Fabrication of Nitrogen-Doped Reduced Graphene Oxide Modified Screen Printed Carbon Electrode (N-rGO/SPCE) as Hydrogen Peroxide Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of N@rGO
2.3. Fabrication of N-rGO/SPCE
3. Results
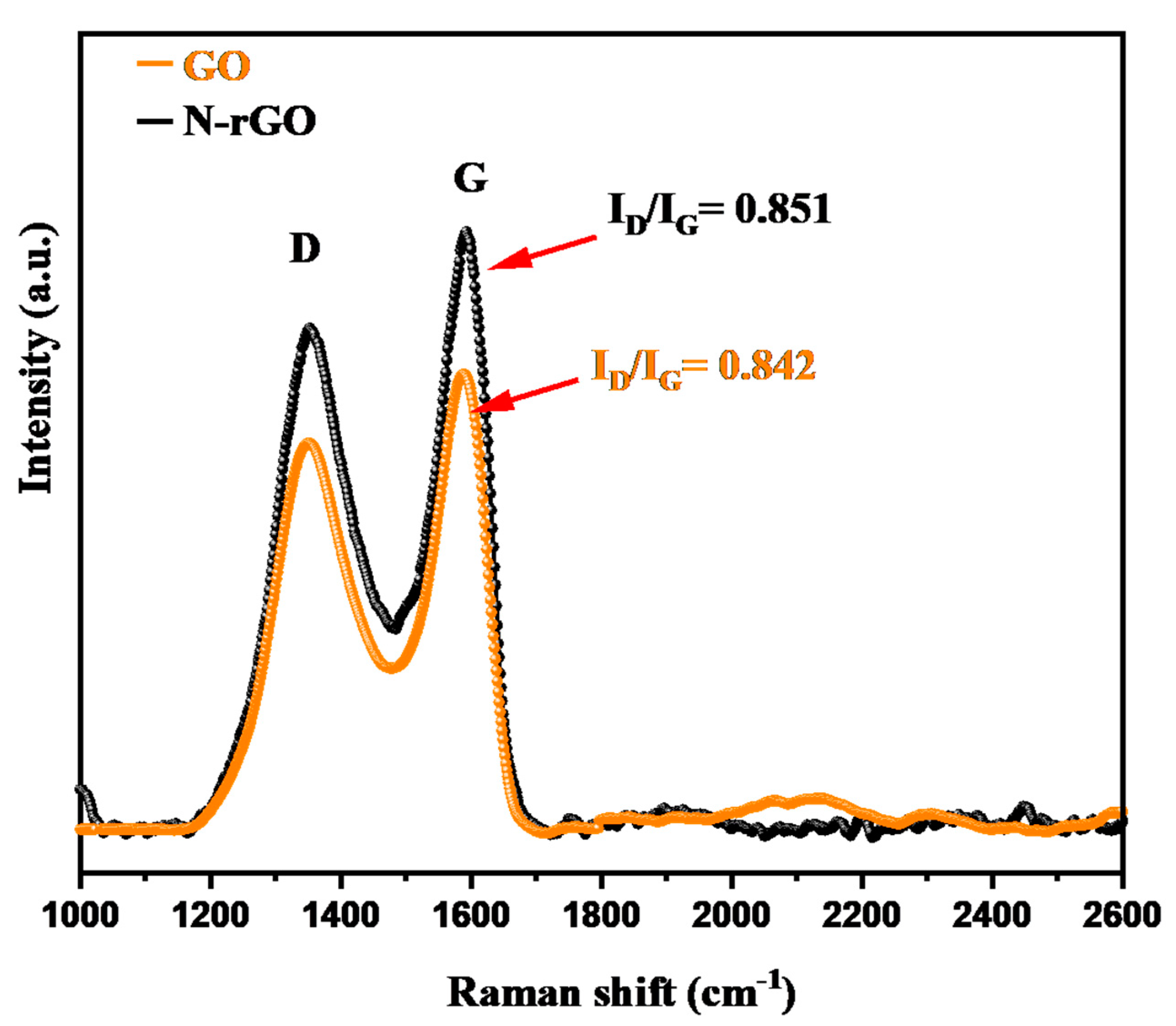
3.1. Physiochemical Characterization of N-rGO
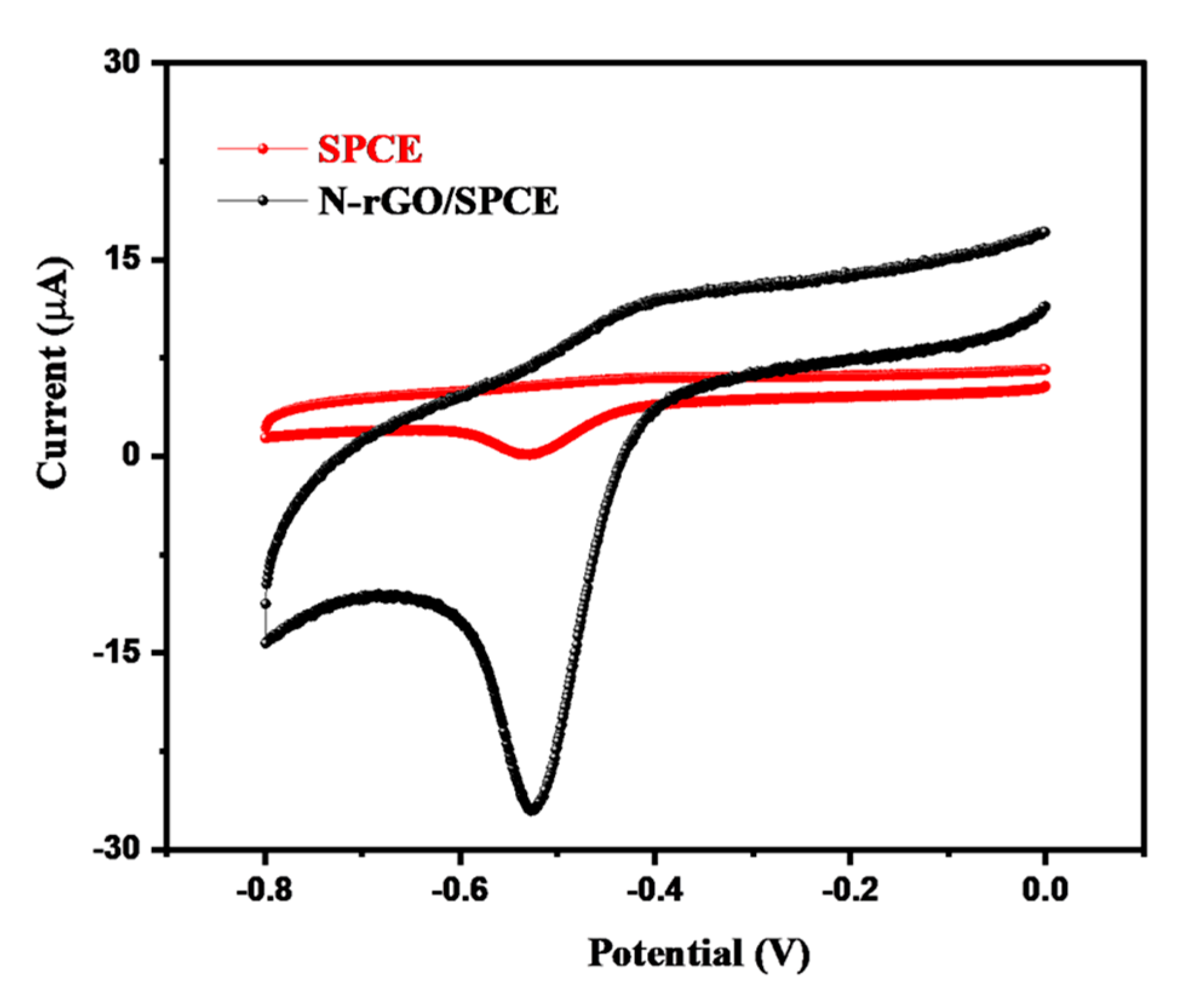
3.2. Electrochemical Performance of N-rGO/SPCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borah, N.; Boruah, P.K.; Kalita, A.J.; Guha, A.K.; Das, M.R.; Tamuly, C. A novel method for the rapid sensing of H2O2 using a colorimetric AuNP probe and its DFT study. Anal. Methods 2021, 13, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Mobin, S.M. Synthesis of MgO microstructures for Congo red dye adsorption and peroxide sensing applications. J Environ. Chem. Eng. 2019, 7, 103347. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, S.K. A structurally aligned nickel oxide encapsulated polypyrrole nanocomposite for hydrogen peroxide sensing. Dalton Trans. 2020, 49, 8744–8754. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hojamberdiev, M.; Geng, D. Recent advances in enzyme-free electrochemical hydrogen peroxide sensors based on carbon hybrid nanocomposites. J. Mater. Chem. C 2021, 9, 6970–6990. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and preparation of g-C3N4/rGO modified screen printed electrode for hydrogen peroxide sensing application. Synth. Met. 2022, 286, 117047. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Yorek, M.A. The Role of Oxidative Stress in Diabetic Vascular and Neural Disease. Free Radic. Res. 2003, 37, 471–480. [Google Scholar] [CrossRef]
- Jenner, P.; Dexter, D.T.; Sian, J.; Schapira, A.H.V.; Marsden, C.D. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. Ann. Neurol. 1992, 32, S82–S87. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, Z. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuators B 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [Green Version]
- Pinkernell, U.; Effkemann, S.; Karst, U. Simultaneous HPLC Determination of Peroxyacetic Acid and Hydrogen Peroxide. Anal. Chem. 1997, 69, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 Determination by the I3-Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Gubitz, G.; Zoonen, P.V.; Gooijer, C.; Velthorst, N.H. Immobilized fluorophores in dynamic chemiluminescence detection of hydrogen peroxide. Anal. Chem. 1985, 57, 2071–2074. [Google Scholar] [CrossRef]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef]
