Abstract
The effect of a plant growth-promoting bacterium (PGPB) Bacillus sp. V2026, a producer of indolyl-3-acetic acid (IAA) and gibberellic acid (GA), on the ontogenesis and productivity of four genotypes of early-maturing spring wheat was studied under controlled conditions. The inoculation of wheat plants with Bacillus sp. V2026 increased the levels of endogenous IAA and GA in wheat of all genotypes and the level of trans-Zeatin in Sonora 64 and Leningradskaya rannyaya cvs but decreased it in AFI177 and AFI91 ultra-early lines. Interactions between the factors “genotype” and “inoculation” were significant for IAA, GA, and trans-Zeatin concentrations in wheat shoots and roots. The inoculation increased the levels of chlorophylls and carotenoids and reduced lipid peroxidation in leaves of all genotypes. The inoculation resulted in a significant increase in grain yield (by 33–62%), a reduction in the time for passing the stages of ontogenesis (by 2–3 days), and an increase in the content of macro- and microelements and protein in the grain. Early-maturing wheat genotypes showed a different response to inoculation with the bacterium Bacillus sp. V2026. Cv. Leningradskaya rannyaya was most responsive to inoculation with Bacillus sp. V2026.
1. Introduction
Under conditions of rapid population growth and climate change, it is essential to ensure food security by increasing the productivity of strategically important grain crops. Wheat is cultivated in many regions of the world, providing more than 50% of dietary energy needs [1]. For wheat plants, early maturity is one of the major mechanisms of avoiding damage by phytopathogens, the destructive effects of summer droughts and dry hotwinds, late spring and early autumn frosts, and the harm associated with excessive moisture during grain maturing [2,3,4]. The developmental rate of soft wheat is mainly ensured by VRN and PPD genetic systems, which control the hereditary variability of plant response to vernalization and photoperiod [5,6,7]. VRN and PPD genes and their combinations affecting growing season duration and heading time could also affect the productivity of common wheat [3,8,9]. A negative correlation between earliness and the number of wheat grains, productive tilling, thousand-grain weight, and the harvest index capacity has been noted [2,10,11,12]. Therefore, increasing the yield of early-maturing wheat cultivars and lines is an urgent problem for agriculture.
A possible way of increasing the productivity of grain crops in sustainable agriculture is the use of biological preparations based on PGPB [13,14,15,16]. PGPBs stimulate plant growth and development through various mechanisms: (1) directly affecting plant growth, e.g., by phytohormone production [17,18,19], ACC deaminase activity, nitrogen-fixing activity [19,20,21,22], and solubilization of potassium, phosphorus, zinc, etc.; and (2) affecting plant growth indirectly through the production of hydrolytic enzymes, HCN, siderophores, and antibiotics [18,23], induced systemic resistance, and biofilm formation [22,24,25]. Up to 80% of bacteria inhabiting the plant root zone (rhizobacteria) synthesize auxins, which stimulate root cell proliferation and increase host plant uptake of minerals and nutrients from the soil [26]. PGPBs can also produce cytokinins, gibberellins, or both for plant growth promotion [17,23,27,28].
Bacteria from the genus Bacillus are among the particularly promising PGPBs. Besides the rhizosphere, they can also live on the surface of the aboveground organs of plants and within plant tissues [15,29]. Bacillus spp. promote plant growth by producing phytohormones, siderophores, lipopeptides, polysaccharides, and enzymes [17,20,23,25]. They also affect plant homeostasis by regulating the proportion of antioxidant enzymes, both under natural plant growth conditions and under various stresses [21,30,31]. Most studies have reported positive effects of Bacillus spp. on wheat growth and productivity [14,32,33,34]. However, the effects of PGPB, and Bacillus spp. in particular, on the rate of wheat development are currently poorly understood and require detailed study. The results available in the literature are contradictory, with both the acceleration of developmental phases in different plants under the influence of PGPB [35,36,37,38] and delayed development [39,40] being reported. These controversial results may be due to different plant hormonal changes caused by PGPB since phytohormones play a pivotal role in various developmental processes in plants [41]. The response of early-maturing spring wheat to bacterial inoculation is not clear because there are insufficient results in the literature on the inoculation with PGPB of early spring wheat. A slight stimulation of the root growth of early-maturing wheat cv. Kazakhstanskaya 10, when inoculated with bacteria Bacillus subtilis 11 BM, was noted on the 30th day of growing [42].
The application of exogenous GA3 significantly promoted the elongation of the root, stem, and leaf cells [43], enhanced expression of cell elongation genes [44], promoted GA biosynthesis [45], and shortened germination time [46] and the time to flowering [47,48] in various plants, including wheat. We proposed that GA-producing Bacillus sp. V2026 can reduce the duration of developmental phases of early-maturing wheat by increasing plant endogenous GA. Various wheat genotypes have been studied to select the optimal yield genotype/bacterial combination. The optimization of such a combination is important to achieve higher wheat productivity.
The paper presents a study of the effect of Bacillus sp. V2026 bacteria on the hormonal status and the development of different genotypes of early-maturing wheat.
2. Results
2.1. Bacterial Identification
The bacterium was isolated from the rhizosphere of wheat plants of cv. Leningradskaya 6. The bacterium was a Gram-positive single spore-forming bacillus identified by 16S rRNA and ITS fragment as Bacillus sp. V2026 (the sequences were submitted to the NCBI databases with accession numbers OM764631 and OM855550, respectively). This bacterium is catalase-positive and oxidase-negative; indole and H2S are not produced. The Voges–Proskauer reaction is negative. The bacterium can utilize glucose, sucrose, xylose, arabinose, maltose, sorbitol, and mannitol. Bacillus sp. V2026 showed antifungal activity against the phytopathogenic micromycetes Fusarium oxisporum and Fusarium culmorum (zone with diameters of 30–40 mm). Bacillus sp. V2026 did not mobilize phosphates. The bacterium showed a phytohormonal activity, producing 43.09 ± 0.35 µg/mL IAA and 20.8 ± 0.41 ng/mL GAS3, and did not produce tZ (Table S1).
2.2. Identification of Alleles VRN-1 and PPD-D1 Loci
The main loci of the VRN and PPD genetic systems that determine different degrees of sensitivity to vernalization and photoperiod and, consequently, different rates of development were identified in the plant material using molecular markers specific to the VRN and PPD genes (Figure S1).
Molecular genetic analysis showed that ultra-early lines AFI177 and AFI91 gave four PCR fragments: 715-bp and 624-bp for Vrn-A1a alleles, 1124-bp for the Vrn-B1a allele, and 1671-bp for the Vrn-D1 allele. The cultivars S64 and LR contain PCR products 1671-bp and 1124-bp, respectively, which may correspond to the Vrn-D1 and Vrn-B1 alleles. We also found that the tested cultivars S64 and LR contain the Vrn-A1a allele since all of them gave 715-bp and 624-bp PCR products with primers VRN1AF//VRN1-1R. Cv. S64 yielded a 414-bp PCR fragment, indicating the presence of the photoperiod-insensitive Ppd-D1a allele. Cv. LR differed from the other genotypes by the presence of a 288-bp fragment, indicative of the photoperiod-sensitive PPD-D1b allele (Figure S1).
2.3. Yield and Yield Components
The effects of inoculation of the Bacillus sp. V2026 on the productivity components of wheat plants are presented in Table 1.

Table 1.
Effect of inoculation with Bacillus sp. V2026 on yield attributes of early-maturing spring wheat.
The results suggest that yield increase after bacterial inoculation was mainly due to an increase in productive tilling capacity: the maximum value was observed in early-maturing cultivars S64 and LR, by 24.6% and 27.3%, respectively (Table 1). Plants of cv. LR characterized by the highest tilling capacity were the most responsive to inoculation judging by this characteristic. The proportion of productive tilling increased in inoculated plants of lines AFI177 and AFI91 by 21.1% and 15.0%, respectively. The effect of inoculation with Bacillus sp. V2026 on plant height was observed only in the AFI91 line; the height increased by 5.8%, but no significant effect on the height of S64, LR, and AFI177 plants was noted.
Spike length significantly increased in inoculated S64 and LR plants: by 9.0% and 6.3%, respectively. Moreover, plants of these cultivars had a longer spike in the control variant. Plants of AFI91 and AFI177, which had a shorter spike, had no significant effect of Bacillus sp. V2026 on the length of the spike.
The number of spikelets in the spike statistically increased significantly in inoculated plants of all genotypes, from 5.6% in AFI91 to 7.5% in LR. Inoculation with Bacillus sp. V2026 was strongly influenced by increasing the number of grains of the spike in early-maturing cultivars S64 and LR (by 21.0% and 16.7%, respectively) than in ultra-early-maturing lines AFI91 and AFI177 (by 8.8% and 8.9%, respectively). The responsiveness of AFI91 and AFI177 plants to inoculation with Bacillus sp. V2026 by grain weight of the spike (22.3% and 27.0%, respectively) was greater than S64 and LR plants (17.2% and 13.5%, respectively).
