3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanocomposites Mixture Preparation
2.2. Specimens’ Preparations
2.3. Testing Procedures
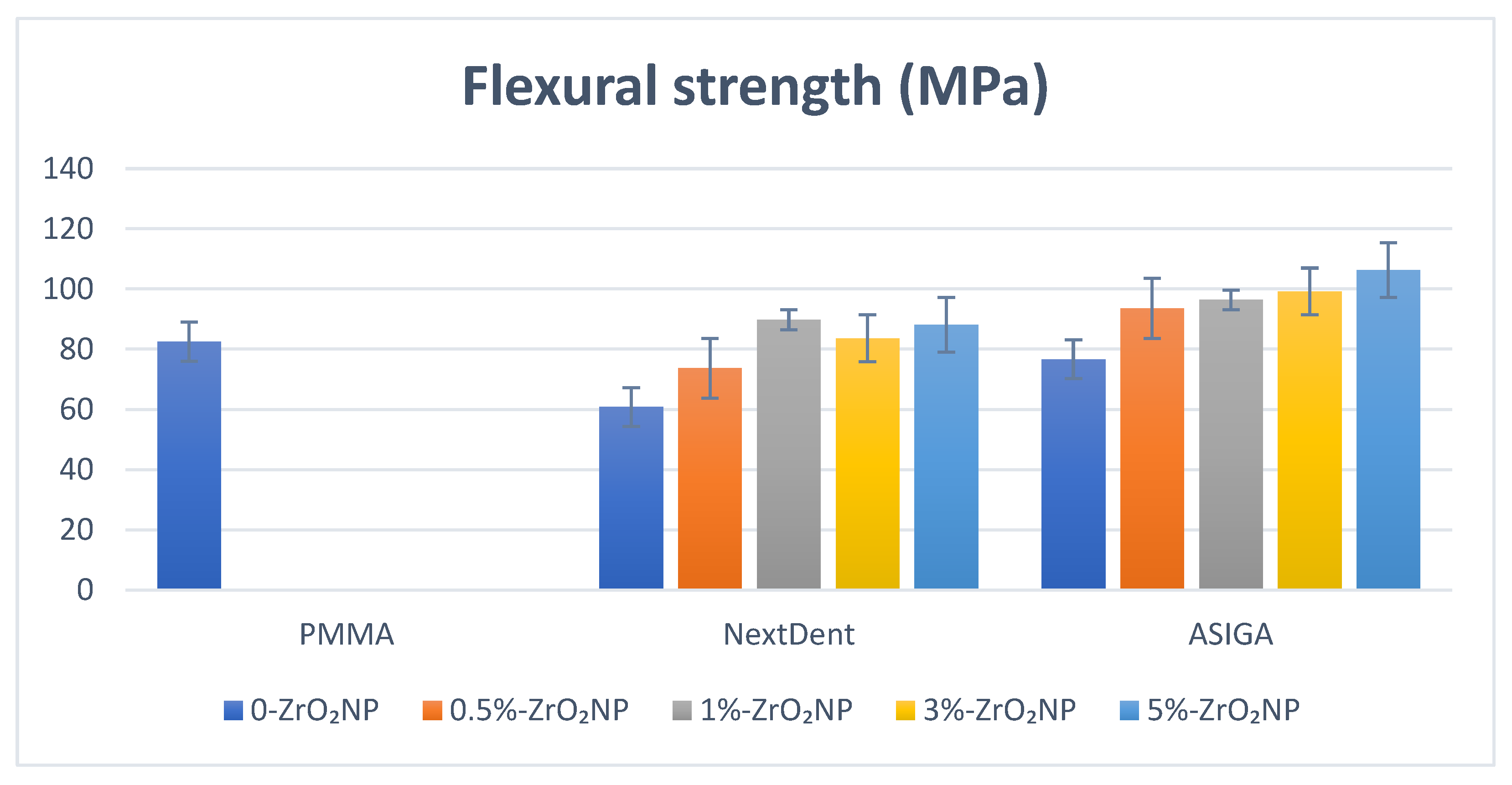
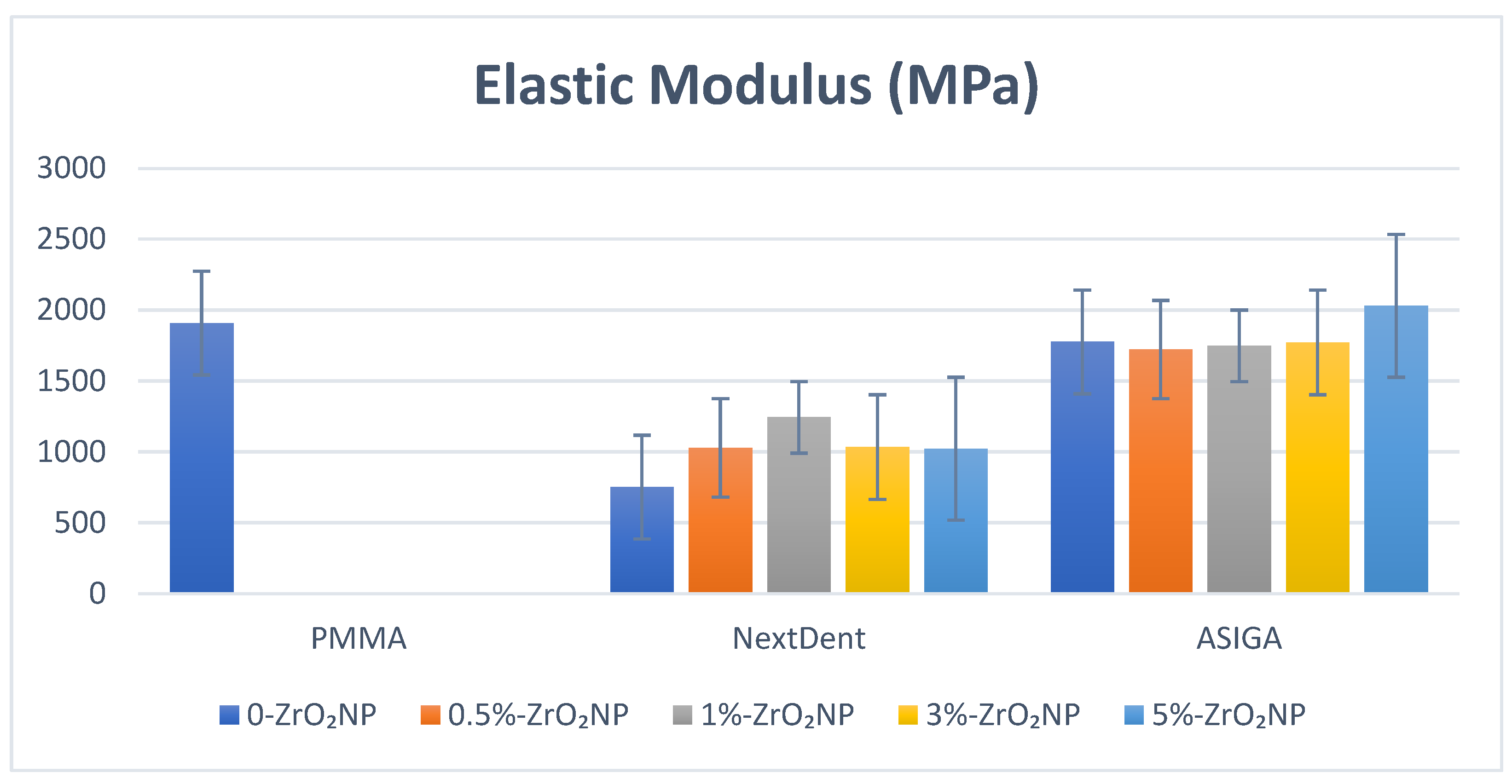
2.3.1. Flexural Strength and Elastic Modulus
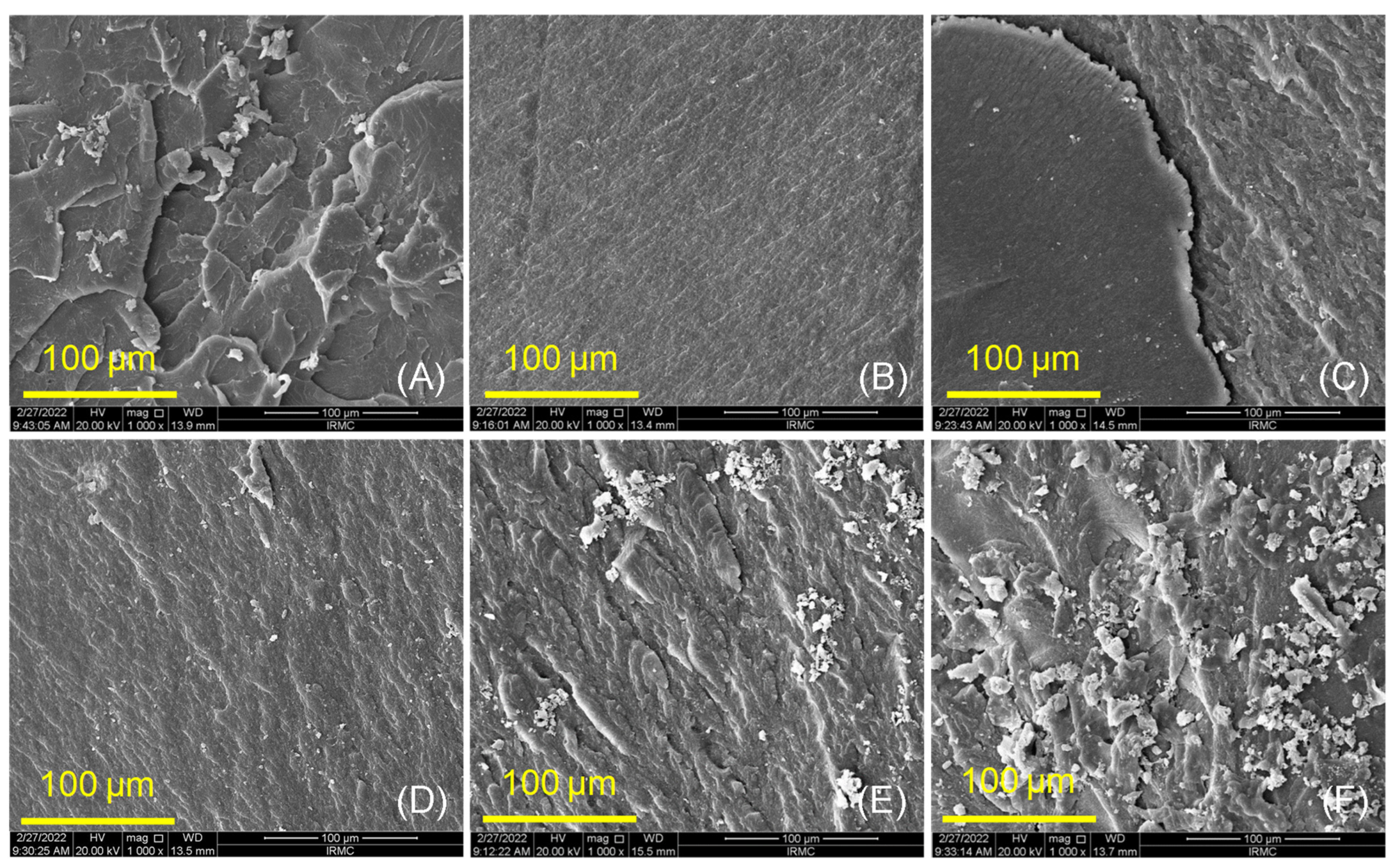
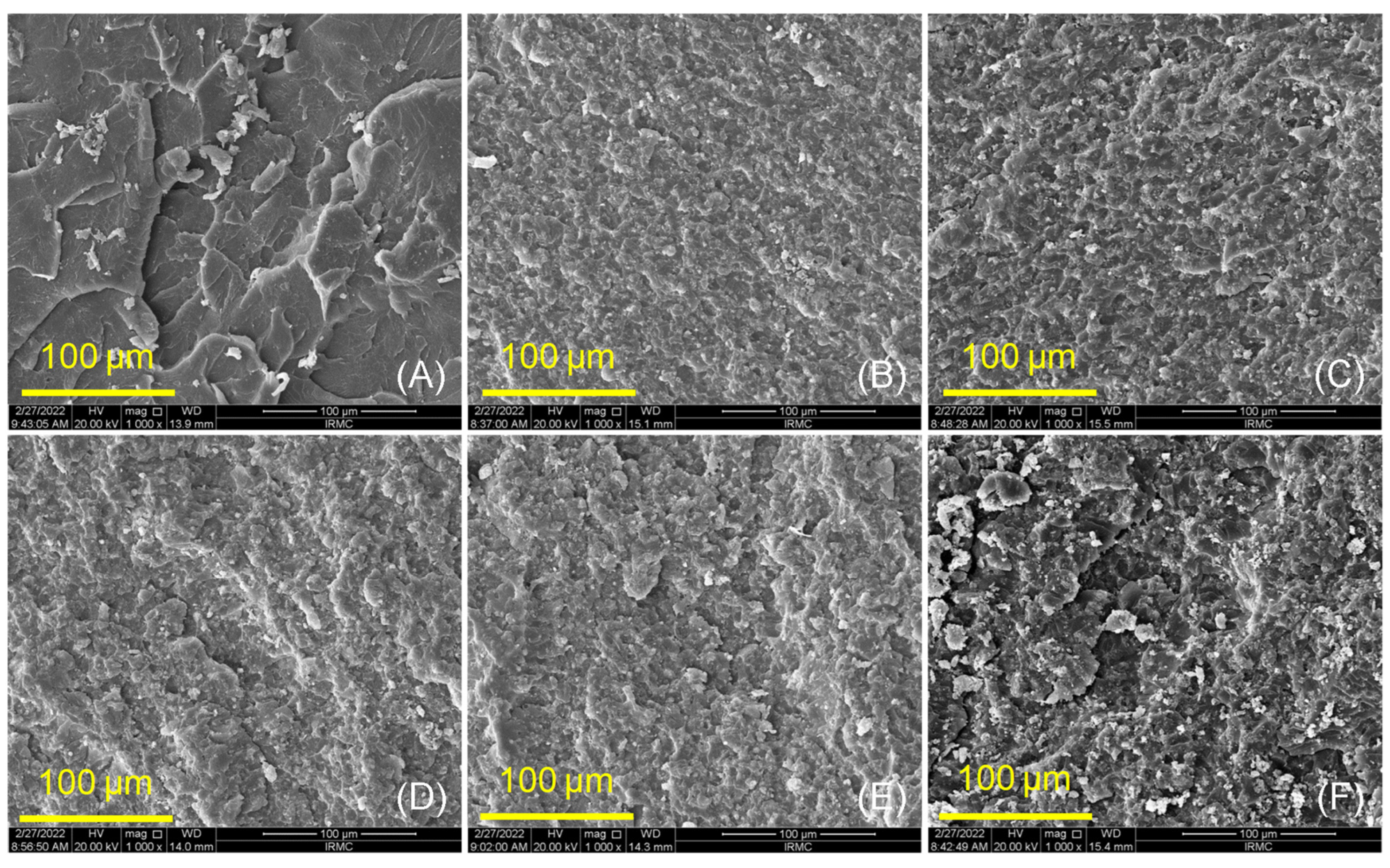
2.3.2. Surface Morphology and Chemical Bonding (SEM and FTIR)
2.3.3. Impact Strength
2.3.4. Hardness
2.3.5. Surface Roughness (Ra, µm)
Statistical Analysis
3. Results
Ftir Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of US edentulism prevalence following 5 decades of decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, L.F. The current and future treatment of edentulism. J. Prosthodont. 2009, 18, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafee, M.A. The epidemiology of edentulism and the associated factors: A literature Review. J. Fam. Med. Prim. Care 2020, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Saponaro, P.C. Management of edentulous patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D printed complete removable dental prostheses: A narrative review. BMC Oral Health 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Diwan, R. Materials Prescribed in the Management of Edentulous Patients. In Prosthodontic Treatment for Edentulous, 12th ed.; PatientsZarb, G., Bolander, C.L., Eds.; Mosby: London, UK, 2004; pp. 190–207. [Google Scholar]
- Jaafar, M. Review on poly-methyl methacrylate as denture base materials. Malays. J. Micros. 2018, 31, 14. [Google Scholar]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.R.; Latta, M.A. Physical properties of four acrylic denture base resins. J. Contemp Dent. Pract. 2005, 6, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Alla, R.; Raghavendra, K.N.; Vyas, R.; Konakanchi, A. Conventional and contemporary polymers for the fabrication of denture prosthesis: Part I—Overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef]
