Fusarium Dry Rot of Garlic Bulbs Caused by Fusarium proliferatum: A Review
Abstract
:1. Introduction
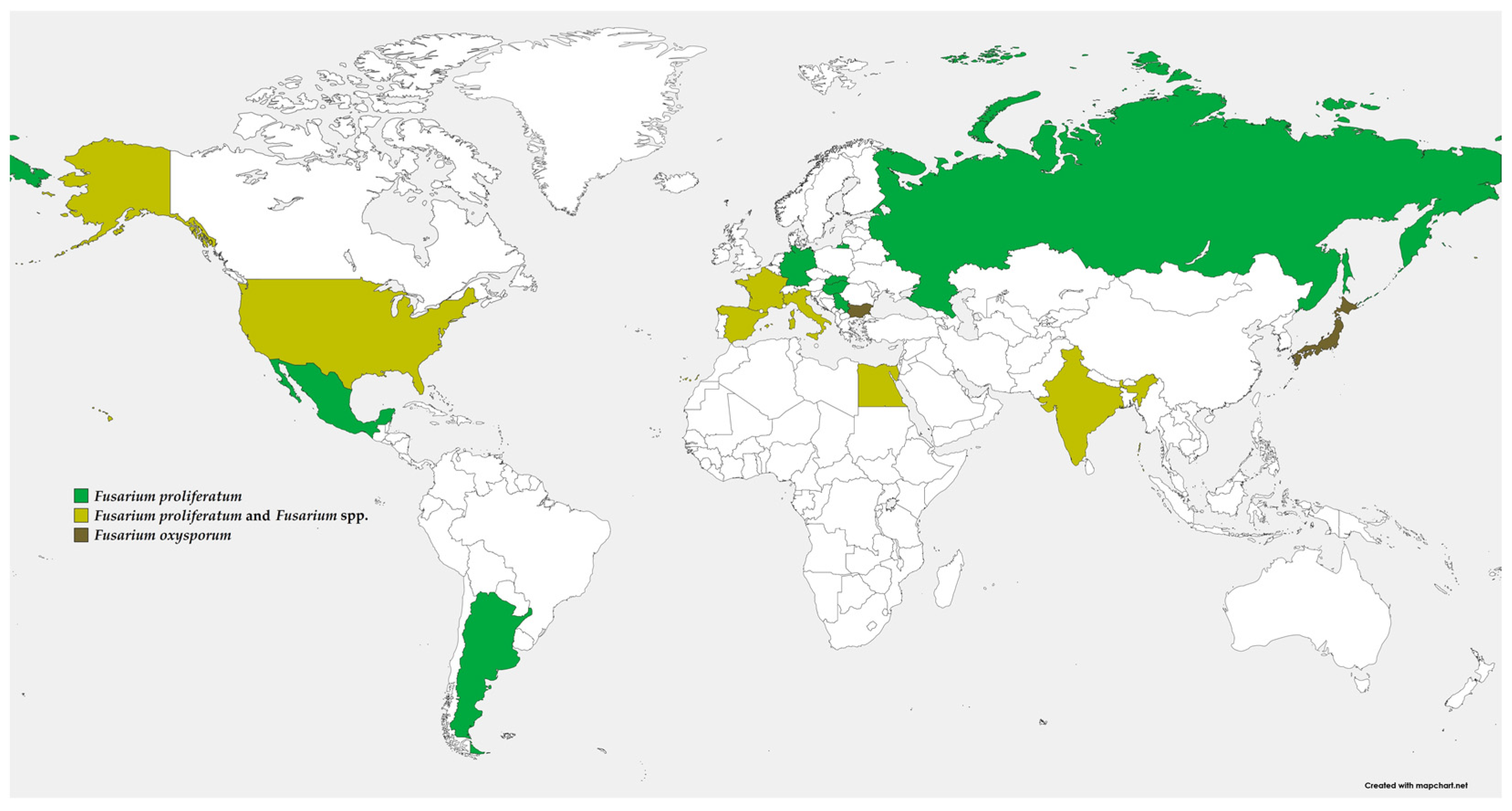
2. Distribution and Importance
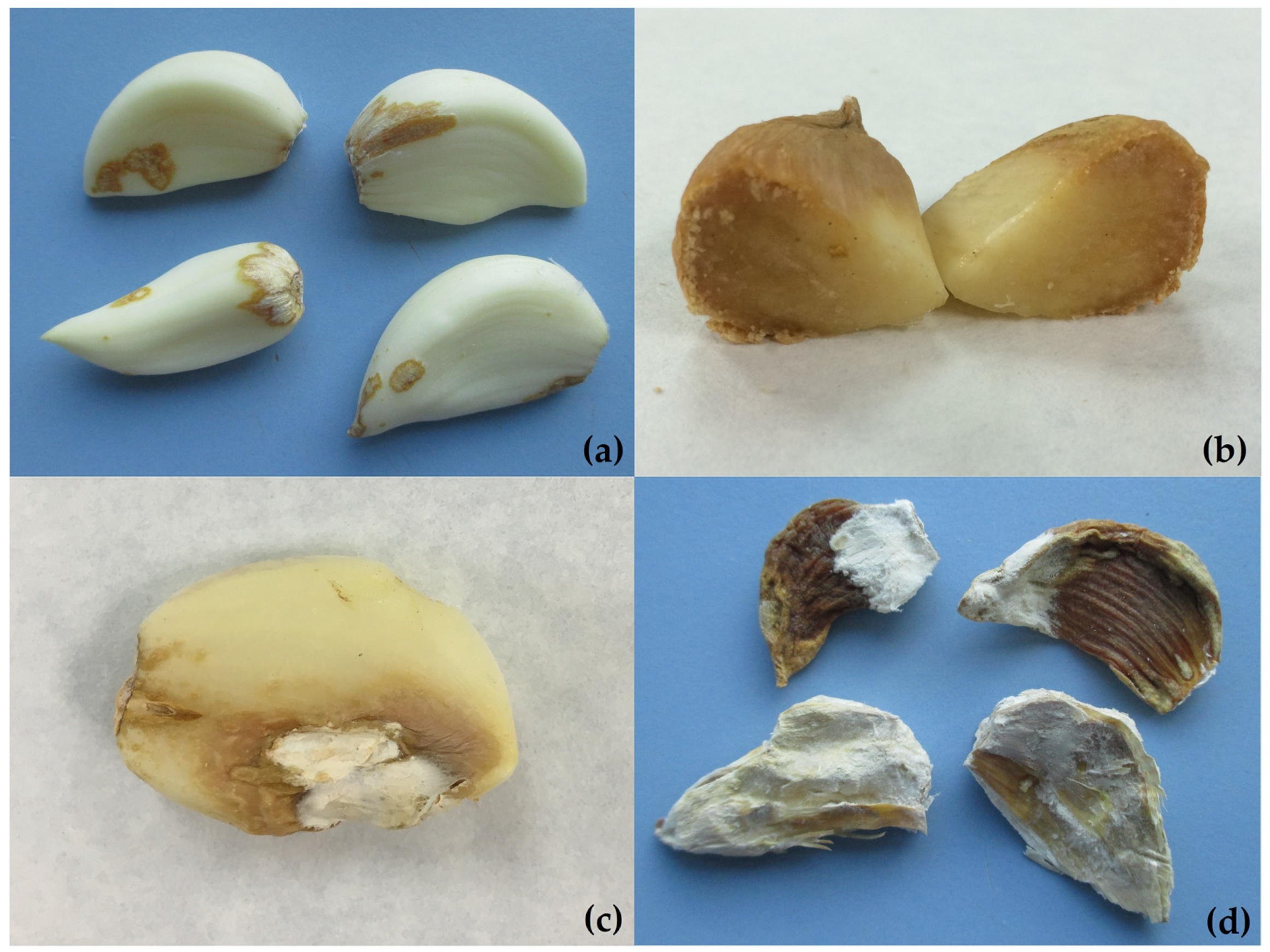
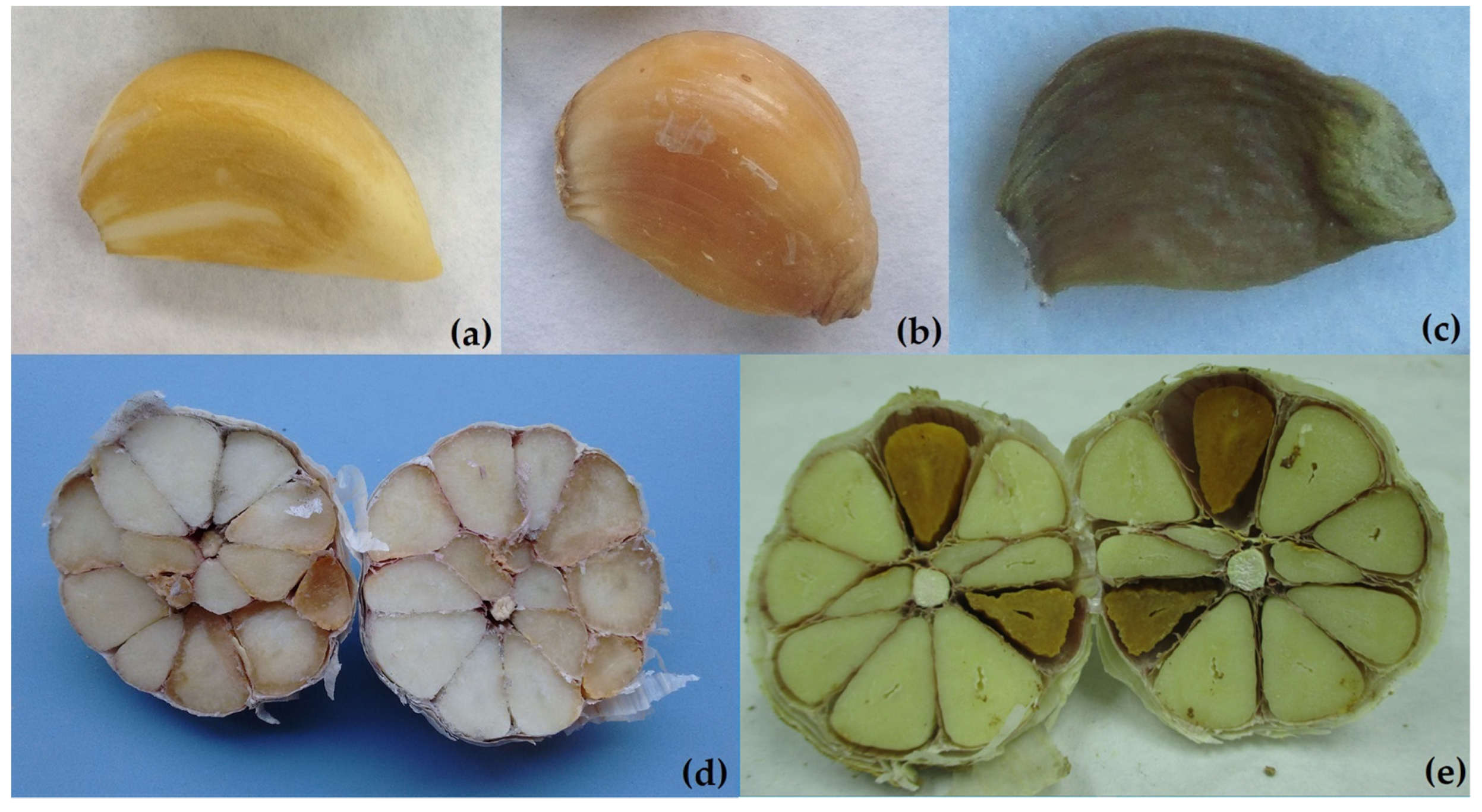
3. Symptomatology
4. Taxonomy and Identification
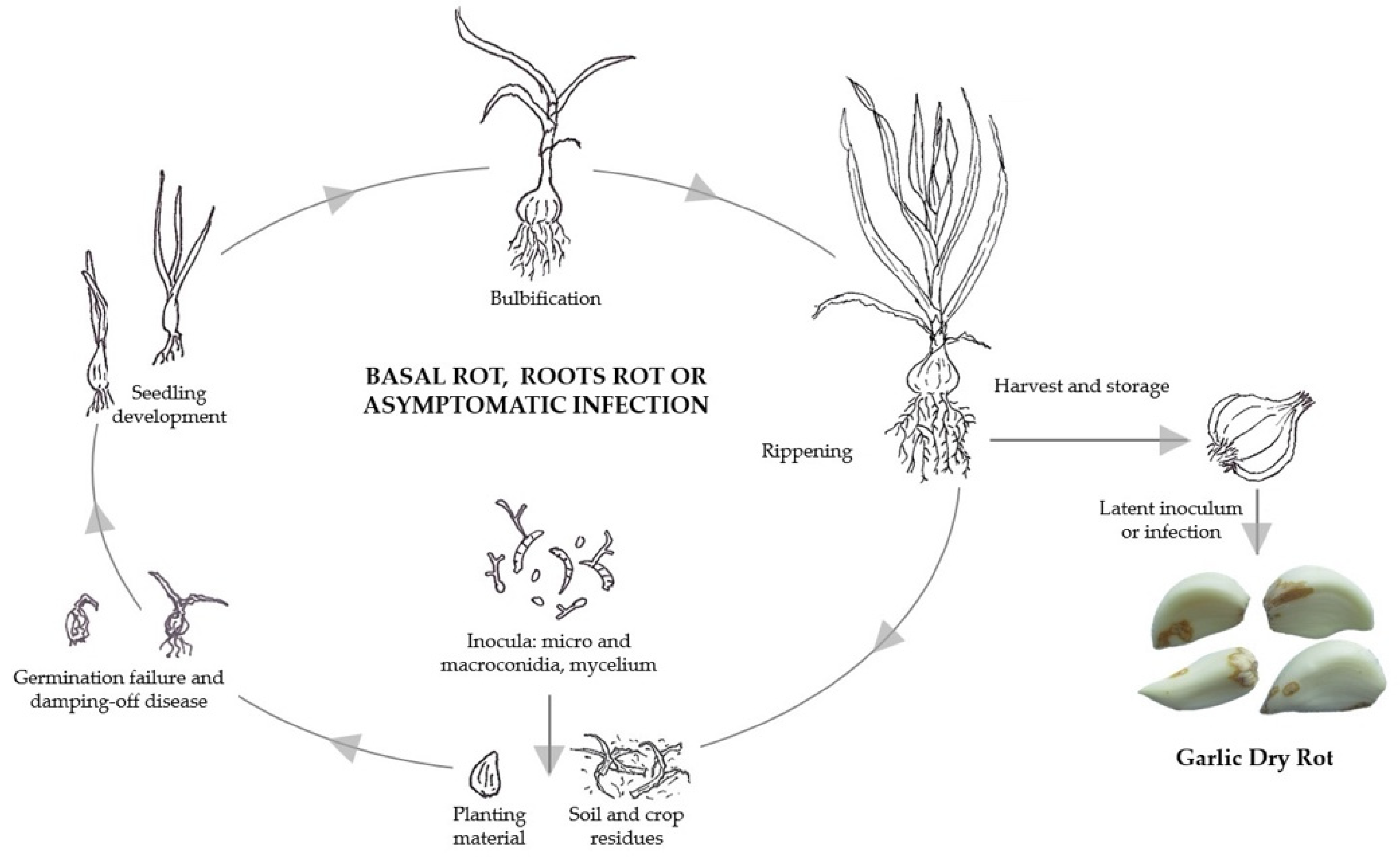