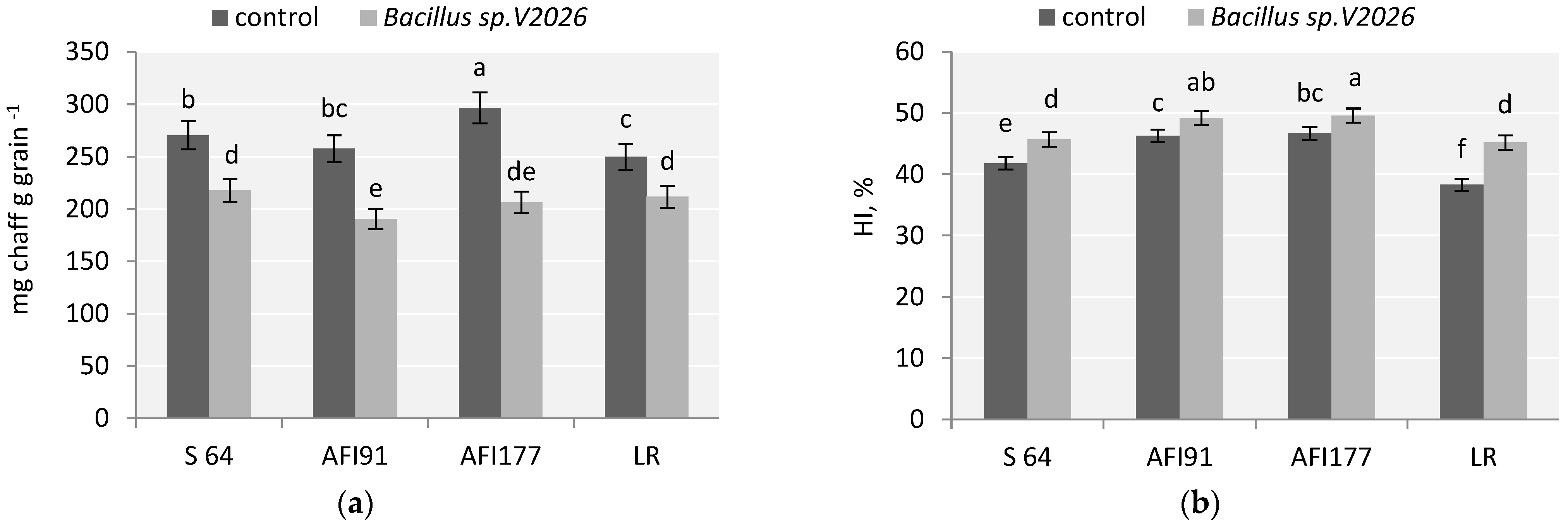
A stimulating effect of Bacillus sp. V2026 on the number of tillers and spike productivity contributed to a higher number of grains per plant (from 16.6% in API177 and up to 57.8% in LR) and grain weight per plant (from 32.5% in S64 and up to 62.2%, in LR) in all early-maturing genotypes. Inoculation with Bacillus sp. V2026 resulted in a greater decrease in the ratio of chaff in the spike of AFI91 and AFI177 plants (by 26.2% and 30.5%, respectively) than in the spike of S64 (19.5%) and LR (15.2%) plants (Figure 1a).
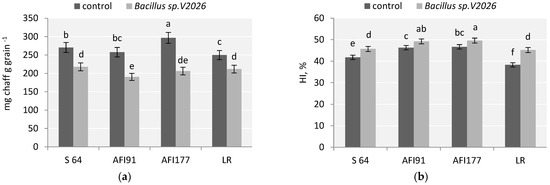
Figure 1.
Effect of inoculation with Bacillus sp. V2026 on chaff to grain ratio (a) and harvest index (HI) (b). The bars are means of two experiments with 50 biological replications per variant. Bars show ± SEM, and different letters (a–f) show a significant difference at the p ≤ 0.05 level, as determined by Duncan’s multiple test.
An increase in wheat grain yield after inoculation with Bacillus sp. V2026 was accompanied by an increase in total biomass (Table 1). Ultra-early-maturing lines AFI91 and AFI177 were the most responsive to bacterial treatment in terms of total dry weight of plants and straw yield, the latter increasing by 27.1% and 26.5%, respectively. HI (harvest index) in the control varied from 38.3% in LR to 47.7% in AFI177. Inoculation with Bacillus sp. V2026 led to a statistically significant increase in HI in all studied cultivars and wheat lines, from 4.2% in AFI177 plants to 18.4% in LR plants (Figure 1b). Two-way ANOVA analysis revealed that all wheat yield components examined in our study were statistically significantly influenced (p ≤ 0.05) by both genotypic differences and inoculation with Bacillus sp. V2026, whereas the interaction of these factors influenced only grain number per spike, number and weight of grains per plant, and HI (Table S2).
2.4. Protein and Macronutrient/Micronutrient Content in Wheat Grain
Genotype-related variation in the macro- and microelement content of grain of early-maturing wheat genotypes investigated in our study was relatively low (Table 2).

Table 2.
Effect of inoculation with Bacillus sp. V2026 on macro- and micronutrient content of wheat grain.
Analysis of the experimental data showed that inoculation with Bacillus sp. V2026 had a strong influence on the accumulation of mineral macronutrients (N, P, K) and micronutrients (Fe, Mg, Zn, and Mn) in wheat grain (Table 2). Significant differences between the genotypes were found for the majority of analyzed mineral elements involved in the analysis. The results suggest that bacterial treatment increases N content from 6.2% in cv. LR to 20.1% in line AFI177 and in K content from 6.9% in cv. LR to 22.9% in cv. S64. Statistically significant differences in the concentration of P in the grain were found in plants of ultra-early lines AFI91 (10.7%) and AFI177 (9.7%), while in plants of cv. S64 and cv. LR, this increase remained insignificant. AFI91 and AFI177 lines were also the most responsive to inoculation with Bacillus sp. V2026 in respect of the content of Fe, Mg, Zn, and Mn in grain. In cv. S64, inoculation resulted in significant changes in Mn concentration (7.2%) but did not have a strong influence on Fe, Mg, and Zn concentrations. Cv. LR was the least sensitive to Bacillus sp. V2026 in terms of changes in concentration of microelements in the grain.
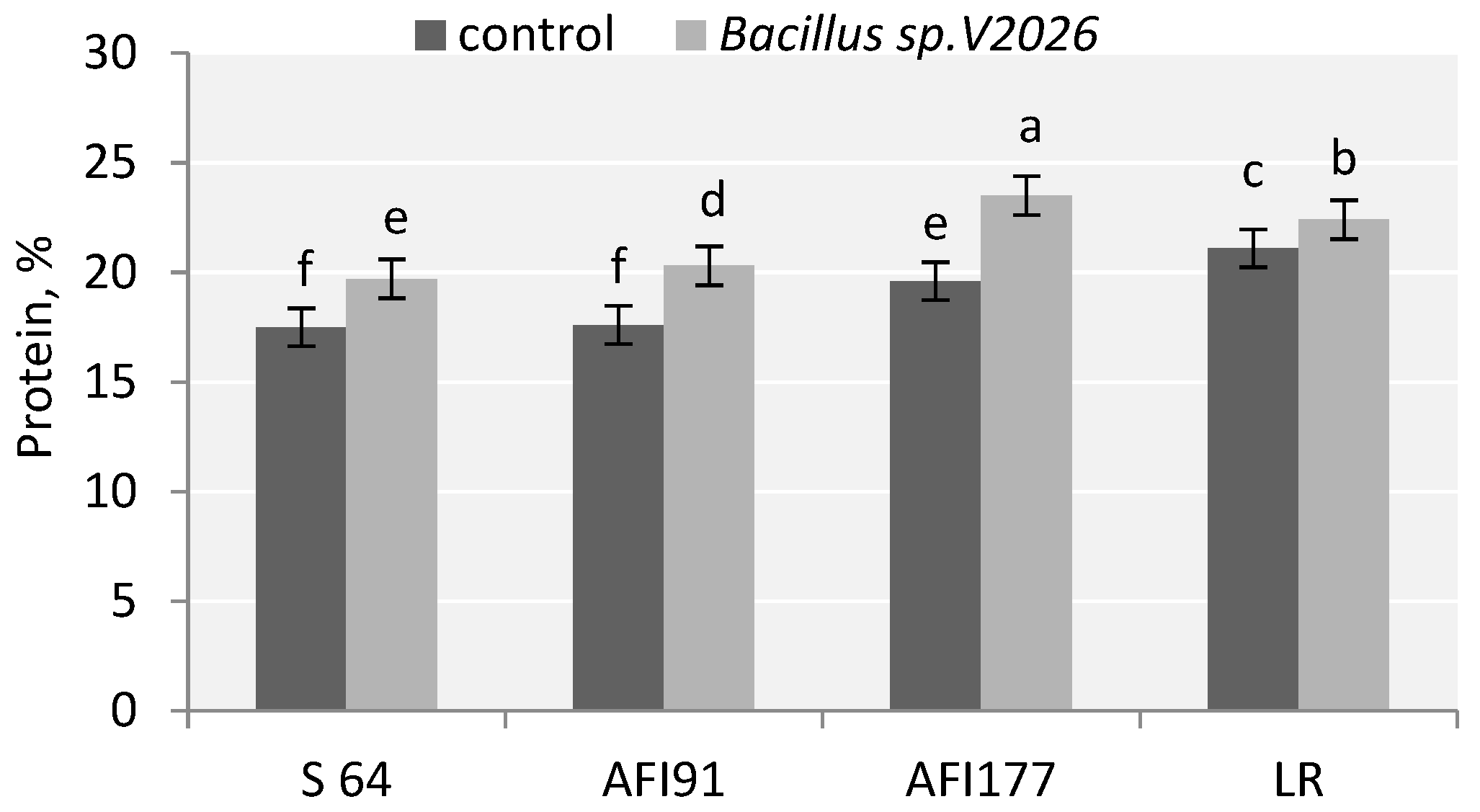
Inoculation with Bacillus sp. V2026 had an effect on grain quality: the protein content in the grain increased significantly in all early-maturing genotypes. These changes were more discovered in lines AFI177 (19.9%) and AFI91 (15.3%) (Figure 2). At the same time, LR and AFI177 genotypes were characterized by a higher grain protein content than S64 and AFI91 genotypes, both in the control variant and in the variant with treatment.