- Williams, D.W.; Chamary, N.; Lewis, M.A.; Milward, P.J.; McAndrew, R. Microbial contamination of removable prosthodontic appliances from laboratories and impact of clinical storage. Br. Dent. J. 2011, 211, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y.; Zhang, X.-J.; Huang, Z.-L.; Zhu, B.-S.; Chen, R.-R. Hybrid effects of zirconia nanoparticles with aluminum borate whiskers on mechanical properties of denture base resin PMMA. Dent. Mater. J. 2014, 33, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abushowmi, T.; AlZaher, Z.A.; Almaskin, D.F.; Qaw, M.S.; Abualsaud, R.; Akhtar, S.; Akhtar, A.M.; Al-Harbi, F.; Gad, M.M.; Baba, N.Z. Comparative Effect of Glass Fiber and Nano-Filler Addition on Denture Repair Strength. J. Prosthodont. 2020, 29, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.A.; Ebrahim, M.I. Effect of zirconium oxide nano-fillers addition on the flexural strength, fracture toughness, and hardness of heat-polymerized acrylic resin. World J. Nano Sci. Eng. 2014, 4, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 17, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Dallago, M.; Fontanari, V.; Torresani, E.; Leoni, M.; Pederzolli, C.; Potrich, C.; Benedetti, M. Fatigue and biological properties of Ti-6Al-4V ELI cellular structures with variously arranged cubic cells made by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 78, 381–394. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Nuvvula, S.; Matinlinna, J.P.; You, C.K.; King, N.M. Bio compatibility of various dental materials in contemporary dentistry: A narrative insight. J. Investig. Clin. Dent. 2013, 4, 9–19. [Google Scholar] [CrossRef]
- Kawai, N.; Lin, J.; Youmaru, H.; Shinya, A.; Shinya, A. Effects of three luting agents and cyclic impact loading on shear bond strengths to zirconia with tribochemical treatment. J. Dent. Sci. 2012, 7, 118–124. [Google Scholar] [CrossRef] [Green Version]