5. Life Cycle
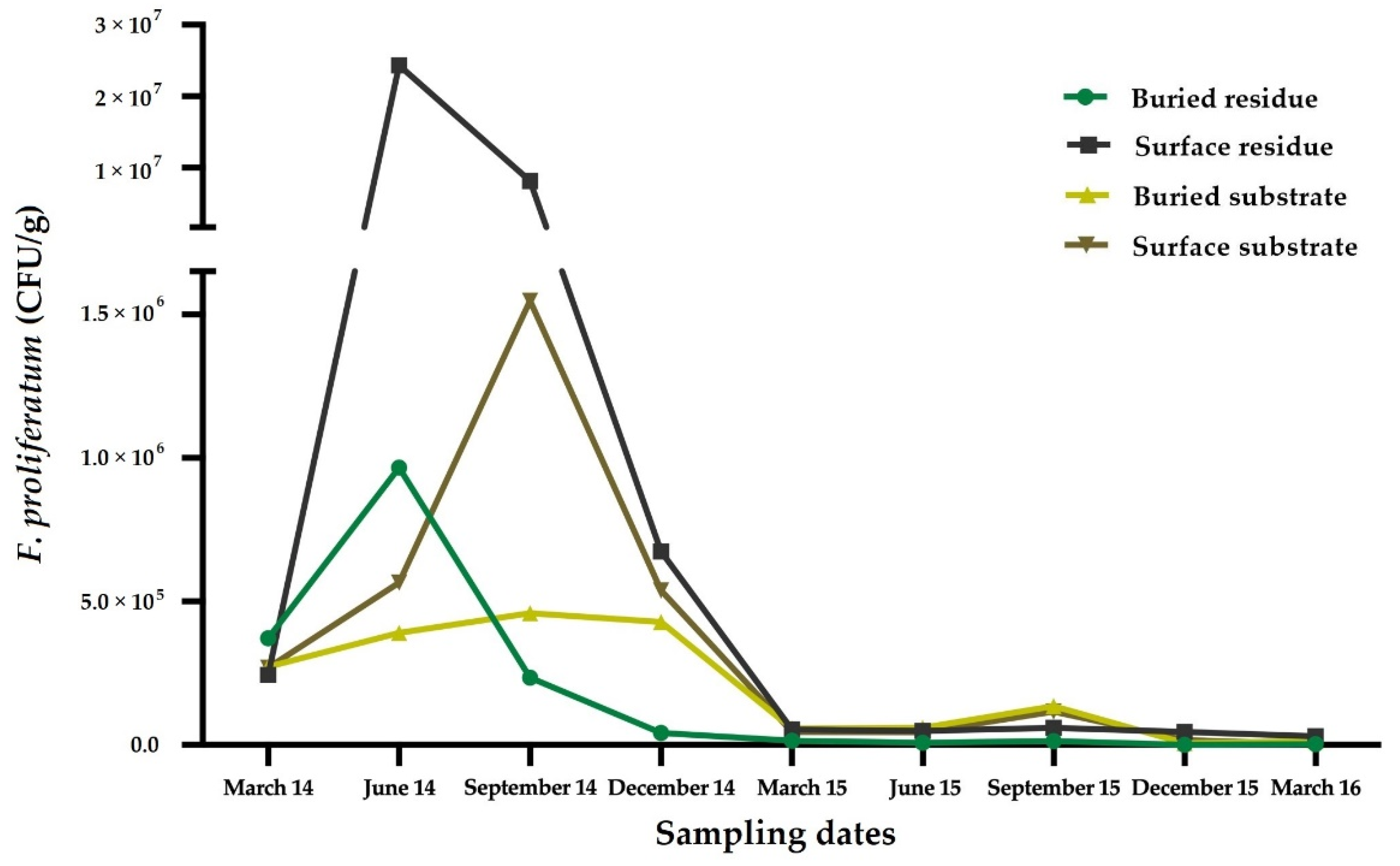
6. Host Range and Varietal Response
7. Cultural Control and Storage Conditions
8. Chemical and Biological Control
9. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential health benefit of garlic based on human intervention studies: A brief overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Chretien, P.L.; Morris, C.E.; Duffaud, M.; Leyronas, C. Aetiology of garlic rot, an emerging disease in France. Plant Pathol. 2021, 70, 1276–1291. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Division 2020. Available online: https://www.fao.org/faostat/es/#data/QC/visualiz (accessed on 25 April 2022).
