella azteca. Environ. Sci. Eur. 2018, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira, J., Jr.; Alves, T. Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health. 2012, 9, 1649–1662. [Google Scholar] [CrossRef] [Green Version]
- Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Li, X.; Zhang, M.; Zhang, Z.; Lu, T.; Pan, X.; Qian, H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci. Total Environ. 2018, 644, 1070–1079. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of silver nanoparticles with different surface properties on monocots and dicots model plants. J. Soil. Sci. Plant Nutr. 2022, 22, 1–18. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Głowacka, K.; Stałanowska, K.; Railean-Plugaru, V.; Horbowicz, M.; Pomastowski, P.; Buszewski, B. The effect of bio-synthesized silver nanoparticles on germination, early seedling development, and metabolome of wheat (Triticum aestivum L.). Molecules 2022, 27, 2303. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef]
- Colman, B.P.; Wang, S.Y.; Auffan, M.; Wiesner, M.R.; Bernhardt, E.S. Antimicrobial effects of commercial silver nanoparticles are attenuated in natural streamwater and sediment. Ecotoxicology 2012, 21, 1867–1877. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Brooks, M.; Gerfen, J.R.; Wang, Q.; Fotis, C.; Sparer, A.; Ma, X.; Berg, R.H.; Geisler, M. Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials 2014, 4, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Li, H.; Li, Y.; Cui, W.; Wang, M.; Li, Z.; Chai, R.; Wang, H. Toxicity of Ag+ on microstructure, biochemical activities and genic material of Trifolium pratense L. seedlings with special reference to phytoremediation. Ecotoxicol. Environ. Saf. 2020, 195, 110499. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1955. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S.; El-Mehasseb, I.M. Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanz. 2020, 72, 113–127. [Google Scholar] [CrossRef]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Mehmood, A.; Murtaza, G. Application of SNPs to improve yield of Pisum sativum L. (pea). IET Nanobiotechnol. 2016, 11, 390–394. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3: A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Mookkan, M.; Andy, G. AgNO3 boosted high-frequency shoot regeneration in Vigna mungo (L.) Hepper. Plant Signal. Behav. 2014, 9, e972284. [Google Scholar] [CrossRef] [Green Version]
- Geetha, G.; Harathi, K.; Naidu, C.V. Role of silver nitrate on in vitro flowering and shoot regeneration of Solanum nigrum (L.)—An important multipurpose medicinal plant. Am. J. Plant Sci. 2016, 7, 1021. [Google Scholar] [CrossRef] [Green Version]
- Harathi, K.; Naidu, C.V. Influence of ethylene inhibitor silver nitrate on direct shoot regeneration from in vitro raised shoot tip explants of Sphaeranthus indicus Linn.—An important antijaundice medicinal plant. Am. J. Plant Sci. 2016, 7, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.M.; Wei, Y.M.; Zheng, Y.L. Effects of silver nitrate on the tissue culture of immature wheat embryos. Russ. J. Plant Physiol. 2006, 53, 530–534. [Google Scholar] [CrossRef]
- Tahoori, F.; Majd, A.; Nejadsattari, T.; Ofoghi, H.; Iranbakhsh, A. Effects of silver nitrate (AgNO3) on growth and anatomical structure of vegetative organs of liquorice (‘Glycyrrhiza glabra’ L.) under in “vitro” condition. Plant Omics. 2018, 11, 153–160. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; Kopittke, P.M.; Wehr, J.B.; Kinraide, T.B.; Menzies, N.W. Rhizotoxic effects of silver in cowpea seedlings. Environ. Toxicol. Chem. 2010, 29, 2072–2078. [Google Scholar] [CrossRef]
- Kotlarz, A.; Sujak, A.; Strobel, W.; Grzesiak, W. Chemical composition and nutritive value of protein of the pea seeds—Effect of harvesting year and variety. Veg. Crop. Res. Bull. 2011, 75, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Kulaeva, O.A.; Zhernakov, A.I.; Afonin, A.M.; Boikov, S.S.; Sulima, A.S.; Tikhonovich, I.A.; Zhukov, V.A. Pea Marker Database (PMD)—A new online database combining known pea (Pisum sativum L.) gene-based markers. PLoS ONE 2017, 12, e0186713. [Google Scholar] [CrossRef] [Green Version]
- Barabanov, P.V.; Gerasimov, A.V.; Blinov, A.V.; Kravtsov, A.A.; Kravtsov, V.A. Influence of nanosilver on the efficiency of Pisum sativum crops germination. Ecotoxicol. Environ. Saf. 2018, 147, 715–719. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S. Ultrastructural and molecular implications of ecofriendly made silver nanoparticles treatments in pea (Pisum sativum L.). J. Genet. Eng. Biotechnol. 2022, 20, 5. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.P.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef] [Green Version]
- Budhani, S.; Egboluche, N.P.; Arslan, Z.; Yu, H.; Deng, H. Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2019, 37, 330–355. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Khalaf, D.M. Assessment of silver nanoparticles contamination on faba bean—Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agric. Ecosyst. Environ. 2016, 218, 163–177. [Google Scholar]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment, an adaptive mechanism for coping with plant water deficits. Plant Cell Environ. 2017, 40, 1–3. [Google Scholar] [CrossRef]
- Larson, L.A.; Beevers, H. Amino acid metabolism in young pea seedlings. Plant Physiol. 1965, 40, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef]