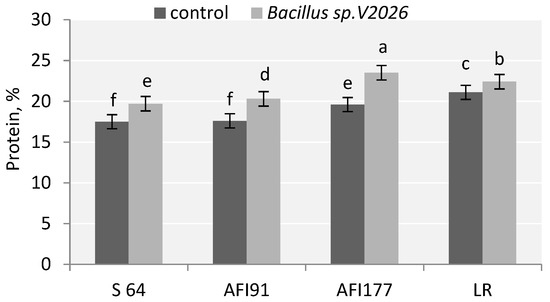
Figure 2.
Effect of inoculation with Bacillus sp. V2026 on content of grain protein of early-maturing wheat. Bars with different letters are significantly different at p ≤ 0.05, as determined by Duncan’s multiple range test.
2.5. Duration of Developmental Phases
Our results showed that the difference in the heading time between ultra-early lines AFI177 and AFI91 and early-maturing cultivars S64 and LR made up 5–9 days (Table 3). Plants of cv. S64 and cv. LR had a longer “stem elongation–heading” period and a shorter “heading–maturing” period compared with plants of the AFI91 and AFI177 lines.

Table 3.
Effect of inoculation with Bacillus sp. 2026 on the duration of developmental phases of early-maturing spring wheat.
We also assessed the effects of inoculation with Bacillus sp. 2026 on the pattern of development of early-maturing genotypes (Table 3). Inoculation with the bacterium significantly shortened the time to reach each growth stage in early-maturing genotypes. Maturation of inoculated plants occurred on average 2–3 days earlier than in control plants, the difference being significant. The number of days from seedling to each growth stage was less in the API91 line than in other genotypes. It is also noteworthy that cv. LR was the most responsive to inoculation with Bacillus sp. 2026, showing the largest reduced duration of the period from seedling to maturing. Heading of inoculated plants of cv. S64, API177, API91, and cv. LR occurred earlier, by 1.5, 2.4, 1.4, and 3.1 days, respectively, than in plants without bacterial treatment, with the cycle reduction being greater from seedlings to stem elongation. Cv. S64, API91, and cv. LR were not responsive to Bacillus sp. V2026 inoculation at the stage of “stem elongation–heading.” The duration of the period from heading to maturing in all four genotypes practically did not differ in the control and experimental variants.
Two-way ANOVA analysis showed that the factor “genotype” had a significant impact on the duration of all studied periods of wheat ontogenesis. At the same time, only genotypic differences contributed significantly to variability in the periods “stem elongation–heading” and “heading–maturing.” The factor “Bacillus sp. V2026” significantly influenced the duration of the initial stages of ontogenesis from seedlings to tillering and from tillering to stem elongation, as well as the timing of heading and maturing (Table S2). The interaction of the factors had a significant effect on the duration of the seedlings–stem elongation and seedlings–heading periods.
2.6. Seedling Growth
Inoculation with Bacillus sp. V2026 increased root and shoot lengths in 14-day-old wheat seedlings of early-maturing genotypes (Figure S2). The relative increase in root length in LR, S64, AFI91, and AFI177 seedlings due to bacterial inoculation made up 20.7%, 11.4%, 20.4%, and 19.1%, respectively, compared with the control (Table 4). The root biomass in these variants increased from 11.0% (S64) to 23.0% (AFI177). AFI177 and LR were more responsive to inoculation. The responsiveness of shoots to the action of the bacterium was less pronounced. The shoot length of cv. LR and cv. S64 treated with Bacillus sp. V2026 was 10.3% and 5.9%, respectively, greater than in control plants. Inoculated plants of AFI177 and AFI91 lines had no statistically significant differences in shoot length compared with the controls (Table 4). A significant increase in shoot biomass after inoculation was observed in AFI177, LR, and S64 seedlings: by 18.8%, 13.4%, and 8.7%, respectively. Thus, inoculation with Bacillus sp. V2026 stimulated the accumulation of root biomass more considerably than the accumulation of shoot biomass. The root/shoot weight ratio increased from 2.9% (S64) to 7.5% (LR).

Table 4.
Effect of Bacillus sp. V2026 on wheat roots and shoots of early-maturing wheat in hydroponic conditions.
The wheat genotypes involved in our study responded to inoculation with Bacillus sp. V2026 in a different manner. Cv. LR and line AFI177 were more sensitive to inoculation with this strain. Inoculation stimulated roots and shoots of LR seedlings, shoots of AFI177 seedlings, and roots of AFI91 seedlings. Cv. S64 was the least responsive to bacterial inoculation compared with other cultivars and lines.
2.7. Photosynthetic Pigments and Lipid Peroxidation
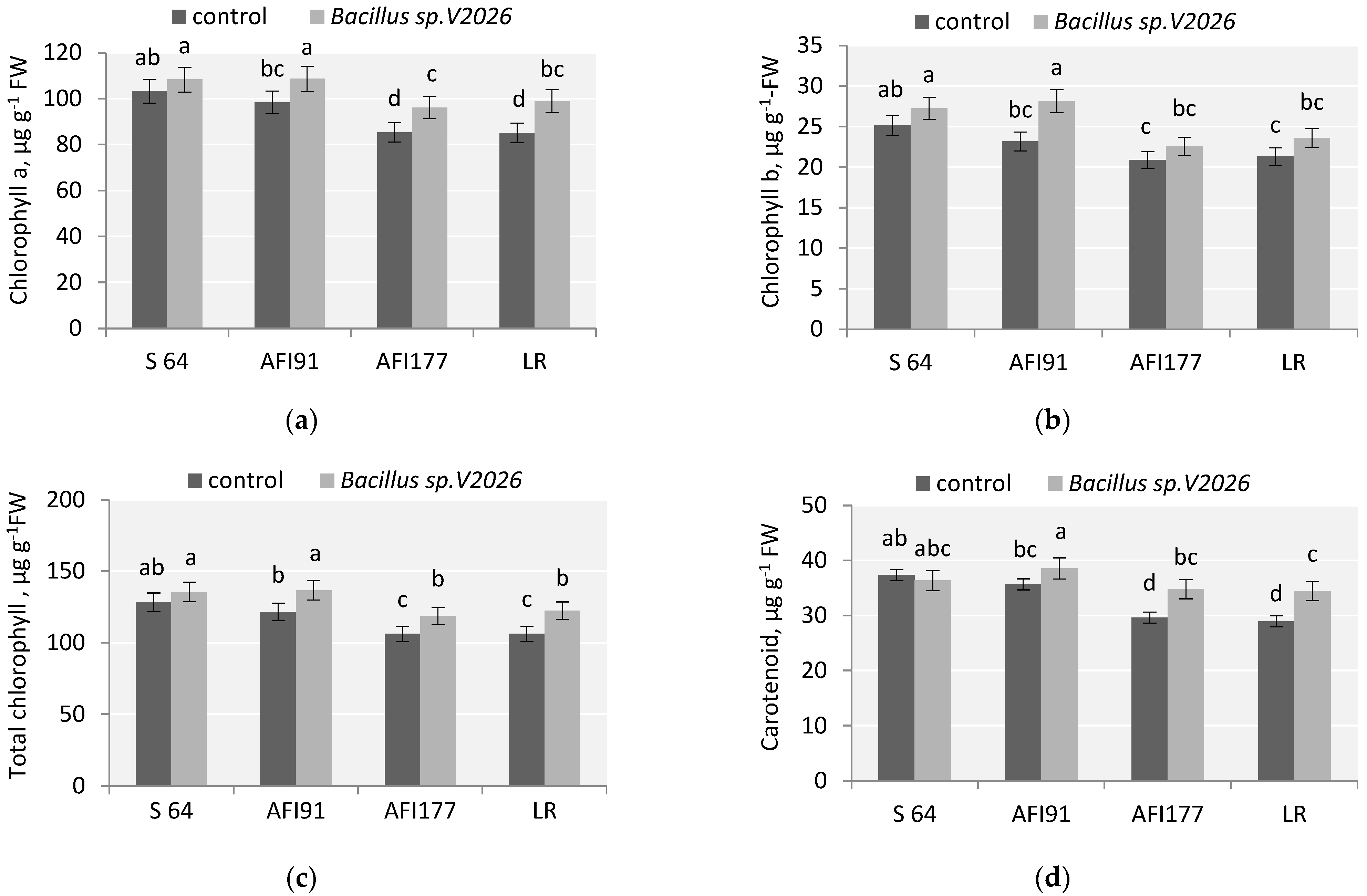
The physiological state of plants after inoculation with Bacillus sp. V2026 was determined by measuring the chlorophyll content and the level of malonic dialdehyde (MDA). Data on the effect of Bacillus sp. V2026 on various biochemical parameters are shown in Figure 3 and Figure 4. The concentration of chlorophyll and carotenoids in leaves significantly increased after bacterial treatment (Figure 3). The increase in total chlorophyll (Chl) and chlorophyll a (Chl a) in samples of AFI91, AFI177, and LR made up, respectively: 12.5%; 11.8%, 15.2% and 10.5%, 12.7%, 16.4% (Figure 3). A statistically significant increase in chlorophyll b (Chl b) levels (21.1%) was observed only in inoculated seedlings of the ultra-early-maturing line AFI91. No significant changes in the content of total Chl, Chl a, and Chl b in the leaves of inoculated S64 plants were noted. An increase in carotenoid content (Car) was observed in LR, AFI177, and AFI91 plants, by 19.4%, 17.6%, and 8.1%, respectively, as compared with the controls. In S64 plants, the level of Car tended to decrease after inoculation. The highest average content of total chlorophyll was registered in cv. S64 and line AFI91 in both the control and the experimental variants.