- Spagnoli, S. La qualità carta vincente contro l’import a basso prezzo. Agricoltura 2014, 6, 36–37. [Google Scholar]
- Lopez-Bellido, F.J.; Lopez-Bellido, R.J.; Muñoz-Romero, V.; Fernandez-Garcia, P.; Lopez-Bellido, L. New phenological growth stages of garlic (Allium sativum). Ann. Appl. Biol. 2016, 169, 423–439. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Battilani, P. Efficacy of chemical and biological spray seed treatments in preventing garlic dry rot. Phytopathol. Mediterr. 2022, 61, 27–37. [Google Scholar] [CrossRef]
- Palmero, D.; Gálvez, L.; Arias, M.; González, C. La roya del ajo (Puccinia allii). Terralia 2016, 108, 56–60. [Google Scholar]
- Gálvez, L.; Gil-Serna, J.; García, M.; Iglesias, C.; Palmero, D. Stemphylium leaf blight of garlic (Allium sativum) in Spain: Taxonomy and in vitro fungicide response. Plant Pathol. J. 2016, 32, 388–395. [Google Scholar] [CrossRef]
- Gálvez, L.; Palmero, D. Incidence and etiology of postharvest fungal diseases associated with bulb rot in garlic (Alllium sativum) in Spain. Foods 2021, 10, 1063. [Google Scholar] [CrossRef]
- Tonti, S.; Prà, M.D.; Nipoti, P.; Prodi, A.; Alberti, I. First report of Fusarium proliferatum causing rot of stored garlic bulbs (Allium sativum L.) in Italy. J. Phytopathol. 2012, 160, 761–763. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology; APS Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Boonzaaijer, G.; van Osenbruggen, W.; Kleinnijenhuis, A.; van Dongen, W. An exploratory investigation of several mycotoxins and their natural occurrence in flavour ingredients and spices, using a multi-mycotoxin LC-MS/MS method. World Mycotoxin J. 2008, 1, 167–174. [Google Scholar] [CrossRef]
- Seefelder, W.; Gossmann, M.; Humpf, H.-U. Analysis of fumonisin B1 in Fusarium proliferatum-infected asparagus spears and garlic bulbs from germany by liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2778–2781. [Google Scholar] [CrossRef] [PubMed]
- Seefelder, W.; Knecht, A.; Goβmann, M.; Kleta, S.; Büttner, C.; Humpf, H. Occurrence of fumonisins in asparagus (Asparagus officinalis L.) and garlic (Allium sativum L.) from Germany. Mycotoxin Res. 2004, 20, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Tonti, S.; Mandrioli, M.; Nipoti, P.; Pisi, A.; Toschi, T.G.; Prodi, A. Detection of fumonisins in fresh and dehydrated commercial garlic. J. Agric. Food Chem. 2017, 65, 7000–7005. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Pietri, A.; Battilani, P. Monitoring the incidence of dry rot caused by Fusarium proliferatum in garlic at harvest and during storage. Postharvest Biol. Technol. 2021, 173, 111407. [Google Scholar] [CrossRef]
- Stankovic, S.; Levic, J.; Petrovic, T.; Logrieco, A.; Moretti, A. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 2007, 118, 165–172. [Google Scholar] [CrossRef]
- Palmero, D.; De Cara, M.; Nosir, W.; Gálvez, L.; Cruz, A.; WoodWard, S.; González-Jaén, M.T.; Tello, J. Fusarium proliferatum isolated from garlic in Spain: Identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol. Mediterr. 2012, 51, 207–218. [Google Scholar]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Waśkiewicz, A.; Stępień, Ł.; Wilman, K.; Kachlicki, P. Diversity of pea-associated F. proliferatum and F. verticillioides populations revealed by FUM1 sequence analysis and fumonisin biosynthesis. Toxins 2013, 5, 488–503. [Google Scholar] [CrossRef] [Green Version]
- Matuo, T.; Miyagawa, M.; Saito, H. Fusarium oxysporum f. sp. garlic nf sp. causing basal rot of garlic. Jpn. J. Phytopathol. 1986, 52, 860–864. [Google Scholar]
- Simey, E.I. Garlic rot caused by Fusarium proliferatum (Matsushima) Nirenferg var. minus Nirenferg in Hungary. Növényvédelem 1990, 26, 397–399. [Google Scholar]
- Koleva, K. Variety of species and spread of fungi of genus Fusarium related to rotting of garlic. Bulg. J. Agric. Sci. 2004, 10, 177–180. [Google Scholar]
- Dugan, F.; Hellier, B.; Lupien, S. First report of Fusarium proliferatum causing rot of garlic bulbs in North America. Plant Pathol. 2003, 52, 426. [Google Scholar] [CrossRef]
- Salvalaggio, A.; Ridao, A.d.C. First report of Fusarium proliferatum causing rot on garlic and onion in Argentina. Plant Dis. 2013, 97, 556. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, Y.M.O.; Ortiz, J.C.D.; Chávez, E.C.; Castillo, F.D.H.; Olivas, A.F.; Morales, G.G.; Martínez, O.V.; Guerra, R.R. The first report of Fusarium proliferatum causing garlic bulb rots in Mexico. Afr. J. Agric. Res. 2013, 8, 570–573. [Google Scholar]
- Palmero, D.; De Cara, M.; Iglesias, C.; Moreno, M.; Gonzalez, N.; Tello, J. First report of Fusarium proliferatum causing rot of garlic bulbs in Spain. Plant Dis. 2010, 94, 277. [Google Scholar] [CrossRef]
- Leyronas, C.; Chrétien, P.L.; Troulet, C.; Duffaud, M.; Villeneuve, F.; Morris, C.; Hunyadi, H. First report of Fusarium proliferatum causing garlic clove rot in France. Plant Dis. 2018, 102, 2658. [Google Scholar] [CrossRef]
- Ignjatov, M.; Milosević, D.; Ivanović, Ž.; Karaman, M.; Vlajić, S.; Nikolić, Z.; Gvozdanović-Varga, J. Morphological and pathogenic properties of Fusarium proliferatum isolates: The causal agent of garlic (Allium sativum L.): Rot in Serbia. Ratar. Povrt. 2018, 55, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Horáková, M.K.; Tancik, J.; Barta, M. Fusarium proliferatum causing dry rot of stored garlic in Slovakia. J. Plant Pathol. 2021, 103, 997–1002. [Google Scholar] [CrossRef]
- Moharam, M.H.A.; Farrag, E.S.H.; Mohamed, M.D.A. Pathogenic fungi in garlic seed cloves and first report of Fusarium proliferatum causing cloves rot of stored bulbs in upper Egypt. Arch. Phytopathol. Plant Prot. 2013, 46, 2096–2103. [Google Scholar] [CrossRef]
- Elshahawy, I.; Saied, N.; Morsy, A. Fusarium proliferatum, the main cause of clove rot during storage, reduces clove germination and causes wilt of established garlic plants. J. Plant Pathol. 2017, 99, 85–93. [Google Scholar]
- Anisimova, O.K.; Seredin, T.M.; Danilova, O.A.; Filyushin, M. first report of Fusarium proliferatum causing garlic clove rot in Russian Federation. Plant Dis. 2021, 105, 3308. [Google Scholar] [CrossRef] [PubMed]
- Sankar, N.R.; Babu, G.P. First report of Fusarium proliferatum causing rot of garlic bulbs (Allium sativum) in India. Arch. Phytopathol. Plant Prot. 2012, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Dugan, F.M.; Hellier, B.C.; Lupien, S.L. Pathogenic fungi in garlic seed cloves from the United States and China, and efficacy of fungicides against pathogens in garlic germplasm in Washington State. J. Phytopathol. 2007, 155, 437–445. [Google Scholar] [CrossRef]
- Cirrincione, M.A.; Guiñazú, M.E. Paralisis Cerosa (Waxy Breakdowm), en Bulbos de Ajo. Available online: http://anapa.com.br/wp-content/uploads/2017/07/paralisis-cerosa.pdf (accessed on 19 April 2022).