- Oh, W.-K.; Jeong, Y.S.; Kim, S.; Jang, J. Fluorescent Polymer Nanoparticle for Selective Sensing of Intracellular Hydrogen Peroxide. ACS Nano 2012, 6, 8516–8524. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mobin, S.M. Hydrothermally grown α-MnO2 nanorods as highly efficient low cost counter-electrode material for dye-sensitized solar cells and electrochemical sensing applications. Electrochim. Acta 2017, 252, 549–557. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and fabrication of WO3/SPE for dopamine sensing application. Mater. Chem. Phys. 2022, 287, 126298. [Google Scholar] [CrossRef]
- Isailović, J.; Vidović, K.; Hočevar, S.B. Simple electrochemical sensors for highly sensitive detection of gaseous hydrogen peroxide using polyacrylic-acid-based sensing membrane. Sens. Actuators B Chem. 2022, 352, 131053. [Google Scholar] [CrossRef]
- Ahmad, K.; Kumar, P.; Mobin, S.M. Hydrothermally grown SnO2 flowers as efficient electrode modifier for simultaneous detection of catechol and hydroquinone. J. Electrochem. Soc. 2019, 166, B1577–B1584. [Google Scholar] [CrossRef]
- Liu, M.; An, M.; Xu, J.; Liu, T.; Wang, L.; Liu, Y.; Zhang, J. Three-dimensional carbon foam supported NiO nanosheets as non-enzymatic electrochemical H2O2 sensors. Appl. Surf. Sci. 2021, 542, 148699. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. High surface area 3D-MgO flowers as the modifier for the working electrode for efficient detection of 4-chlorophenol. Nanoscale Adv. 2019, 1, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, A.; Khan, M.E.; Yoon, T.; Cho, M.H. Na,O-co-doped-graphitic-carbon nitride (Na,O-g-C3N4) for nonenzymatic electrochemical sensing of hydrogen peroxide. Appl. Surf. Sci. 2020, 525, 146353. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Hydrothermally synthesized MoSe2/rGO composite as electrode modifier for the construction of non-enzymatic urea sensor. Mater. Chem. Phys. 2022, 286, 126206. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, L.; Xu, H.; Lin, M. AuNPs-NH2/Cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, M.Q.; Khan, R.A.; Kim, H. Benign approach for the synthesis of ZnO hexagonal plates for electrochemical sensing of l-tryptophan. Mater. Chem. Phys. 2022, 287, 126297. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Synthesis of MoS2/WO3 hybrid composite for hydrazine sensing applications. Mater. Sci. Semicond. Process. 2022, 148, 106803. [Google Scholar] [CrossRef]