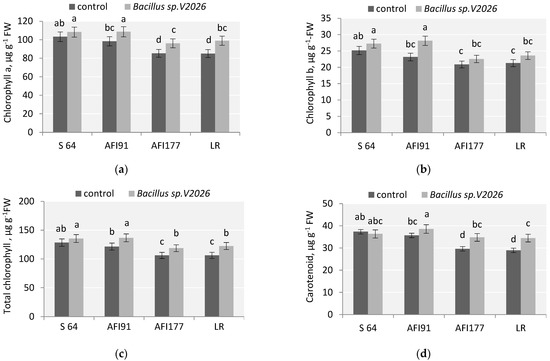
Figure 3.
Effect of Bacillus sp. V2026 on chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), and carotenoid (d) content of early-maturing wheat plants grown under hydroponic conditions. Bars with different letters are significantly different at p ≤ 0.05, as determined by Duncan’s multiple range test.
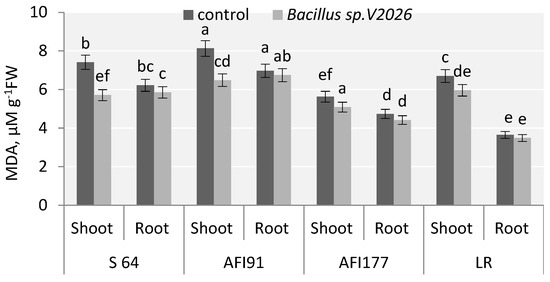
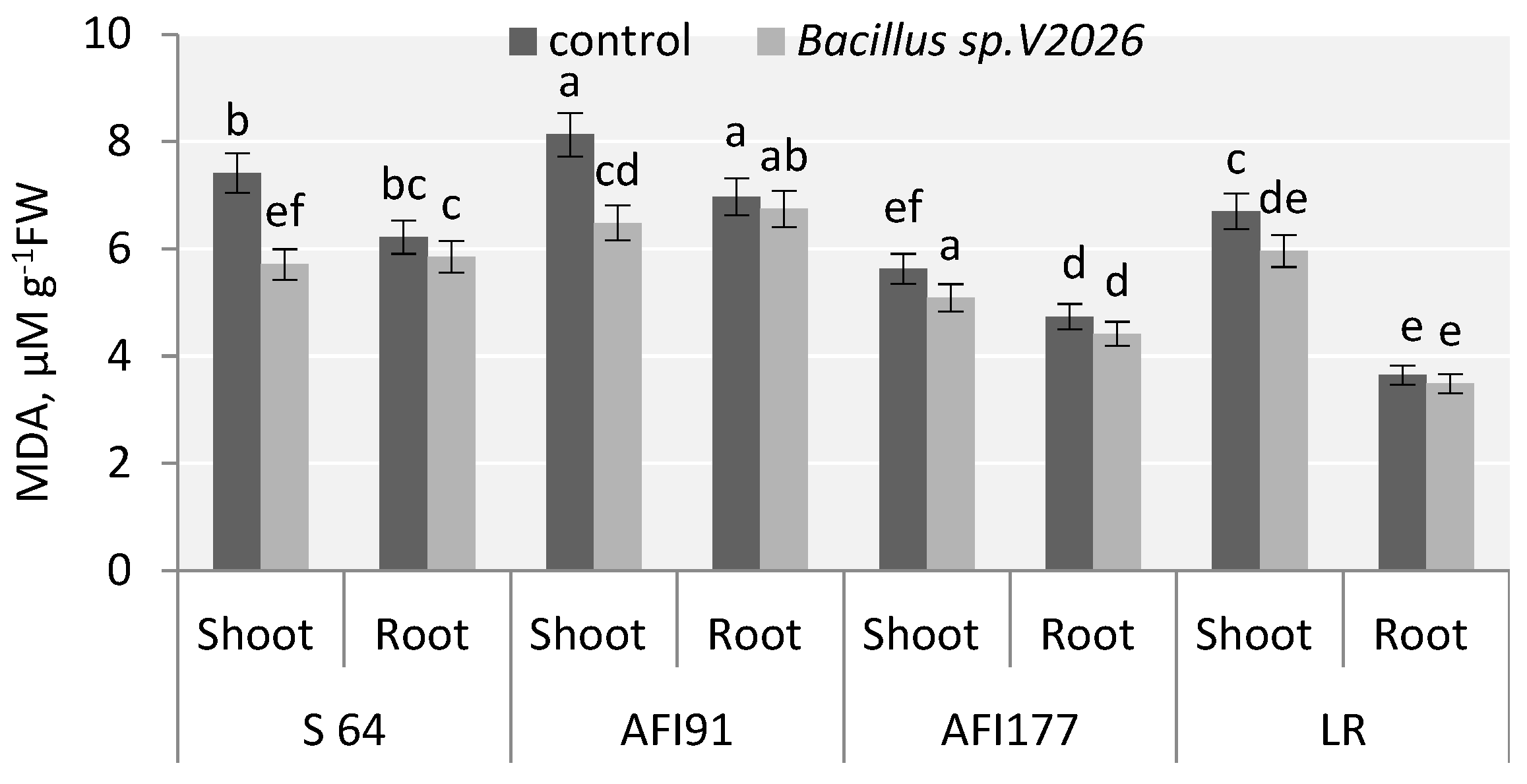
Figure 4.
Effect of Bacillus sp. V2026 on malondialdehyde (MDA) content of early-maturing wheat plants grown under hydroponic conditions. Bars with different letters are significantly different at p ≤ 0.05, as determined by Duncan’s multiple range test.
One of the metabolites of lipid peroxidation is malondialdehyde (MDA); a statistically significant decrease in MDA level in the shoots of inoculated plants was registered. The decrease was the most obvious in S64 and AFI91 plants: by 22.9% and 20.3%, respectively (Figure 4). Inoculation with Bacillus sp. V2026 tended to decrease MDA levels in the roots of all genotypes involved in the study, ranging from 3.3% in AFI91 to 6.8% in AFI177.