- Melcher, I.M. Homoserine synthesis in dark-grown and light-grown seedlings of Pisum sativum. New Phytol. 1985, 100, 157–162. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Gawłowska, M.; Lahuta, L.B.; Święcicki, W.; Krajewski, P. Variability in the oligosaccharide concentration in seeds of the mapping population of pea (Pisum sativum L.). Czech J. Genet. Plant Breed. 2014, 50, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, A.; Peterbauer, T.; Hofmann, J.; Richter, A. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 2008, 228, 99–110. [Google Scholar] [CrossRef]
- Górecki, R.J.; Obendorf, R.L. Galactosyl cyclitols and raffinose family oligosaccharides in relation to desiccation tolerance of pea and soybean seedlings. In Basic and Applied Aspects of Seed Biology; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 119–128. [Google Scholar]
- Gould, N.; Thorpe, M.R.; Koroleva, O.; Minchin, P.E.H. Phloem hydrostatic pressure relates to solute loading rate: A direct test of the Münch hypothesis. Funct. Plant Biol. 2005, 32, 1019–1026. [Google Scholar] [CrossRef]
- Smeekens, S.; Hellmann, H.A. Sugar sensing and signaling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.H.; Loewus, F. Synthesis of methyl D-galacturonate from D-galactose or D-galacturonic acid: Preparation of methyl-14C-galacturonate using methyl-14C-iodide. Carbohydr. Res. 1967, 4, 401–407. [Google Scholar] [CrossRef]
- Scheller, H.V.; Jensen, J.K.; Sorensen, S.O.; Harholt, J.; Geshi, N. Biosynthesis of pectin. Physiol. Plant. 2007, 129, 283–295. [Google Scholar] [CrossRef]
- Melcher, I.M. Changes in nitrogen-containing compounds of the garden pea (Pisum sativum L.) during germination. Z. Pflanzenphysiol. 1983, 112, 95–102. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Çekiç, F.Ö.; Ekinci, S.; İnal, M.S.; Ünal, D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk. J. Biol. 2017, 41, 700–707. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: A review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Pluskota, W.E.; Stelmaszewska, J.; Szablińska, J. Dehydration induces expression of GALACTINOL SYNTHASE and RAFFINOSE SYNTHASE in seedlings of pea (Pisum sativum L.). J. Plant Physiol. 2014, 171, 1306–1314. [Google Scholar] [CrossRef]
- Pluskota, W.E.; Szablińska, J.; Obendorf, R.L.; Górecki, R.J.; Lahuta, L.B. Osmotic stress induces genes, enzymes and accumulation of galactinol, raffinose and stachyose in seedlings of pea (Pisum sativum L.). Acta Physiol. Plant. 2014, 37, 156. [Google Scholar] [CrossRef] [Green Version]
- Aliyari Rad, S.; Dehghanian, Z.; Asgari Lajayer, B.; Nobaharan, K.; Astatkie, T. Mitochondrial respiration and energy production under some abiotic stresses. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Obendorf, R.L.; Górecki, R.J. Soluble carbohydrates in legume seeds. Seed Sci. Res. 2012, 22, 219–242. [Google Scholar] [CrossRef]
- Charlton, A.J.; Donarski, J.A.; Harrison, M.; Jones, S.A.; Godward, J.; Oehlschlager, S.; Arques, J.L.; Ambrose, M.; Chinoy, C.; Mullineaux, P.M.; et al. Responses of the pea (Pisum sativum L.) leaf metabolome to drought stress assessed by nuclear magnetic resonance spectroscopy. Metabolomics 2008, 4, 312–327. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate valves: Old shuttles with new perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimian, E.; Bybordi, A. Effect of organic acids on heavy-metal uptake and growth of canola grown in contaminated soil. Commun. Soil Sci. Plant Anal. 2014, 45, 1715–1725. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Dung, V.V.; Kuchaeva, L. The role of organic acids in heavy metal tolerance in plants. Biol. Commun. 2018, 63, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Jócsák, I.; Végvári, G.; Droppa, M. Heavy metal detoxification by organic acids in barley seedlings. Acta Biol. Szeged. 2005, 49, 99–101. [Google Scholar]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Giordano, C.; Musetti, R. In vivo synthesis of nanomaterials in plants: Location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014, 9, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lott, J.N.A.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic acid and phosphorus in crop seeds and fruits: A global estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar] [CrossRef]
- Frank, A.W. Phytic Acid. In Chemistry of Plant Phosphorus Compounds; Frank, A.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Sun, X.; Weckwerth, W. COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 2012, 8, 81–93. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablińska-Piernik, J.; Lahuta, L.B.; Stałanowska, K.; Horbowicz, M. The Imbibition of Pea (Pisum sativum L.) Seeds in Silver Nitrate Reduces Seed Germination, Seedlings Development and Their Metabolic Profile. Plants 2022, 11, 1877. https://doi.org/10.3390/plants11141877
Szablińska-Piernik J, Lahuta LB, Stałanowska K, Horbowicz M. The Imbibition of Pea (Pisum sativum L.) Seeds in Silver Nitrate Reduces Seed Germination, Seedlings Development and Their Metabolic Profile. Plants. 2022; 11(14):1877. https://doi.org/10.3390/plants11141877
Chicago/Turabian StyleSzablińska-Piernik, Joanna, Lesław Bernard Lahuta, Karolina Stałanowska, and Marcin Horbowicz. 2022. "The Imbibition of Pea (Pisum sativum L.) Seeds in Silver Nitrate Reduces Seed Germination, Seedlings Development and Their Metabolic Profile" Plants 11, no. 14: 1877. https://doi.org/10.3390/plants11141877