- El-Tamimi, K.M.; Bayoumi, D.A.; Ahmed, M.M.; Albaijan, I.; El-Sayed, M.E. The Effect of Salinized Nano ZrO2 Particles on the Microstructure, Hardness, and Wear Behavior of Acrylic Denture Tooth Nanocomposite. Polymers 2022, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.; Gandhi, R.R.; Sundrarajan, M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry-State of the Art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef]
- Bassoli, E.; Gatto, A.; Iuliano, L.; Violante, M.G. 3D printing technique applied to rapid casting. Rapid Prototyp. J. 2007, 13, 148–155. [Google Scholar] [CrossRef]
- Bae, E.J.; Jeong, I.D.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; Bowman, J.; White, A.R.; George, R.; George, R. Characterization of the double bond conversion of acrylic resins for 3D printing of dental prostheses. Compend. Contin. Educ. Dent. 2019, 40, 7–11. [Google Scholar]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3D-Printed vs. Heat-Polymerizing and Autopolymerizing Denture Base Acrylic Resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and Surface Properties of a 3D-Printed Denture Base Polymer. J. Prosthodont. 2021, 31, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Belles, D.; Gonzalez, M.; Kiat-Amnuay, S.; Dugarte, A.; Ontiveros, J. Impact strength of 3D printed and conventional heat-cured and cold-cured denture base acrylics. Int. J. Prosthodont. 2022, 35, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, S.; Dhamodharan, D.; Kale, M.B.; Divakaran, N.; Senthil, T.; Wu, L.; Wang, J. A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal-Metal Oxide Nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. A study of 3D-printable reinforced composite resin: PMMA modified with silver nanoparticles loaded cellulose nanocrystal. Materials 2018, 11, 2444. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.M.; Al-Harbi, F.A.; Akhtar, S.; Fouda, S.M. 3D-Printable Denture Base Resin Containing SiO2 Nanoparticles: An In Vitro Analysis of Mechanical and Surface Properties. J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Hada, T.; Kanazawa, M.; Miyamoto, N.; Liu, H.; Iwaki, M.; Komagamine, Y.; Minakuchi, S. Effect of Different Filler Contents and Printing Directions on the Mechanical Properties for Photopolymer Resins. Int. J. Mol. Sci. 2022, 23, 2296. [Google Scholar] [CrossRef]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the Mechanical Properties of ZrO2-Impregnated PMMA Nanocomposite for Denture-Based Applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Akhtar, S.; Fouda, S.M. Double-layered acrylic resin denture base with nanoparticle additions: An in vitro study. J. Prosthet. Dent. 2022, 127, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.A.; Gad, M.M.; Al-Thobity, A.M.; Akhtar, S.; Kashkari, A.; Alzoubi, F.; Yilmaz, B. Effect of treated zirconium dioxide nanoparticles on the flexural properties of autopolymerized resin for interim fixed restorations: An in vitro study. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Rahman, H.A. The effect of addition nano particle ZrO2 on some properties of autoclave processed heat cure acrylic denture base material. J. Bagh. Coll. Dent. 2015, 27, 32–39. [Google Scholar]
- Ayad, N.M.; Badawi, M.F.; Fatah, A.A. Effect of reinforcement of high-impact acrylic resin with zirconia on some physical and mechanical properties. Arch. Oral Res. 2008, 24, 245–250. [Google Scholar]
- International Organization for Standardization (ISO). Dentistry—Base Polymers—Part 1: Denture Base Polymers. ISO 20795-1:2013. 16 October 2019. Available online: https://www.iso.org/standard/62277.html/ (accessed on 11 July 2022).