- Le, D.; Audenaert, K.; Haesaert, G. Fusarium basal rot: Profile of an increasingly important disease in Allium spp. Trop. Plant Pathol. 2021, 46, 241–253. [Google Scholar] [CrossRef]
- Armengol, J.; Moretti, A.; Perrone, G.; Vicent, A.; Bengoechea, J.; García-Jiménez, J. Identification, incidence and characterization of Fusarium proliferatumon ornamental palms in Spain. Eur. J. Plant Pathol. 2005, 112, 123–131. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Battilani, P. Fungi associated with garlic during the cropping season, with focus on Fusarium proliferatum and F. oxysporum. Plant Health Prog. 2021, 22, 37–46. [Google Scholar] [CrossRef]
- Chretien, P.L.; Laurent, S.; Bornard, I.; Troulet, C.; El Maâtaoui, M.; Leyronas, C. Unraveling the infection process of garlic by Fusarium proliferatum, the causal agent of root rot. Phytopathol. Mediterr. 2020, 59, 285–293. [Google Scholar]
- Schwartz, H.F.; Mohan, S.K. Compendium of Onion and Garlic Diseases and Pests; APS Press: St. Paul, MN, USA, 2008. [Google Scholar]
- Delgado-Ortiz, J.C.; Ochoa-Fuentes, Y.M.; Cerna-Chávez, E.; Beltrán-Beache, M.; Rodríguez-Guerra, R.; Aguirre-Uribe, L.A.; Vázquez-Martínez, O. Patogenicidad de especies de Fusarium asociadas a la pudrición basal del ajo en el centro norte de México. Rev. Argent. Microbiol. 2016, 48, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Baker, S.E.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J. One fungus, one name: Defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 2013, 103, 400–408. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Lima, C.S.; Pfenning, L.H.; Costa, S.S.; Abreu, L.M.; Leslie, J.F. Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia 2012, 104, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Desjardins, A.E.; Moretti, A. Biological and chemical complexity of Fusarium proliferatum. In The Role of Plant Pathology in Food Safety and Food Security; Strange, R.N., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 97–111. [Google Scholar]
- Seifert, K.A.; Aoki, T.; Baayen, R.P.; Brayford, D.; Burgess, L.W.; Chulze, S.; Gams, W.; Geiser, D.; De Gruyter, J.; Leslie, J.F. The name Fusarium moniliforme should no longer be used. Mycol. Res. 2003, 107, 643–644. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Kenényi, Z.; Mulé, G.; Moretti, A.; Waalwijk, C.; Hornok, L. Fertility and mating type assessment within Fusarium proliferatum isolates from different host plants. In Proceedings of the the Seventh International Symposium, Poznán, Poland, 4–7 September 2002; pp. 55–68. [Google Scholar]
- Gálvez, L.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł.; Palmero, D. Fusarium proliferatum–Causal agent of garlic bulb rot in Spain: Genetic variability and mycotoxin production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Marín, P.; Callejas, C.; Moretti, A.; Vázquez, C.; González-Jaén, M.T. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 2010, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.D.O.; Reis, G.M.; Da Silva, V.N.; Braghini, R.; Teixeira, M.M.G.; Correa, B. Molecular characterization and fumonisin production by Fusarium verticillioides isolated from corn grains of different geographic origins in Brazil. Int. J. Food Microbiol. 2011, 145, 9–21. [Google Scholar] [CrossRef]
- Geiser, D.M.; del Mar Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiol. Lett. 2004, 230, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Jurado, M.; Vázquez, C.; Marín, S.; Sanchis, V.; González-Jaén, M.T. PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in maize. Syst. Appl. Microbiol. 2006, 29, 681–689. [Google Scholar] [CrossRef]
- Gálvez, L.; Clarkson, J.P.; Palmero, D. IGS region polymorphisms are responsible for failure of commonly used species-specific primers in Fusarium proliferatum isolates from diseased garlic. Plant Pathol. 2020, 69, 713–722. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Whitaker, B.K.; Laraba, I.; Proctor, R.H.; Brown, D.W.; Broders, K.; Kim, H.-S.; McCormick, S.P.; Busman, M.; Aoki, T.; et al. DNA sequence-based identification of Fusarium: A work in progress. Plant Dis. 2022, 106, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cruz, T.J.; Whitaker, B.K.; Proctor, R.H.; Broders, K.; Laraba, I.; Kim, H.-S.; Brown, D.W.; O’Donnell, K.; Estrada-Rodríguez, T.L.; Lee, Y.-H. FUSARIUM-ID v. 3.0: An updated, downloadable resource for Fusarium species identification. Plant Dis. 2022, 106, 1610–1616. [Google Scholar] [CrossRef]
- Nyvall, R.F.; Kommedahl, T. Individual thickened hyphae as survival structures of Fusarium moniliforme in corn. Phytopathology 1968, 58, 1704–1707. [Google Scholar]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Fernández, M.D.; Stenglein, S.A.; Dinolfo, M.I. Fusarium species richness in mono-and dicotyledonous weeds and their ability to infect barley and wheat. Mycol. Prog. 2021, 20, 1203–1216. [Google Scholar] [CrossRef]
- Ooka, J.; Kommedahl, T. Wind and rain dispersal of Fusarium moniliforme in corn fields. Phytopathology 1977, 67, 1023–1026. [Google Scholar] [CrossRef]
- Funnell-Harris, D.L.; Pedersen, J.F. Presence of Fusarium spp. in air and soil associated with sorghum fields. Plant Dis. 2011, 95, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Palmero, D.; Rodríguez, J.; De Cara, M.; Camacho, F.; Iglesias, C.; Tello, J. Fungal microbiota from rain water and pathogenicity of Fusarium species isolated from atmospheric dust and rainfall dust. J. Ind. Microbiol. Biotechnol. 2011, 38, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Gil-Serna, J.; Gálvez, L.; París, M.; Palmero, D. Fusarium proliferatum from rainwater and rooted garlic show genetic and pathogenicity differences. Eur. J. Plant Pathol. 2016, 146, 199–206. [Google Scholar] [CrossRef]
- Leslie, J.F.; Pearson, C.A.; Nelson, P.E.; Toussoun, T. Fusarium spp. from corn, sorghum, and soybean fields in the central and eastern United States. Phytopathology 1990, 80, 343–350. [Google Scholar] [CrossRef]
- Gaige, A.R.; Todd, T.; Stack, J. Interspecific competition for colonization of maize plants between Fusarium proliferatum and Fusarium verticillioides. Plant Dis. 2020, 104, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Dugan, F.M. Diseases and disease management in seed garlic: Problems and prospects. Am. J. Plant Sci. Biotechnol. 2007, 1, 47–51. [Google Scholar]
- Gilbert, J.; Fernando, W. Epidemiology and biological control of Gibberella zeae/Fusarium graminearum. Can. J. Plant Pathol. 2004, 26, 464–472. [Google Scholar] [CrossRef]