- Karuppiah, C.; Venkatesh, K.; Arunachalam, P.; Ramaraj, S.K.; Al-Mayouf, A.M.; Yang, C.-C. Optimization of S-dopant on N, S co-doped graphene/CNT-Fe3C nanocomposite electrode for non-enzymatic H2O2 sensor. Mater. Lett. 2021, 285, 129001. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Ipekci, H.H. The use of CeO2-modified Pt/C catalyst inks for the construction of high-performance enzyme-free H2O2 sensors. J. Electroanal. Chem. 2019, 848, 113302. [Google Scholar] [CrossRef]
- Patella, P.; Buscetta, M.; Vincenzo, S.D.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical sensor based on rGO/Au nanoparticles for monitoring H2O2 released by human macrophages. Sens. Actuators B Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, Y.; Zhao, P.; Zhou, S.; Ji, Z.; Huo, D.; Zhong, D.; Hou, C. Electrochemical sensor based on reduced graphene oxide supported dumbbell-shaped CuCo2O4 for real-time monitoring of H2O2 released from cells. Microchem. J. 2021, 160, 105521. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Zhao, G.; Wu, C. In situ growth of FeOOH nanoparticles on physically-exfoliated graphene nanosheets as high performance H2O2 electrochemical sensor. Sens. Actuators B Chem. 2020, 313, 128038. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Deng, D.; Ye, J.; Wu, Z.; Wang, J. Liqiang Luo, A novel non-enzymatic H2O2 sensor using ZnMn2O4 microspheres modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2019, 179, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, J.; Guan, X.; Peng, W.; Fan, X.; Zhang, G.; Zhang, F.; Li, Y. A novel H2O2 electrochemical sensor based on NiCo2S4 functionalized reduced graphene oxide. J. Alloys Compd. 2019, 784, 827–833. [Google Scholar] [CrossRef]
- Xiao, F.; Li, H.; Yan, X.; Yan, L.; Zhang, X.; Wang, M.; Qian, C.; Wang, Y. Graphitic carbon nitride/graphene oxide(g-C3N4/GO) nanocomposites covalently linked with ferrocene containing dendrimer for ultrasensitive detection of pesticide. Anal. Chim. Acta 2020, 1103, 84–96. [Google Scholar] [CrossRef]
- Atta, N.F.; Gawad, S.A.A.; Galal, A.; Razik, A.A.; El-Gohary, A.R.M. Efficient electrochemical sensor for determination of H2O2 in human serum based on nano iron-nickel alloy/carbon nanotubes/ionic liquid crystal composite. J. Electroanal. Chem. 2021, 881, 114953. [Google Scholar] [CrossRef]