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Matin, A.; Gad, M.M. Antifungal efficacy and physical properties of poly(methylmethacrylate) denture base material reinforced with SiO2 nanoparticles. J. Prosthodont. 2021, 30, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Bui, P.H.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.M.; Lai, Y.L.; Lee, S.Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Kim, J.Y.; Mangal, U.; Seo, J.Y.; Lee, M.J.; Jin, J.; Yu, J.H.; Choi, S.H. Durable Oral Biofilm Resistance of 3D-Printed Dental Base Polymers Containing Zwitterionic Materials. Int. J. Mol. Sci. 2021, 22, 417. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Silva, C.D.; Machado, A.L.; Chaves, C.D.; Pavarina, A.C.; Vergani, C.E. Effect of thermal cycling on denture base and autopolymerizing reline resins. J. Appl. Oral Sci. 2013, 21, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of Mechanical Properties of 3D-Printed, CAD/CAM, and Conventional Denture Base Materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, I.; Gurbuz, O.; Unalan, F.; Koksal, T. Impact strength of denture polymethyl methacrylate reinforced with different forms of E-glass fibers. Acta Odontol. Scand. 2013, 71, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Mowade, T.K.; Dange, S.P.; Thakre, M.B.; Kamble, V.D. Effect of fiber reinforcement on impact strength of heat polymerized polymethyl methacrylate denture base resin: In vitro study and SEM analysis. J. Adv. Prosthodont. 2012, 4, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujahid, A.; Choudhary, T.; Mehmood, M.; Irshad, M.; Hussain, T.; Bajwa, S.Z.; Ahmad, M.N. Nickel sulfide nanoparticles incorporated poly (methyl methacrylate)-zirconia membranes for ultra deep desulfurization of dibenzothiophene. MRS Adv. 2019, 4, 369–375. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Mbese, J.Z. Synthesis and characterization of metal sulfides nanoparticles/poly (methyl methacrylate) nanocomposites. Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Chitchumnong, P.; Brooks, S.C.; Stafford, G.D. Comparison of three- and four-point flexural strength testing of denture-base polymers. Dent. Mater. 1989, 5, 2–5. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N. Stawarczyk: 3D-printed material for temporary restorations: Impact of print layer thickness and post-curing method on degree of conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar]
- Zeidan, A.A.E.; Sherif, A.F.; Baraka, Y.; Abualsaud, R.; Abdelrahim, R.A.; Gad, M.M.; Helal, M.A. Evaluation of the Effect of Different Construction Techniques of CAD-CAM Milled, 3D-Printed, and Polyamide Denture Base Resins on Flexural Strength: An In Vitro Comparative Study. J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Mangal, U.; Seo, J.Y.; Yu, J.; Kwon, J.S.; Choi, S.H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials 2020, 10, 827. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; ArRejaie, A.S.; Al-Thobity, A.M. Comparative Effect of Different Polymerization Techniques on the Flexural and Surface Properties of Acrylic Denture Bases. J. Prosthodont. 2019, 28, 458–465. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef] [PubMed]
- KEßLER, A.; Hickel, R.; Ilie, N. In vitro investigation of the influence of printing direction on the flexural strength, flexural modulus and fractographic analysis of 3D-printed temporary materials. Dent. Mater. J. 2021, 40, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Faot, F.; Costa, M.A.; Cury, A.A.; Garcia, R.C.R. Impact strength and