- Blok, W.J.; Bollen, G.J. Inoculum sources of Fusarium oxysporum f.sp. asparagi in asparagus production. Ann. Appl. Biol. 1996, 128, 219–231. [Google Scholar]
- Cramer, C.S. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Claflin, L.E. Fusarium root and stalk rot. In Compendium of Sorghum Diseases; Frederiksen, R.A., Odvody, G.N., Eds.; APS Press: St. Paul, MN, USA, 2000; pp. 28–30. [Google Scholar]
- Kintega, K.R.; Zida, P.E.; Soalla, R.; Tarpaga, V.W.; Sankara, P.; Sereme, P. Determination of Fusarium species associated with onion plants (Allium cepa) in field in Burkina Faso causing damping-off and bulb rots. Am. J. Plant Sci. 2020, 11, 64. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Ritieni, A.; Bottalico, A.; Corda, P. Occurrence and toxigenicity of Fusarium proliferatum from preharvest maize ear rot, and associated mycotoxins, in Italy. Plant Dis. 1995, 79, 727–731. [Google Scholar] [CrossRef]
- Conner, R.; Hwang, S.; Stevens, R. Fusarium proliferation: A new causal agent of black point in wheat. Can. J. Plant Pathol. 1996, 18, 419–423. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D.; Nelson, P.E. Production of fumonisin B and moniliformin by Gibberella fujikuroi from rice from various geographic areas. Appl. Environ. Microbiol. 1997, 63, 1838–1842. [Google Scholar] [CrossRef] [Green Version]
- Leslie, J.F. Gibberella fujikuroi: Available populations and variable traits. Can. J. Bot. 1995, 73, 282–291. [Google Scholar] [CrossRef]
- Elmer, W.H. Fusarium proliferatum as a causal agent in Fusarium crown and root rot of asparagus. Plant Dis. 1990, 74, 938. [Google Scholar] [CrossRef]
- Abdalla, M.; Al-Rokibah, A.; Moretti, A.; Mule, G. Pathogenicity of toxigenic Fusarium proliferatum from date palm in Saudi Arabia. Plant Dis. 2000, 84, 321–324. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, L.J.; Inglis, D.; Pelter, G. Fusarium proliferatum pathogenic on onion bulbs in Washington. Plant Dis. 2003, 87, 750. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Morita, Y.; Kashiwa, T.; Teraoka, T.; Arie, T. Fusarium proliferatum, an additional bulb rot pathogen of Chinese chive. J. Gen. Plant Pathol. 2013, 79, 431–434. [Google Scholar] [CrossRef]
- Merlington, A.; Hanson, L.; Bayma, R.; Hildebrandt, K.; Steere, L.; Kirk, W. First report of Fusarium proliferatum causing dry rot in Michigan commercial potato (Solanum tuberosum) production. Plant Dis. 2014, 98, 843. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, G.; Zhang, Y.; Zhang, J.; Zheng, H.; Jing, L.; Zhou, H.; Zhao, J. First report of sunflower wilt caused by Fusarium proliferatum in Inner Mongolia, China. Plant Dis. 2015, 99, 1275. [Google Scholar] [CrossRef]
- Arias, M.D.; Munkvold, G.; Leandro, L. First report of Fusarium proliferatum causing root rot on soybean (Glycine max) in the United States. Crop Prot. 2015, 67, 52–58. [Google Scholar]
- Shrestha, U.; Butler, D.; Ownley, B. First report of dry root and stem rot of cowpea caused by Fusarium proliferatum in the United States. Plant Dis. 2016, 100, 860. [Google Scholar] [CrossRef]
- Molnár, O. Fusarium proliferatum causing head blight on oat in Hungary. Eur. J. Plant Pathol. 2016, 146, 699–703. [Google Scholar] [CrossRef]
- Kamel, M.A.M.; Cortesi, P.; Saracchi, M. Etiological agents of crown rot of organic bananas in Dominican Republic. Postharvest Biol. Technol. 2016, 120, 112–120. [Google Scholar] [CrossRef]
- Pérez, B.; Berretta, M.; Carrión, E.; Wright, E. First report of root rot caused by Fusarium proliferatum on blueberry in Argentina. Plant Dis. 2011, 95, 1478. [Google Scholar] [CrossRef]
- Cong, L.; Sun, Y.; Kang, J.; Li, M.; Long, R.; Zhang, T.; Yang, Q. First report of root rot disease caused by Fusarium proliferatum on alfalfa in China. Plant Dis. 2016, 100, 2526. [Google Scholar] [CrossRef]
- Borrero, C.; Capote, N.; Gallardo, M.; Avilés, M. First report of vascular wilt caused by Fusarium proliferatum on strawberry in Spain. Plant Dis. 2019, 103, 581. [Google Scholar] [CrossRef]
- Ghuffar, S.; Irshad, G.; Zhai, F.; Aziz, A.; Asadullah, H.M.A.M.; Mehmood, N.; Yang, H.; Bashir, A.; Ahmed, M.Z.; Aslam, M.F. First report of Fusarium proliferatum causing fruit rot of grapes (Vitis vinifera) in Pakistan. Int. J. Phytopathol. 2018, 7, 85–88. [Google Scholar] [CrossRef]
- Zhu, Y.; Abdelraheem, A.; Sanogo, S.; Wedegaertner, T.; Nichols, R.; Zhang, J. First report of cotton (Gossypium) wilt caused by Fusarium proliferatum in New Mexico, USA. Plant Dis. 2019, 103, 2679. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Guo, L.; Yu, A.; Wang, X.; Xiang, W.; Zhao, J. First report of Fusarium proliferatum causing fruit rot on Muskmelon (Cucumis melo L.) in China. Plant Dis. 2021, 106, 1305. [Google Scholar] [CrossRef] [PubMed]
- Yan, L. First report of vascular wilt caused by Fusarium proliferatum on cauliflower in China. Plant Dis. 2020, 104, 3065. [Google Scholar] [CrossRef]
- Lahuf, A. First report of Fusarium proliferatum causing stem and root rot on lucky bamboo (Dracaena braunii) in Iraq. Plant Prot. J. 2019, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.; Li, H. First report of Fusarium proliferatum causing trunk canker on Ilex cornuta in China. Plant Dis. 2019, 103, 155. [Google Scholar] [CrossRef]
- Punja, Z.K. First report of Fusarium proliferatum causing crown and stem rot, and pith necrosis, in cannabis (Cannabis sativa L., marijuana) plants. Can. J. Plant Pathol. 2021, 43, 236–255. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Okello, P.N.; Mathew, F.M. Cross pathogenicity studies show South Dakota isolates of Fusarium acuminatum, F. equiseti, F. graminearum, F. oxysporum, F. proliferatum, F. solani, and F. subglutinans from either soybean or corn are pathogenic to both crops. Plant Health Prog. 2019, 20, 44–49. [Google Scholar] [CrossRef]
- Dissanayake, M.; Tanaka, S.; Ito, S. Fumonisin B1 production by Fusarium proliferatum strains isolated from Allium fistulosum plants and seeds in Japan. Lett. Appl. Microbiol. 2009, 48, 598–604. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A. Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Galván, G.A.; Koning-Boucoiran, C.F.; Koopman, W.J.; Burger-Meijer, K.; González, P.H.; Waalwijk, C.; Kik, C.; Scholten, O.E. Genetic variation among Fusarium isolates from onion, and resistance to Fusarium basal rot in related Allium species. Eur. J. Plant Pathol. 2008, 121, 499–512. [Google Scholar] [CrossRef]