- Song, J.; Feng, S.; Zhu, C.; Lee, J.-I.; Fu, S.; Dong, P.; Song, M.-K.; Lin, Y. Tuning the structure and composition of graphite-phase polymeric carbon nitride/reduced graphene oxide composites towards enhanced lithium-sulfur batteries performance. Electrochim. Acta 2017, 248, 541–546. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Huang, H.; Li, F.; Deng, K.; Wang, X. Palladium nanoparticles supported on graphitic carbon nitride-modified reduced graphene oxide as highly efficient catalysts for formic acid and methanol electrooxidation. J. Mater. Chem. A 2014, 2, 19084–19094. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, J.; Hu, C.; Wu, X.; Wang, X. Covalently coupled hybrid of graphitic carbon nitride with reduced graphene oxide as a superior performance lithium-ion battery anode. Nanoscale 2014, 6, 12555–12564. [Google Scholar] [CrossRef]
- Hang, T.; Xiao, S.; Yang, C.; Li, X.; Guo, C.; He, G.; Li, B.; Yang, C.; Chen, H.; Liu, F.; et al. Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sens. Actuators B Chem. 2019, 289, 15–23. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Electrochemical sensors based on nitrogen-doped reduced graphene oxide for the simultaneous detection of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2020, 842, 155873. [Google Scholar] [CrossRef]
- Wiench, P.; González, Z.; Menéndez, R.; Grzyb, B.; Gryglewicz, G. Beneficial impact of oxygen on the electrochemical performance of dopamine sensors based on N-doped reduced graphene oxides. Sens. Actuators B Chem. 2018, 257, 143–153. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Enhanced stability of MAPbI3 based perovskite solar cells. Mater. Lett. 2022, 318, 132187. [Google Scholar] [CrossRef]
- Park, H.-Y.; Singh, K.P.; Yang, D.-S.; Yu, J.-S. Simple approach to advanced binder-free nitrogen doped graphene electrode for lithium batteries. RSC Adv. 2015, 5, 3881–3887. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhao, P.; Lei, D.Y.; Li, W.; Bai, J.; Ren, Z.; Xu, X. Searching for magnetism in pyrrolic N-doped graphene synthesized via hydrothermal reaction. Carbon 2015, 84, 460–468. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, V. Heteroatom Doped Multi-Layered Graphene Material for Hydrogen Storage Application. Graphene 2016, 5, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Wang, F.; Zhang, Y.; Sui, Y.; Liu, P.; Zhang, Z.; Xu, C.; Yang, C. High-performance hydrogen peroxide micro-sensors based on laser-induced fabrication of graphene@Ag electrodes. Appl. Surf. Sci. 2021, 565, 150565. [Google Scholar] [CrossRef]