2.8. Endogenous Levels of Plant Hormones in Seedlings
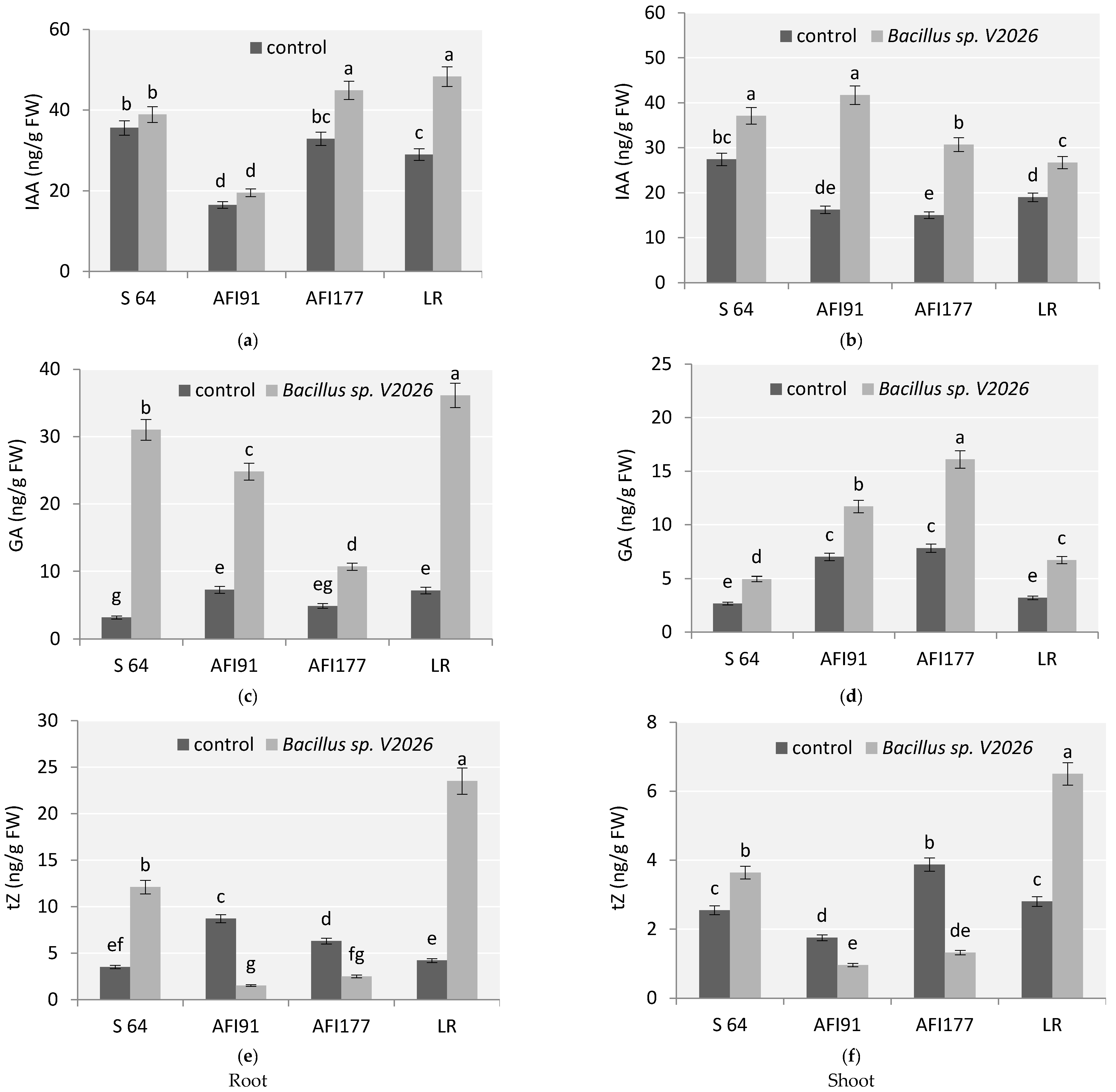
This study revealed that with inoculation Bacillus sp. V2026, the content of endogenous hormones in wheat plants significantly varied depending on the genotype (Figure 5). An increase in indolyl-3-acetic acid (IAA) and gibberellic acid (GA) content was observed for all genotypes studied. The most evident change in IAA levels was noted in the roots of cv. LR (by 66%) (Figure 5a) and shoots of lines AFI177 and AFI91 (2–2.5-fold, respectively) (Figure 5b). A high level of basal auxins in shoots was detected in cv. S64 (Figure 5b). Plants of line AFI91 differed significantly from other genotypes in having a reduced basal level of IAA in the roots (Figure 5a).
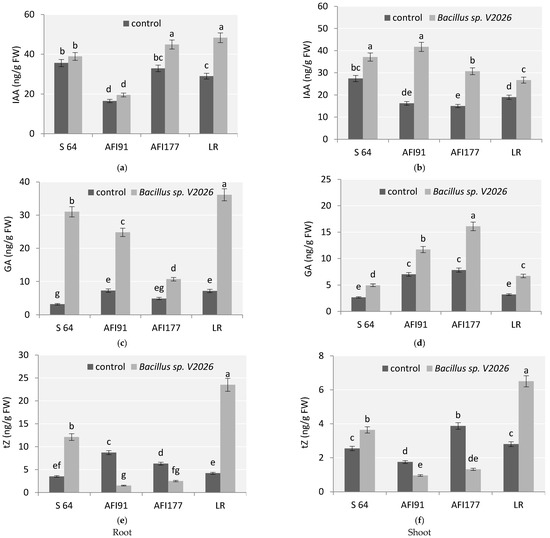
Figure 5.
Effect of Bacillus sp. V2026 on concentrations of plant hormones: (a,b) indole-3-acetic acid (IAA); (c,d) gibberellic acid (GA); and (e,f) trans-Zeatin (tZ) in roots (a,c,e) and shoots (b,d,f) of early-maturing wheat plants grown under hydroponic conditions. Bars with different letters are significantly different at p ≤ 0.05, as determined by Duncan’s multiple range test.
The most significant increase in GA was observed in the roots of plants of early-maturing cultivars LR and S64 (Figure 5c). The basal level of GA in the roots differed significantly between cv. S64 and cv. LR, as well as between cv. S64 and line AFI91. The basal level of GA in shoots of lines AFI177 and AFI91 was higher than in shoots of cv. S64 and cv. LR (Figure 5d).
The content of cytokinins (tZ, trans-Zeatin) also showed significant differences in all four early-maturing wheat genotypes after inoculation compared with the control (Figure 5e,f). Inoculation with Bacillus sp. V2026 significantly increased tZ content in roots and leaves of cv. S64 and cv. LR, whereas in roots and shoots of ultra-early-maturing lines AFI177 and AFI91, a 2.5–5-fold decrease in tZ concentration, respectively, was observed. Changes in tZ content were most noticeable in cv. LR; its level increased two-fold in shoots and five-fold in roots (Figure 5e,f).
Two-way ANOVA revealed that a statistically significant contribution to the variation in the concentration of all endogenous hormones was made by genotype-related differences (factor 1), by inoculation with Bacillus sp. V2026 (factor 2), and by the combination of these two factors (Table S2).
3. Discussion
Bacterium Bacillus sp. V2026 was found to produce plant hormones, such as IAA (at a concentration of 40.3 µg·mL−1) and GA3 (at a concentration of 20.8 ng·mL−1). There are data in the literature on the production of phytohormones IAA (at concentrations 0.1 to 92 µg·mL−1) [49,50] and different GA (at concentrations 0.13–17.9 ng mL−1) [24] by bacteria from genera Bacillus. Thus, concentrations of GA3 57 and 51 ng mL−1 were revealed for B. licheniformis and B.pumilus accordingly [51].
The four early-maturing genotypes of spring wheat studied in this work differed not only in morphological characteristics, yield structure indicators, and duration of individual development phases but also in the combination of allelic forms of the VRN-1 and PPD-D1 genes (Figure S1). It is known that different alleles of VRN-1 and PPD-D1 and their combinations have different effects on the timing of heading, duration of individual development phases, and yield structure in wheat [3,8,9]. Early-maturing genotypes are characterized by a certain allelic composition of these genes [3,52]. The observed stimulating effect of Bacillus sp. V2026 on grain yield and yield-related traits of early-maturing wheat genotypes is consistent with the results of several other studies on the effect of PGPB on wheat productivity under normal and stress conditions [22,53,54,55,56]. PGPB inoculation has been shown to positively affect grain yield [14,32,54,57,58,59,60], number of tillers, plant height and biomass [14,22,32,34], spike length [53,61], number of spikelets and grains in the spike [54,62,63], weight of thousand grains [57,58,61,63] and the content of nutrients [32,34,57,59,60].
We found that inoculation with Bacillus sp. V2026 and genotypic differences had a statistically significant impact (p ≤ 0.05) on all the indices of wheat productivity recorded in our experiments. The interaction of factors had a significant effect on the number of grains per spike, the number and weight of grains per plant, and HI (Table S3). It is of interest that the increase in grain yield of wheat genotypes after inoculation was mainly associated with a higher number of productive shoots and the number and weight of grains per plant. At the same time, the effect on plant height, spike length, and weight of thousand grains was much less significant.