- Palmero, D.; Gálvez, L.; García, M.; Gil-Serna, J.; Benito, S. The effects of storage duration, temperature and cultivar on the severity of garlic clove rot caused by Fusarium proliferatum. Postharvest Biol. Technol. 2013, 78, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Conci, V.; Nome, S.F. Virus free garlic (Allium sativum L.) plants obtained by thermotherapy and meristem tip culture. J. Phytopathol. 1991, 132, 186–192. [Google Scholar] [CrossRef]
- Yulianingsih, R.; Hidayat, S.; Dinarti, D. Elimination of Garlic common latent virus from garlic through meristem culture and thermotherapy. IOP Conf. Ser. Earth Environ. Sci. 2020, 468, 012028. [Google Scholar] [CrossRef]
- Roberts, P.; Matthews, W. Disinfection alternatives for control of Ditylenchus dipsaci in garlic seed cloves. J. Nematol. 1995, 27, 448. [Google Scholar]
- Zouhar, M.; Douda, O.; Dlouhý, M.; Lišková, J.; Maňasová, M.; Stejskal, V. Using of hydrogen cyanide against Ditylenchus dipsaci nematode present on garlic. Plant Soil Environ. 2016, 62, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Dugan, F.; Lupien, S.; Hellier, B. Infection by Fusarium proliferatum in aerial garlic bulbils is strongly reduced compared to rates in seed cloves when both originate from infected bulbs. Crop Prot. 2019, 116, 43–48. [Google Scholar] [CrossRef]
- Molinero-Ruiz, L.; Rubio-Pérez, E.; González-Domínguez, E.; Basallote-Ureba, M.J. Alternative hosts for Fusarium spp. causing crown and root rot of Asparagus in Spain. J. Phytopathol. 2011, 159, 114–116. [Google Scholar] [CrossRef]
- Gálvez, L. Etiología, Epidemiología y Estrategias de Control de la Podredumbre del Diente de ajo. Ph.D. Thesis, Agro-Environmental Technology for Sustainable Agriculture, Universidad Politécnica de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Cotten, T.; Munkvold, G. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Manzo, S.; Claflin, L. Survival of Fusarium moniliforme hyphae and conidia in grain Sorghum stalks. Plant Dis. 1984, 68, 866–867. [Google Scholar] [CrossRef]
- Taylor, A.; Vagany, V.; Barbara, D.J.; Thomas, B.; Pink, D.; Jones, J.; Clarkson, J.P. Identification of differential resistance to six Fusarium oxysporum f. sp. cepae isolates in commercial onion cultivars through the development of a rapid seedling assay. Plant Pathol. 2013, 62, 103–111. [Google Scholar]
- Reis, E.M.; Baruffi, D.; Remor, L.; Zanatta, M. Decomposition of corn and soybean residues under field conditions and their role as inoculum source. Summa Phytopathol. 2011, 37, 65–67. [Google Scholar] [CrossRef] [Green Version]
- Khonga, E.; Sutton, J. Inoculum production and survival of Gibberella zeae in maize and wheat residues. Can. J. Plant Pathol. 1988, 10, 232–239. [Google Scholar] [CrossRef]
- Onana, B.; Essono, G.; Nyegue, M.; Tchikoua, R.; Ambang, Z.; Ayodele, M. Natural occurrence of Fusarium species and fumonisins in stored cassava chips. Afr. J. Microbiol. Res. 2015, 9, 2257–2269. [Google Scholar]
- Liddell, C.M.; Burgess, L.W. Survival of Fusarium moniliforme at controlled temperature and relative humidity. Trans. Br. Mycol. Soc. 1985, 84, 121–130. [Google Scholar] [CrossRef]
- Nyvall, R.F.; Kommedahl, T. Saprophytism and survival of Fusarium moniliforme in corn stalks. Phytopathology 1970, 60, 1233–1235. [Google Scholar] [CrossRef]
- Han, X.; Cheng, Z.; Meng, H. Soil properties, nutrient dynamics, and soil enzyme activities associated with garlic stalk decomposition under various conditions. PLoS ONE 2012, 7, e50868. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V. Modelling the effect of weather on moisture fluctuations in maize stalk residues, an important inoculum source for plant diseases. Agric. For. Meteorol. 2015, 207, 83–93. [Google Scholar] [CrossRef]
- Reyes Gaige, A.; Giraldo, M.; Todd, T.; Stack, J.P. Growth and colonization of organic matter in soil by Fusarium proliferatum. Can. J. Plant Pathol. 2019, 41, 242–250. [Google Scholar] [CrossRef]
- BEST4SOIL. Una Red de Profesionales Creada Para Compartir Conocimientos Sobre la Prevención y Control de las Enfermedades Edáficas. Available online: https://www.best4soil.eu/ (accessed on 4 May 2022).
- Carrieri, R.; Raimo, F.; Pentangelo, A.; Lahoz, E. Fusarium proliferatum and Fusarium tricinctum as causal agents of pink rot of onion bulbs and the effect of soil solarization combined with compost amendment in controlling their infections in field. Crop Prot. 2013, 43, 31–37. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.I.; Melero-Vara, J.M.; Basallote-Ureba, M.J. Organic amendments conditions on the control of Fusarium crown and root rot of asparagus caused by three Fusarium spp. Span. J. Agric. Res. 2015, 13, 1009. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, R.A.; Evans, G.; Graz, M.; Marti, G.; Martínez, P.C.; Rogers, H.J.; Müller, C.T. From laboratory to industrial storage–Translating volatile organic compounds into markers for assessing garlic storage quality. Postharvest Biol. Technol. 2022, 191, 111976. [Google Scholar] [CrossRef]
- Marin, S.; Sanchis, V.; Magan, N. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maize. Can. J. Microbiol. 1995, 41, 1063–1070. [Google Scholar] [CrossRef]
- Lee, H.B.; Magan, N. The influence of environmental factors on growth and interactions between Embellisia allii and Fusarium oxysporum f. sp. cepae isolated from garlic. Int. J. Food Microbiol. 2010, 138, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmero, D.; Gálvez, L.; Bango, D.; Jurado, M.; De Cara, M. Evaluación del efecto del diferentes materias activas fungicidas sobre la inhibición del crecimiento micelial de Fusarium proliferatum. In Proceedings of the XVII Congreso de la Sociedad Española de Fitopatología, Lleida, Spain, 7–10 October 2014; p. 279. [Google Scholar]
- Mondani, L.; Chiusa, G.; Battilani, P. Chemical and biological control of Fusarium species involved in garlic dry rot at early crop stages. Eur. J. Plant Pathol. 2021, 160, 575–587. [Google Scholar] [CrossRef]
- Patón, L.G.; Marrero, M.D.R.; Llamas, D.P. In vitro and field efficacy of three fungicides against Fusarium bulb rot of garlic. Eur. J. Plant Pathol. 2017, 148, 321–328. [Google Scholar] [CrossRef]
- Müllenborn, C.; Steiner, U.; Ludwig, M.; Oerke, E.C. Effect of fungicides on the complex of Fusarium species and saprophytic fungi colonizing wheat kernels. Eur. J. Plant Pathol. 2008, 120, 157–166. [Google Scholar] [CrossRef]