- Du, X.; Chen, Y.; Dong, W.; Han, B.; Liu, M.; Chen, Q.; Zhou, J. A nanocomposite-based electrochemical sensor for non-enzymatic detection of hydrogen peroxide. Oncotarget 2017, 8, 13039–13047. [Google Scholar] [CrossRef] [Green Version]
- Promsuwan, K.; Soleh, A.; Saisahas, K.; Saichanapan, J.; Thiangchanya, A.; Phonchai, A.; Limbut, W. Micro-colloidal catalyst of palladium nanoparticles on polyaniline-coated carbon microspheres for a non-enzymatic hydrogen peroxide sensor. Microchem. J. 2021, 171, 106785. [Google Scholar] [CrossRef]
- Yin, H.; Shi, Y.; Dong, Y.P.; Chu, X.F. Synthesis of spinel-type CuGa2O4 nanoparticles as a sensitive non-enzymatic electrochemical sensor for hydrogen peroxide and glucose detection. J. Electroanal. Chem. 2021, 885, 115100. [Google Scholar] [CrossRef]
- Bohlooli, F.; Yamatogi, A.; Mori, S. Manganese oxides/carbon nanowall nanocomposite electrode as an efficient non-enzymatic electrochemical sensor for hydrogen peroxide. Sens. Bio-Sens. Res. 2021, 31, 100392. [Google Scholar] [CrossRef]
- Xiao, X.; Song, Y.; Liu, H.; Xie, M.; Hou, H.; Wang, L.; Li, Z. Electrospun carbon nanofibers with manganese dioxide nanoparticles for nonenzymatic hydrogen peroxide sensing. J. Mater. Sci. 2013, 48, 4843–4850. [Google Scholar] [CrossRef]
- Sinha, G.N.; Subramanyam, P.; Sivaramakrishna, V.; Subrahmanyam, C. Electrodeposited copper bismuth oxide as a low-cost, non-enzymatic electrochemical sensor for sensitive detection of uric acid and hydrogen peroxide. Inorg. Chem. Commun. 2021, 129, 108627. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Bao, J.; Ma, Y.; Zhou, J.; Shen, C.; Luo, H.; Yang, M.; Hou, C.; Huo, D. A simple hydrothermal one-step synthesis of 3D-MoS2/rGO for the construction of sensitive enzyme-free hydrogen peroxide sensor. Microchem. J. 2021, 162, 105746. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zheng, J. One-pot fabrication of AuNPs-Prussian blue-Graphene oxide hybrid nanomaterials for non-enzymatic hydrogen peroxide electrochemical detection. Microchem. J. 2021, 160, 105595. [Google Scholar] [CrossRef]
- Nia, P.M.; Woi, P.M.; Alias, Y. Facile one-step electrochemical deposition of copper nanoparticles and reduced graphene oxide as nonenzymatic hydrogen peroxide sensor. Appl. Surf. Sci. 2017, 413, 56–65. [Google Scholar]
- Mutyala, S.; Mathiyarasu, J. A reagentless non-enzymatic hydrogen peroxide sensor presented using electrochemically reduced graphene oxide modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 69, 398–406. [Google Scholar] [CrossRef]