Under the conditions of inoculation with Bacillus sp. V2026, the main impact to change in the grain yield of early-maturing genotypes was made by the increase in productive tilling capacity (Table 1), which is an important trait determining the yield of wheat. The positive effect of PGPB on shoot formation has been reported in other studies [32,53,54,57,58,61,63]. Cytokinins are central to the regulation of wheat shoot growth, and the IAA/CK ratio is used to determine plant response in this respect [64,65]. Stimulation of productive tilling capacity observed after inoculation with PGPB is associated with their ability to produce IAA and CK, as well as with their influence on the hormonal balance of plants [66]. The evidence suggests that the promotion of productive tilling capacity induced by the Bacillus sp. V2026 could be mediated by bacterial IAA and GAs. Effective stimulation of tiller formation by Bacillus sp. V2026 seems to be associated with its IAA-producing activity, which resulted in an increase in endogenous IAA and tZ in wheat roots and shoots (Figure 5). Tiller numbers increased after inoculation in all the genotypes involved in our study, but the response varied depending on the degree of hormonal changes. The highest stimulation of productive tilling capacity was registered in plants of cv. LR and was associated with more pronounced changes in hormonal levels in roots and shoots of plants of this cultivar, especially changes in the level of CK.
Similar to IAA, CK are among the main regulators of primary root growth due to their participation in cell division and differentiation in the root meristem. Accumulation of CK in inoculated wheat plants has been shown to be associated with an increase in shoot weight [17]. Even though Bacillus sp. V2026 was found to not produce trans-Zeatin, the concentration of cytokinins in shoot and root tissues of plants of early-maturing genotypes after inoculation with the bacterium changed considerably more than IAA concentration. Interestingly, the accumulation of root tZ found in our experiments in cv. S64 and cv. LR did not inhibit root growth (Table 4), although high concentrations of CK are known to do so [67]. However, both the increase in the levels of tZ in the roots of S64 and LR plants and their decrease in roots of AFI177 and AFI91 plants were accompanied by stimulation rather than inhibition of root growth. These ambiguous results can be explained by the literature data indicating that, on the one hand, auxins decrease the levels of CK by inhibiting their synthesis [68], and, on the other hand, the auxin-induced increase in the volume of the root where CK are synthesized promotes the accumulation of cytokinins [62]. Zeatin levels in leaves increased after the inoculation of A. thaliana plants with the PGPB strain Burkholderia phytofirmans, which has also not been shown to be able to synthesize CK [69]. The increased level of endogenous CK in wheat roots after inoculation with Paenibacillus illinoisensis IB 1087 has been explained by its IAA activity [18].
An increase in the productivity of an individual spike associated with an increase in the number and weight of grains is thought to be a promising direction for increasing the productivity of early-maturing genotypes [2]. Grain number is more variable than grain weight [70,71], and the yield is much more often associated with the number of grains than with the average grain weight [72,73]. We found that inoculation with Bacillus sp. V2026 had a greater effect on increasing the number of grains in the spike in S64 and LR, while in lines API91 and API177, it had a greater effect on increasing the grain weight of the spike (Table 2). Grain number in wheat is largely determined in the stem elongation phase, with the process of spike growth before heading being crucial for the number of grains [74,75]. It should be noted that in our study, the duration of the period from stem elongation to heading, which has a decisive influence on spike productivity after inoculation of the plants with Bacillus sp. V2026, did not change significantly.
The stimulating effect of Bacillus sp. V2026 on stem and spike productivity resulted in a significant increase in the number of grains per plant and plant grain weight in all early-maturing genotypes. A significant increase in grain yield after inoculation with Bacillus sp. V2026 in early-maturing cultivars S64 and LR was determined by more intensive stem formation. In contrast, in API91 and API177 lines, the increase in grain weight of the spike (Table 1) was due to a more effective redistribution of nutrients between the structural components of the spike, as evidenced by a significantly improved ratio of grain/chaff in the spike of plants of these two lines (Figure 1B). It is currently thought that fruiting efficiency and the ratio between the productive and the vegetative components of the spike (which is associated with fruiting efficiency) are among the characteristics important for achieving an increased yield in wheat [12,76,77]. PGPB Bacillus sp. V2026 increased grain weight by intensifying the redistribution of substances between the structural components of the spike. Differences between wheat genotypes in respect of this characteristic were observed.
Inoculation with Bacillus sp. V2026 resulted in a statistically significant increase of HI in all wheat cultivars and lines involved in our study. This increase seems to be associated with a change in donor-acceptor relationships between the spike and the vegetative mass and the redirection of the supply of nutrients mainly towards the spike. We showed that bacteria Bacillus sp. V2026 stimulated nutrient supply to the grain and affected the level of N, P, K, Fe, Mg, Zn, and Mn in wheat grain (Table 2), which is consistent with our earlier results obtained on Bacillus subtilis N2 [78,79]. Our results accord with those of [14], who reported an increase in the content of macro- and micronutrients in wheat grain after inoculation with Bacillus megaterium. There is evidence of positive effects of PGPB on nutrient uptake (mainly N), yield and grain quality [32,34], protein, P, K and Fe concentration in wheat grain [57,59,60].One of the core processes in primary plant metabolism that is directly related to productivity is photosynthesis. The content of Chl and Car, while indirect, is the most important biochemical indicator of plant photosynthetic activity. Our results also showed an increase in Chl and Car content in all wheat genotypes involved in the study (Figure 3) and a decrease in MDA accumulation and, thus, lipid peroxidation, indicating a lowered stress load (Figure 4). Increased concentration of chlorophyll in leaves activates photosynthesis, ensuring a more rapid accumulation of plant biomass. It has been shown that bacterial inoculation can positively affect the content of photosynthetic pigments in plants [61]. This is reflected in the activity of photosynthetic apparatus, which affects the rate of accumulation of assimilates, plant growth, and productivity. Increased chlorophyll content may be associated with positive effects of PGPB on water and mineral uptake [61,80].
It was found that the bacterium Bacillus sp. V2026 had a statistically significant effect on the duration of the vegetation period of ultra-early-maturing lines and early-maturing cultivars of common wheat, resulting in the acceleration of heading and maturating (Table 3).
Inoculation with Bacillus sp. V2026 accelerated the growth of wheat plants by shortening the vegetation period at the early stages of development from seedlings to stem elongation. It did not affect the rate of development at later ontogenetic stages. Bacterium Bacillus sp. V2026 shortened the growing season of early-maturing wheat plants, conceivably due to the production of the GA hormone. It is known that the application of exogenous GA accelerated flowering in winter wheat cultivars [81], significantly influenced spike development, and shortened the duration of the preheading phase in spring cultivars [82,83]. GAs are a major class of phytohormones regulating plant development, from seed germination and vegetative growth (including initiation and stimulation of flowering) to fruit and seed setting [84,85].
In our study, inoculation with Bacillus sp. V2026 impacted the change in endogenous GA, significantly increasing its concentration in roots and shoots of all wheat genotypes involved in the study, with the most significant increase observed in roots (Figure 5). In addition, increased GA concentration was found in plants treated with GA-producing bacteria, confirming their effects on plant hormonal status [23,28]. To note, under conditions of bacterial inoculation, the ultra-early-maturing lines AFI177 and AFI91 demonstrated a higher level of endogenous GA in shoots, while early-maturing cultivars S64 and LR showed a higher level of GA in roots. These differences may be associated both with a more intense growth of ultra-early-maturing genotypes at the early ontogenetic stages (Table 3) and with greater responsiveness of early-maturing cultivars, especially LR, to inoculation (Figure 5).
Similar results were obtained by [38], who showed that inoculation with Bacillus subtilis B26 resulted in a shortening of the growing season of Brachypodium distachyon. Some PGPBs are known to synthesize gibberellins [23,86], which promotes plant growth. The positive effect of a PGPB Bacillus methylotrophicus on plants through the secretion of several gibberellins has been confirmed by the increased percentage of seed germination in lettuce, melon, soybean, and vegetable mustard [87]. GA-producing Bacillus sp. strains have been reported to stimulate the growth of red pepper [88] and rice [28].
As shown in our experiments, inoculation of wheat plants with Bacillus sp. V2026, contributed to a change in the level of endogenous IAA in plant shoot and root tissues (Figure 5). Since plants are capable of auxin uptake from the nutrient medium [18,89], increased concentration of auxins in plants treated with auxin-producing Bacillus sp. V2026 may be attributed to the uptake of microbial hormones by plants and appeared to depend on the characteristics of each genotype. IAA is a phytohormone so important for plant development and growth, performing multiple functions, including the response of roots and shoots to light and gravity [90,91], initiation of lateral and adventitious roots [92,93,94], stimulation of cell division and elongation of stems and roots [95,96], vascular tissue differentiation [96], apical dominance, and flower morphogenesis [97,98]. Auxin levels in lines AFI177 and AFI91 increased to a greater extent in the shoots; those in cv. LR increased to a greater extent in the roots, while the changes in auxin levels in cv. S64 were less pronounced (Figure 5). A weak positive correlation between the increase in IAA concentration and the acceleration of development was observed, indicating a possible effect of bacterial IAA on the ontogenesis duration.