- Amini, J.; Sidovich, D. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Hoffmann, L.; Beyer, M. Evidence for natural resistance towards trifloxystrobin in Fusarium graminearum. Eur. J. Plant Pathol. 2011, 130, 239–248. [Google Scholar] [CrossRef]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Casanova, A.; Hoffmann, L.; Beyer, M. Differences between the succinate dehydrogenase sequences of isopyrazam sensitive Zymoseptoria tritici and insensitive Fusarium graminearum strains. Pestic. Biochem. Physiol. 2013, 105, 28–35. [Google Scholar] [CrossRef]
- Pasquali, M.; Spanu, F.; Scherm, B.; Balmas, V.; Hoffmann, L.; Hammond-Kosack, K.E.; Beyer, M.; Migheli, Q. FcStuA from Fusarium culmorum controls wheat foot and root rot in a toxin dispensable manner. PLoS ONE 2013, 8, e57429. [Google Scholar] [CrossRef] [Green Version]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? Fungicide Resistance Action Committee: Belgium, Brussels, 1995. [Google Scholar]
- Fought, L.; Musson, G.H.; Young, H. Fluopyram fungicides for the control of diseases of horticultural and row crops. Phytopathology 2011, 101, S54. [Google Scholar]
- Avenot, H.F.; Thomas, A.; Gitaitis, R.D.; Langston, D.B.; Stevenson, K.L. Molecular characterization of boscalid-and penthiopyrad-resistant isolates of Didymella bryoniae and assessment of their sensitivity to fluopyram. Pest Manag. Sci. 2012, 68, 645–651. [Google Scholar] [CrossRef]
- Amiri, A.; Heath, S.M.; Peres, N.A. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2014, 98, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Maitlo, S.; Syed, R.; Rustamani, M.; Khuhro, R.; Lodhi, A. Comparative efficacy of different fungicides against Fusarium wilt of chickpea (Cicer arietinum L.). Pak. J. Bot. 2014, 46, 2305–2312. [Google Scholar]
- Chen, Y.; Zhang, A.F.; Gao, T.C.; Zhang, Y.; Wang, W.X.; Ding, K.J.; Chen, L.; Sun, Z.; Fang, X.Z.; Zhou, M.G. Integrated use of pyraclostrobin and epoxiconazole for the control of Fusarium head blight of wheat in Anhui Province of China. Plant Dis. 2012, 96, 1495–1500. [Google Scholar] [CrossRef] [Green Version]
- Marin, P.; de Ory, A.; Cruz, A.; Magan, N.; Gonzalez-Jaen, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef]
- Ivić, D.; Sever, Z.; Kuzmanovska, B. In vitro sensitivity of Fusarium graminearum, F. avenaceum and F. verticillioides to carbendazim, tebuconazole, flutriafol, metconazole and prochloraz. Pestic. Fitomed. 2011, 26, 35–42. [Google Scholar] [CrossRef]
- Vyas, S.C. Nontarget Effects of Agricultural Fungicides; CRC Press, Inc.: Boca Raton, FL, USA, 1988. [Google Scholar]
- Palmero, D.; de Cara, M.; Galvez, L.; Tello, J. Effect of pre-sowing treatment on postharvest garlic rot caused by Fusarium proliferatum. In Proceedings of the International Congress of Postharvest Pathology, Lleida, Spain, 11–14 April 2011; p. 156. [Google Scholar]
- Bjelić, D.; Ignjatov, M.; Marinković, J.; Milošević, D.; Nikolić, Z.; Gvozdanović-Varga, J.; Karaman, M. Bacillus isolates as potential biocontrol agents of Fusarium clove rot of garlic. Zemdirb. Agric. 2018, 105, 369–376. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Wu, J.; Yu, J.; Xu, M.; Chen, D.; Zhang, Z.; Li, X.; Chi, Y.; Wan, S. In vitro inhibitory effect of the bacterium Serratia marcescens on Fusarium proliferatum growth and fumonisins production. Biol. Control 2020, 143, 104188. [Google Scholar] [CrossRef]
- Ahmed, N.G.; Gouda, H.; Hussein, M. Efficiency of silver nanoparticles synthesized by using Pleurotus ostreatus nanoparticles to manage fungal garlic cloves rot. SVU-Int. J. Agric. Sci. 2022, 4, 211–222. [Google Scholar] [CrossRef]
| Year | Symptom | Species | Country | Reference |
|---|---|---|---|---|
| 1976 | Basal plate rot | F. culmorum | United States | Schwartz and Mohan [41] |
| 1986 | Bulb rot | F. oxysporum | Japan | Matuo et al. [21] |
| 1990 | Bulb rot | F. proliferatum var. minus | Hungary | Simey [22] |
| 2003 | Bulb rot | F. proliferatum | United States | Dugan et al. [24] |
| 2004 | Bulb rot | F. proliferatum | Germany | Seefelder et al. [13] |
| 2004 | Seedling | F. oxysporum, F. moniliforme, F. solani | Bulgaria | Koleva [23] |
| Bulb rot | F. oxysporum, F. culmorum | |||
| 2007 | Seedling root rot | F. proliferatum | Serbia | Stankovic et al. [17] |
| 2007 | Bulb rot | F. proliferatum, F. verticillioides, F. oxysporum | United States | Dugan et al. [35] |
| 2010 | Bulb rot | F. proliferatum | Spain | Palmero et al. [27] |
| 2012 | Bulb rot | F. proliferatum | Italy | Tonti et al. [10] |
| 2012 | Bulb rot | F. proliferatum | India | Sankar et al. [34] |
| 2013 | Bulb rot | F. proliferatum | Argentina | Salvalaggio et al. [25] |
| 2013 | Clove germination and damping-off | F. proliferatum, F. oxysporum, F. solani | Egypt | Moharam et al. [31] |
| Bulb rot | ||||
| 2013 | Bulb rot | F. proliferatum | México | Fuentes et al. [26] |
| 2016 | Basal plate rot | F. proliferatum, F. verticillioides, F. oxysporum, F. solani, F. acuminatum | México | Delgado-Ortiz et al. [42] |
| 2017 | Clove germination and seedling wilt | F. proliferatum | Egypt | Elshahawy et al. [32] |
| Bulb rot | ||||
| 2018 | Bulb rot | F. proliferatum | France | Leyronas et al. [28] |
| 2018 | Bulb rot | F. proliferatum | Serbia | Ignjatov et al. [29] |
| 2021 | Bulb rot | F. proliferatum, F. oxysporum, F. solani | Spain | Gálvez and Palmero [9] |
| 2021 | Bulb rot | F. proliferatum, F. oxysporum | France | Chrétien et al. [2] |
| 2021 | Basal plate rot | F. proliferatum, F. oxysporum | Italy | Mondani et al. [39] |
| 2021 | Basal plate rot | F. proliferatum, F. oxysporum | Italy | Mondani et al. [39] |
| Bulb rot | Mondani et al. [16] | |||
| 2021 | Bulb rot | F. proliferatum | Russia | Anisimova et al. [33] |
| 2021 | Bulb rot | F. proliferatum | Slovakia | Horáková et al. [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gálvez, L.; Palmero, D. Fusarium Dry Rot of Garlic Bulbs Caused by Fusarium proliferatum: A Review. Horticulturae 2022, 8, 628. https://doi.org/10.3390/horticulturae8070628
Gálvez L, Palmero D. Fusarium Dry Rot of Garlic Bulbs Caused by Fusarium proliferatum: A Review. Horticulturae. 2022; 8(7):628. https://doi.org/10.3390/horticulturae8070628
Chicago/Turabian StyleGálvez, Laura, and Daniel Palmero. 2022. "Fusarium Dry Rot of Garlic Bulbs Caused by Fusarium proliferatum: A Review" Horticulturae 8, no. 7: 628. https://doi.org/10.3390/horticulturae8070628
APA StyleGálvez, L., & Palmero, D. (2022). Fusarium Dry Rot of Garlic Bulbs Caused by Fusarium proliferatum: A Review. Horticulturae, 8(7), 628. https://doi.org/10.3390/horticulturae8070628