fracturemorphology of denture acrylic resins. J. Prosthet. Dent. 2006, 96, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Hamanaka, I.; Takahashi, Y.; Kawaguchi, T. Effect of long-term water immersion or thermal shock on mechanical properties of high-impact acrylic denture base resins. Dent. Mater. J. 2016, 35, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dwairi, Z.N.; Ebrahim, A.A.A.H.; Baba, N.Z. A Comparison of the Surface and Mechanical Properties of 3D Printable Denture-Base Resin Material and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M. Current perspectives and the future of Candida albicans-associated denture stomatitis treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef]

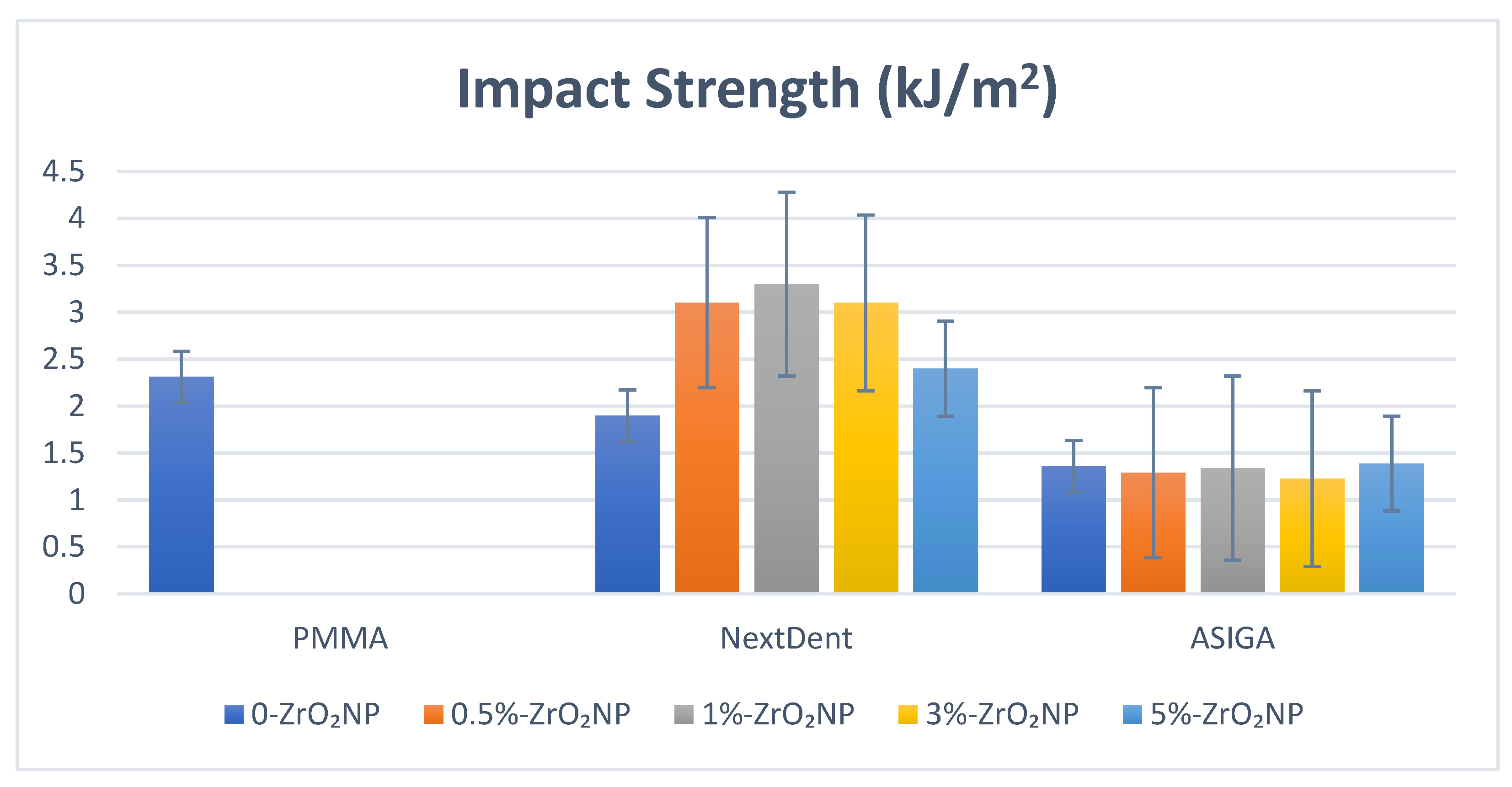
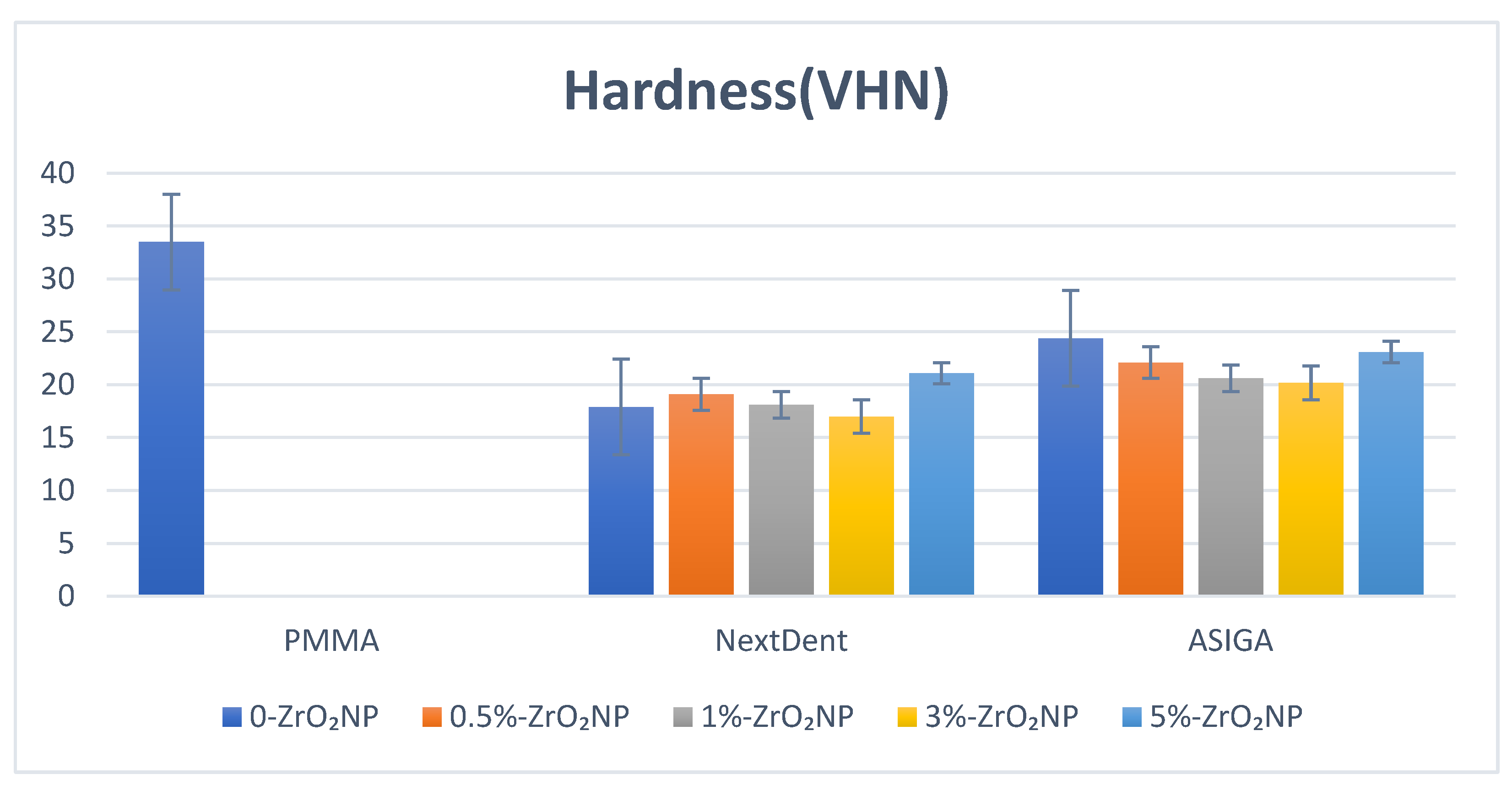
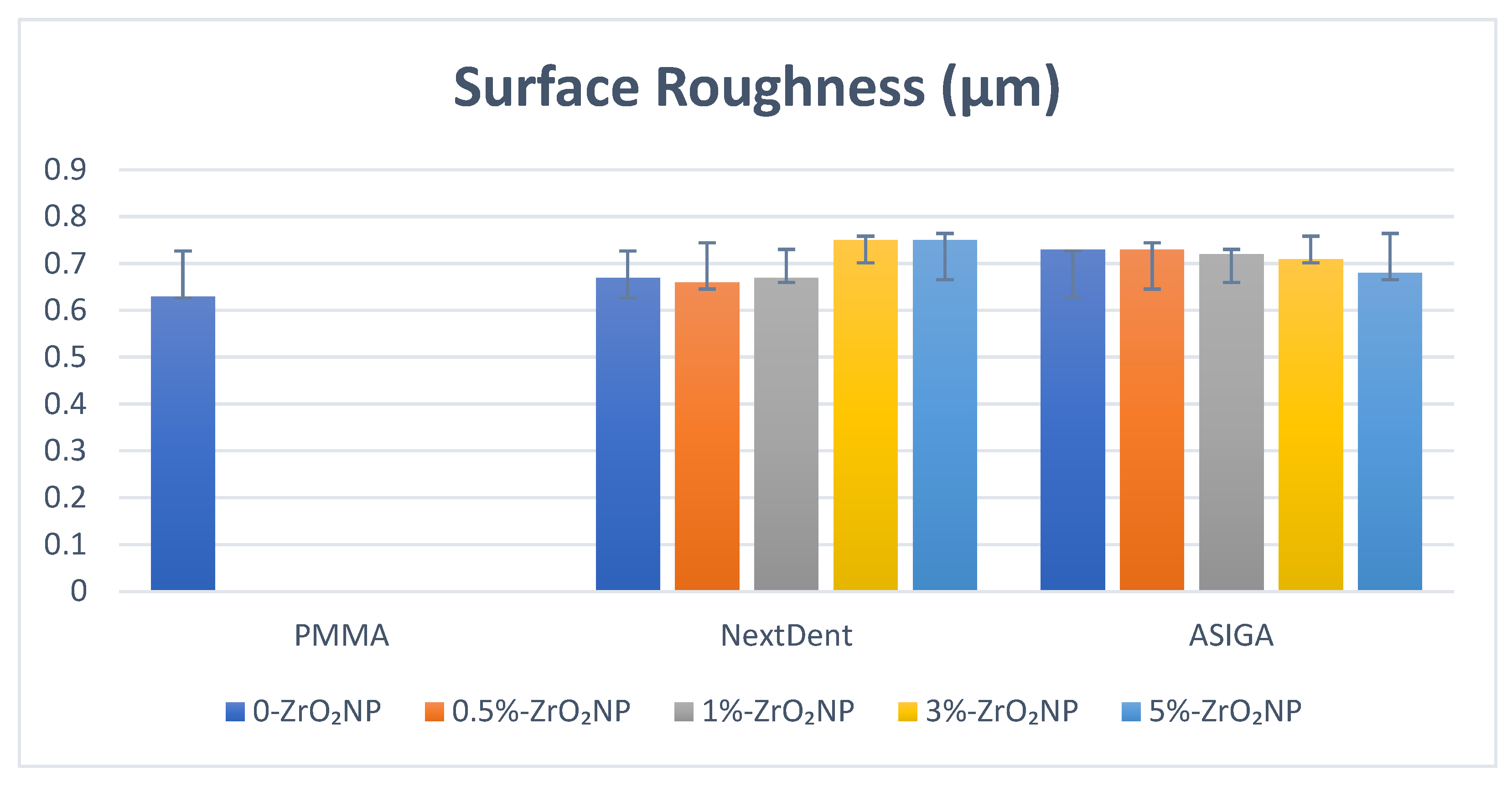
| ZrO2 Nanoparticles | Flexural Strength | Elastic Modulus | Impact Strength | Hardness | Surface Roughness |
|---|---|---|---|---|---|
| Control | 82.5 (7.6) a | 1909.4 (679.3) a,b,c,d,e | 2.31 (0.2) a | 33.5 (3.8) a,b,c,d,e | 0.63 (0.1) |
| 0% | 60.8 (4.7) a,b,c,d,e | 751.8 (35.2) a,f | 1.9 (0.7) b,c,d | 17.9 (0.81) a | 0.67 (0.03) |
| 0.5% | 73.7 (5.6) b,f,g | 1029.1 (109.9) b | 3.1 (0.67) b | 19.1 (2.7) b | 0.66 (0.03) |
| 1% | 89.8 (2.1) c,f | 1244.3 (105.6) c,f | 3.3 (0.58) a,c | 18.1 (1.6) c | 0.67 (0.08) |
| 3% | 83.6 (5.9) d | 1034.2 (49.1) d | 3.1 (0.56) d | 17.0 (3.1) d,f | 0.75 (0.09) |
| 5% | 88.1 (15.6) e,g | 1022.9 (46.7) e | 2.4 (0.22) | 21.1 (1.9) e,f | 0.75 (0.12) |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.11 |
| ZrO2 Nanoparticles | Flexural Strength | Elastic Modulus | Impact Strength | Hardness | Surface Roughness |
|---|---|---|---|---|---|
| Control | 82.5 (7.6) a,b | 1909.4 (679.3) | 2.31 (0.2) a,b,c,d,e | 33.5 (3.8) a,b,c,d,e | 0.63 (0.1) a,b |