Stimulation of rhizogenesis is one of the best-known effects of auxins [92,99]. The increase in length and weight of the roots observed after inoculation with Bacillus sp. V2026 in our experiments (Table 4) was associated with changes in auxin levels in the roots of plants of all the genotypes involved in the study (Figure 5). A greater increase in root length and weight in LR and AFI177 plants was apparently due to a higher and more stable increase in endogenous IAA in plants of these genotypes after inoculation with Bacillus sp. V2026. Similar data have been obtained in experiments with inoculation of wheat plants with auxin-producing Paenibacillus illinoisensis IB 1087 and Pseudomonas extremaustralis IB-K13-1A: increased root weight and increased auxin levels in the roots have been registered [18]. Inoculation with IAA-producing Bacillus strains on plant roots enhances root length as well as the number of lateral roots [50,53,93]. IAA-producing bacterium Bacillus spp. controls endogenous IAA levels in plant roots by regulating auxin-responsive genes, which changes the root architecture [100]. Auxin-producing bacteria are known to enhance both root and shoot growth [101]. Similarly, in our study, inoculation with Bacillus sp. V2026 resulted in an increase in shoot length and weight, with plants of LR and AFI177 genotypes being more susceptible to bacterial inoculation (Table 4). In our experiments, inoculation with Bacillus sp. V2026 resulted in a greater increase in the biomass of roots compared with that of shoots, which contributed to an increase in the root/shoot weight ratio. According to the available literature data, after inoculation of plants with cytokinin (CK)-producing bacteria, an opposite pattern is observed: a greater increase in the biomass of shoots compared with that of roots results in a decreased root/shoot weight ratio [102].
It is important to note that the effect of auxins on shoot growth is seldom discussed, probably due to the fact that IAA transport from roots to shoots is less studied than cytokinin transport. IAA- and CK-producing PGPBs are known to reconstruct the architecture of the root system by altering the hormonal balance of plants [17,25,103,104].
Thus, Bacillus sp. V2026 promotes growth, accelerates development, and increases the yield of early-maturing genotypes of spring soft wheat. The effect of this strain on the duration of wheat ontogenesis is particularly interesting, as it provides an additional opportunity of simulating the timing of heading and maturation of cultivars depending on the region of cultivation. Our results suggest that PGPB Bacillus sp. V2026 stimulate the ontogenesis of early-maturing genotypes by increasing the concentration of endogenous GA in the early stages of wheat ontogenesis. According to data available in the literature, an increase in bioactive GA results in an upregulation of the expression of transcription factors required to initiate the transition of the wheat apical meristem to generative development [105,106]. In general, the findings allowed us to assume that GA activity of Bacillus sp. V2026 explains their ability to influence the GA-dependent signaling pathway, which regulates various aspects of plant development, including the duration of early stages of ontogenesis [82,107,108].
4. Materials and Methods
4.1. Plant Material
Seeds of early-maturing soft spring wheat (Triticum aestivum L.) cv. Sonora 64 (S64) (k-45398) and cv. Leningradskaya rannyaya (LR) (k-142751) were provided for research by the Department of Wheat Genetic Resources of the N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR) (St. Petersburg, Russia). Ultra-early-maturing lines of soft spring wheat AFI91 and AFI177 were obtained from the Agrophysical Research Institute [109]. Heading time in these lines is comparable with that of typical representatives of ultra-early-maturing wheat from the VIR collection [2].
4.2. Identification of Alleles VRN-1 and PPD-D1 Loci
Using allele-specific primers, the presence of alleles of the VRN-1 and PPD-D1 loci in cultivars S64 and LR and lines AFI177 and AFI91 was determined. For molecular genetic analysis, genomic DNA was isolated from 5-day-old seedlings by the CTAB method after [110]. Published allele-specific primers were used to detect dominant and recessive alleles of Ppd-D1, Vrn-A1, Vrn-B1, and Vrn-D1 genes (Table S3). Reaction mixture preparation protocols and PCR conditions followed the recommendations of the authors of the molecular markers [5,111,112].
4.3. Screening and Isolation of PGPB
PGPB were isolated from roots of spring wheat cv. Leningradskaya 6. Flasks containing 100 mL of sterile phosphate buffer and 10 g of wheat roots were placed in an ultrasonic bath (Bandelin; 50 Hz) for 10 min. The phosphate buffer solution containing microorganisms washed from the roots was serially diluted. Then, 0.1 mL of various dilutions was inoculated on Petri dishes containing LB (Luria Bertani, Sigma-Aldrich, St. Louis, MO, USA) agar medium.
4.4. Identification and Characteristics of Bacteria
Genomic DNA of Bacillus sp. V2026 was isolated using the Monarch® Genomic DNA Purification Kit (NEB, Ipswich, MA, USA) according to the manufacturer’s protocol.
PCR amplification of DNA fragments was performed according to a standard protocol using universal primers [113,114] (Table S3). PCR parameters were: (1) matrix pre-denaturation—95 °C, 3 min; (2) 30 cycles: denaturation—94 °C, 30 s; primer annealing—54 °C, 30 s; elongation—72 °C, 30 s; and (3) final elongation—72 °C, 5 min. The obtained PCR fragments were isolated from agarose gel [115] and sequenced using an ABI PRISM 3500xl automatic sequencer (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s protocol. The strain was identified by comparing the obtained nucleotide sequences of the 16S rRNA gene and ITS fragment with the RDP (https://rdp.cme.msu.edu, accessed on 1 June 2022) and GenBank databases (https://blast.ncbi.nlm.nih.gov, accessed on 1 June 2022). The sequences were submitted to the NCBI databases with accession numbers OM764631 and OM855550, respectively.
The Gram reaction was determined using the Gram-staining method with the help of a bioMe’rieux Gram-staining kit. Catalase activity was examined via the production of oxygen bubbles using H2O2 (3%, v/v), and oxidase activity was detected using a commercial oxidase strip (Sigma-Aldrich, St. Louis, USA). H2S production was determined according to [116]; indole production was assessed by the Ehrlich method [117]. Phosphate solubilizing activity was assayed on Pikovskaya medium [118]. Bacterial biochemical characteristics such as the utilization of D-glucose D-sucrose, maltose, arabinose, D-galactose, xylose, inositol, dulcitol, sorbitol, glycerol, and mannitol were determined according to [119]. For the agar well diffusion assay, PDA (potato dextrose) agar plates containing 104 Fusarium conidia per mL agar were prepared. Then the wells with a diameter of 5 mm were cut in agar. Liquid culture of Bacillus sp. V2026 was added to the wells [120]. The plates were incubated at 28 °C for 72 h and verified every 12 h. The diameter of the zone of inhibition of the growth of the mycelium of the fungus was measured in mm.
The bacterial phytohormones IAA, tZ, and GA in the extract were determined using a VARIAN 212 LC high-performance liquid chromatograph with a mass selective detector (Varian 500 MS system). Detection of IAA was carried out using ESI- (electrospray) ion at 174 m/z. The detection of tZ and GA3 was carried out using ESI+ for ions at 220 m/z and 345 m/z, respectively. To determine phytohormones, 50 mL of liquid culture (and 50 mL sterile liquid medium, used as a control) was taken and centrifuged at a speed of 3000–5000 rpm for 5 min. The supernatant was drained into a dividing funnel. The precipitate was shaken twice with 30 mL of distilled water and centrifuged after combining the supernatant in a dividing funnel. The combined supernatant in the dividing funnel was acidified with a 10% solution of acetic acid to a pH of 2, after which phytohormones were extracted three times with 10 mL of ethyl acetate. The upper ethyl acetate layer was drained through anhydrous sodium sulfate and evaporated until dry on a rotary evaporator at a temperature of no more than 40 °C. The extraction was performed three times. Chromatography was carried out in the gradient mode (phase A, methanol + 0.1% formic acid; phase B, deionized water +0.1% formic acid). The chromatographic system used a Cosmosil C18 4.6 ID 150 mm column. The chromatograph was calibrated using the Sigma-Aldrich internal standards for pure hormone substances. The identification of hormones was carried out in the mass–mass mode.
4.5. Experimental Design
4.5.1. Pot Experiments
The plants were grown in vegetative light units with the following parameters: illumination with lamps DNaZ-400 (Reflax, Moscow, Russia), illumination intensity 23–25 klx, 16-h photoperiod, temperature 23–24 °C (day) and 19–20 °C (night), and humidity 70–80%.
The plants were grown in 4 L pots, five plants per pot. The pots were placed randomly in five replications per variant. The experimental design included two variants: wheat plants without treatment (control) and wheat plants treated with Bacillus sp. V2026 (treatment). The experiment was repeated twice. Wheat seeds, after surface sterilization in 70% ethanol for 2 min, were washed with water and placed in Petri dishes for germination in a thermostat at 26 °C for 48 h. Germinated seeds were planted in pots, 10 plants per pot; 7 days after sowing, 5 identical plants per pot were left. Soddy-podzolic light loamy soil was used in the root layer containing mobile phosphorus 198 mg/kg, mobile potassium 112 mg/kg, nitrate nitrogen 18.2 mg/kg, and ammonium nitrogen 34.6 mg/kg.