- Woo, S.; Kim, Y.R.; Chung, T.D.; Piao, Y.; Kim, H. Synthesis of a graphene carbon nanotube composite and its electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2012, 59, 509–523. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Zhang, Y.W.; Sun, X.P. Stable aqueous dispersion of graphene nanosheets: Noncovalent functionalization by a polymeric reducing agent and their subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Macromolecules 2010, 43, 10078–10083. [Google Scholar] [CrossRef]
- Karimi, M.A.; Banifatemeh, F.; Mehrjardi, A.H.; Tavallali, H.; Eshaghia, Z.; Rad, G.D. A novel rapid synthesis of Fe2O3/graphene nanocomposite using ferrate(VI) and its application as a new kind of nanocomposite modified electrode as electrochemical sensor. Mater. Res. Bull. 2015, 70, 856–864. [Google Scholar] [CrossRef]












| Electrode Material | LOD (µM) | Sensitivity | Working Electrode Area | References |
|---|---|---|---|---|
| Graphene@Ag | 2.8 | 28.6 µM mM−1 cm−2 | 5 mm | [49] |
| Pt-PANI/rGO | 1.1 | 257 μA mM−1 cm−2 | - | [50] |
| Pd/PANI/(CMF) | 0.70 | 234 μA mM−1 cm−2 | - | [51] |
| CuGa2O4/GCE | 5 | - | 3 mm | [52] |
| MnOx/carbon nanowall | 0.55 | 698 μA mM−1 cm−2 | 10 mm | [53] |
| MnO2-CNF/GCE | 1.1 | 71 μA mM−1 cm−2 | 3 mm | [54] |
| CuBi2O4/FTO | 380 | 280 μA mM−1 cm−2 | 0.5 cm2 | [55] |
| MoS2/rGO | 0.19 | 3 mm | [56] | |
| AuNPs-PB-GO | 1.3 | 7.6 μA mM− 1 cm−2 | - | [57] |
| CuNP-rGO | 600 | - | - | [58] |
| ERGO/GC | 0.7 | - | - | [59] |
| Graphene/MWCNT | 9.4 | - | 3 mm | [60] |
| Ag/rGO | 28 | - | - | [61] |
| rGO/Fe2O3 | 6 | - | 2 mm | [62] |
| N-rGO/SPCE | 0.83 | 4.34 µA µM−1 cm−2 | 3 mm | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Kim, H. Fabrication of Nitrogen-Doped Reduced Graphene Oxide Modified Screen Printed Carbon Electrode (N-rGO/SPCE) as Hydrogen Peroxide Sensor. Nanomaterials 2022, 12, 2443. https://doi.org/10.3390/nano12142443
Ahmad K, Kim H. Fabrication of Nitrogen-Doped Reduced Graphene Oxide Modified Screen Printed Carbon Electrode (N-rGO/SPCE) as Hydrogen Peroxide Sensor. Nanomaterials. 2022; 12(14):2443. https://doi.org/10.3390/nano12142443
Chicago/Turabian StyleAhmad, Khursheed, and Haekyoung Kim. 2022. "Fabrication of Nitrogen-Doped Reduced Graphene Oxide Modified Screen Printed Carbon Electrode (N-rGO/SPCE) as Hydrogen Peroxide Sensor" Nanomaterials 12, no. 14: 2443. https://doi.org/10.3390/nano12142443