| 0% | 76.7 (11.2) c,d,e,f | 1776.9 (99.6) | 1.36 (0.17) a | 24.4 (4.5) a | 0.73 (0.03) a |
| 0.5% | 93.6 (11.0) c | 1722.7 (122.1) | 1.29 (0.12) b | 22.1 (1.9) b | 0.73 (0.03) b |
| 1% | 96.4 (15.8) d | 1749.0 (66.3) | 1.34 (0.1) c | 20.6 (1.9) c | 0.72 (0.08) |
| 3% | 99.2 (9.6) a,e | 1773.1 (59.8) | 1.23 (0.27) d | 20.2 (2.3) d | 0.71 (0.09) |
| 5% | 106.3 (16.9) b,f | 2031.2 (77.2) | 1.39 (0.3) e | 23.1 (2.2) e | 0.68 (0.13) |
| p-value | 0.000 * | 0.15 | 0.000 * | 0.000 * | 0.025 * |
| Materials | ZrO2NP | Tested Properties | ||||
|---|---|---|---|---|---|---|
| FS | EM | SR | SH | IS | ||
| NextDent | Modified vs. PMMA | 8.8% | −34.9% | −4.7% | −37.1% | 42.8% |
| Modified vs. Unmodified | 47.6% | 65.5% | 1.5% | 17.8% | 73.6% | |
| ASIGA | Modified vs. PMMA | 28.8% | 6.3% | −7.9% | −31.1% | −39.9% |
| Modified vs. Unmodified | 38.5% | 14.3% | 6.9% | −5.4% | 2.2% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro. Nanomaterials 2022, 12, 2451. https://doi.org/10.3390/nano12142451
Alshaikh AA, Khattar A, Almindil IA, Alsaif MH, Akhtar S, Khan SQ, Gad MM. 3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro. Nanomaterials. 2022; 12(14):2451. https://doi.org/10.3390/nano12142451
Chicago/Turabian StyleAlshaikh, Ali A., Abdulrahman Khattar, Ibrahim A. Almindil, Majed H. Alsaif, Sultan Akhtar, Soban Q. Khan, and Mohammed M. Gad. 2022. "3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro" Nanomaterials 12, no. 14: 2451. https://doi.org/10.3390/nano12142451
APA StyleAlshaikh, A. A., Khattar, A., Almindil, I. A., Alsaif, M. H., Akhtar, S., Khan, S. Q., & Gad, M. M. (2022). 3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro. Nanomaterials, 12(14), 2451. https://doi.org/10.3390/nano12142451