The PGPB were grown at 28 °C for 48 h at 140 rpm in a broth of Luria–Bertani (LB) medium in a rotatory shaker. The bacterial cells were centrifuged at 3900× g for 5 min; pelleted bacteria were rinsed in 10 mM MgSO4 and diluted in Knop solution. The final PGPB concentration was monitored by counting the bacterial colonies grown on LB–agar medium and was 3 × 108 CFU/mL. Wheat plants were inoculated with the bacterial strain Bacillus sp. V2026 twice: at the time of planting and at the tillering stage. Bacterial cell suspension at a rate of 1 mL (3 × 108 CFU/mL) per seedling was applied to the soil surface around the roots of each plant. In total, 5 mL of bacterial suspension in a concentration of 3 × 108 CFU/mL was added to each pot.
Ontogenetic phases of spring soft wheat were observed using the conventional Eucarpia scale. Conducting vegetative experiments under controlled conditions allows more accurate determination of the time of onset of developmental phases [121,122]. The dates of the phases of development were observed individually for each plant. Tillering was noted on the day when the second shoot emerged from the main shoot. Stem elongation was recorded when the first node rose to a height of about 1 cm. Heading was recorded on the day when the ear fully emerged from the flag leaf. Days before the beginning of maturing were recorded as the number of days from the date of sowing to the date of yellowing of the upper internode of the main stem. The plants were harvested in the phase of full ripeness. In this study on wheat yield structure, data were analyzed on productive tilling capacity, plant height, spike length, number of spikelets in a spike, spike weight, and number and weight of grains per spike and per plant. After drying the samples at 70 °C for 48 h, the dry weight of the plant was determined. We calculated the weight of thousand grains, straw yield per plant (the difference between biological yield and grain yield), harvest index (HI, the ratio of grain yield to aboveground biomass yield expressed as a percentage), and the ratio of unproductive to productive spike weight (ratio chaff to grain).
4.5.2. Hydroponic Experiment
Hydroponic experiments were performed in Knop solution (containing CaNO3 1 g, KH2PO4 0.25 g, MgSO4 0.25 g, KCl 0.125 g, and FeCl3 0.0125 g per 1 L) to study the effect of Bacillus sp. V2026 on the growth of wheat plants and their biochemical and hormonal status. The experimental design included two variants: control—wheat plants without treatment grown on Knop medium; treatment—wheat plants grown in Knop solution and treated with Bacillus sp. V2026 at a concentration of 3 × 105 cells per·mL. The experiments were performed in three independent replications, and from 35 to 45 plants per variant were used in each replication.
For the experiment, undamaged and calibrated seeds were selected, surface-sterilized in 70% ethanol for 2 min, and washed with sterile water. The seeds were then soaked in 2% sodium hypochlorite solution for 20 min and washed five times with sterile water. After that, the seeds were placed in Petri dishes and germinated in a thermostat at 26 °C. After 48 h, the germinated seeds were placed between two layers of hydrophilic tissue at a distance of 2 cm from each other; then, the tissue was rolled up and placed in 300 mL vessels with Knop nutrient solution. For the hydroponic experiments, bacteria were diluted to a concentration of 3 × 105 cells per 1 mL of Knop’s solution. On the 14th day after the beginning of the experiment and on the 10th day after adding the bacterium, the length and the biomass of plant shoots and roots were measured, and samples were taken to determine the content of photosynthetic pigments and hormone levels.
4.6. Analysis of Plant Phytohormones
To determine the phytohormones, 10 g of leaves were homogenized with 80% methanol at 4 °C and evaporated at a rotary evaporator under vacuum. The remaining aqueous phase was divided into two parts. One half was acidified with 10% solution of muriatic acid to pH 2.5–3 and extracted three times in a separating funnel with 30 mL diethyl ether for determination of IAA, tZ, and GA. The second part was diluted with 10% potassium hydroxide to pH 8.0 and extracted three times in a separating funnel with n-butanol (30 mL each), after which the extract was purified with Dowex 50W*8 ion-exchange resin. The final extracts were evaporated to a dry residue and dissolved in 2 mL of mobile phase A. Concentrations of IAA, tZ, and GA in the extract were determined by high-performance liquid chromatography with mass selective detection (VARIAN 212 LC liquid chromatograph with mass selective detector [Varian 500 MS system]). IAA was detected using an ESI- (electrospray) at 174 m/z ion. tZ and GA were detected using ESI+ (electrospray) at 220 m/z and 345 m/z ions, respectively. Chromatography was performed in gradient mode (phase A, methanol + 0.1% formic acid; phase B, deionized water +0.1% formic acid). Cosmosil C18 4.6 ID × 150 mm column was used in the chromatographic system. The chromatograph was calibrated using Sigma-Aldrich internal standards for pure hormones. The hormones were identified in MS/MS mode.
4.7. Chlorophyll and Carotenoids Analysis
Photosynthetic pigments chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Car) were analyzed in acetone extract using the spectrophotometric method [123]. A total of 0.2 g of leaves were ground in a porcelain mortar with a small amount of acetone and sand in the presence of calcium carbonate. The ground mass was transferred to a centrifuge tube and centrifuged at 4000 rpm−1. The supernatant was transferred into a 50 mL volumetric flask and made up to the mark with acetone. Optical density was measured on a spectrophotometer PE-3000UF at 662, 644, and 440.5 nm wavelengths.
4.8. Lipid Peroxidation
The level of lipid peroxidation (LPO) was assessed based on the content of malonic dialdehyde (MDA), which is a product of LPO [124]. Plant material (0.3 g of raw leaves) was homogenized in 1 mL of reaction medium consisting of thiobarbituric acid and trichloroacetic acid. The total volume of the homogenate was 4 mL. One sample of reaction medium (4 mL) contained 0.4 g of trichloroacetic acid (10%) and 1.0 mg of thiobarbituric acid (0.25%). The homogenate was placed in a water bath at 95–100 °C for 30 min, after which the samples were cooled and centrifuged for 10 min at 10,000 g. Absorbance was measured at 532 nm and 600 nm. The TBA reactive product concentration was calculated using an extinction coefficient of 155 mM−1 cm−1.
4.9. Analysis of Protein and Macronutrient/Micronutrient Content in Wheat Grain
Wheat grain was dried in an oven to constant weight at a temperature of 105 °C, crushed in a mill, and sifted through a sieve with a mesh diameter of 1 mm. The prepared sample was used to determine the concentration of trace elements Fe, Mg, Zn, and Mn. For analysis, a sample weighing 1 g was transferred into precalcined crucibles, and dry ashing was carried out in a muffle furnace at a temperature of 520 °C for 5 h until complete ashing. After the crucibles cooled down, an 18% HCl solution was added to them, dissolving the ash; the contents of the crucibles were transferred with deionized water into a 100 mL volumetric flask. The resulting solution was filtered through an ash-free blue ribbon filter. The measurements were carried out on a Varian AA240FS atomic absorption spectrophotometer with flame atomization. The device was calibrated using standard solutions of elements with a given concentration. Trace elements were measured at the most sensitive wavelengths of Fe (248.7 nm), Mg (324.6 nm), Zn (213.7 nm), and Mn (279.5 nm) using hollow cathode lamps.
4.10. Statistical Analysis
For the statistical analysis, we used two-factor analysis of variance (ANOVA) and Duncan’s multiple range test to determine the significance of differences between the mean values. The number of repeats for each characteristic is shown in the tables and figures. The mean ± SE values presented in the tables and the figures were calculated using MS Excel.
5. Conclusions
PGPB Bacillus sp. V2026 producing indole-3-acetic acid and gibberellin influenced the development dynamics and productivity of early-maturing cultivars S64 and LR and ultra-early-maturing lines AFI91 and AFI177 of wheat, as well as their physiological and biochemical responses and endogenous hormone levels at the early stages of ontogenesis. Inoculation with the bacterium significantly shortened the time to reach each growth stage in early-maturing genotypes, with cycle reduction being greater from seedlings to stem elongation. Inoculation of plants with Bacillus sp. V2026 significantly affected the content of endogenous hormones IAA, GA, and tZ in roots and shoots of early-maturing wheat genotypes. The stimulating effect of Bacillus sp. V2026 on the cultivars S64 and LR was mostly expressed as an increase in the number of grains, while the effect on the plants of lines AFI91 and AFI177 was mainly expressed as an increase in grain weight. The contents of macro- and microelements and protein in the grain of AFI91 and AFI177 were maximal compared with other genotypes. The bacterium Bacillus sp. V2026 could be used to increase the yield and the grain quality of early-maturing genotypes of spring soft wheat. However, further studies are necessary to select the most effective association for growing high-yielding early-maturing wheat plants, since there are the differences in the response of early-maturing wheat genotypes to inoculation with PGPB Bacillus sp. V2026.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11141817/s1. Table S1: morphological, physiological, and biochemical characteristics of the studied strain, Table S2: analysis of variance (ANOVA) for genotype and Bacillus sp. V2026 effect on various traits of four spring wheat genotypes, Table S3, primers used in the study, Figure S1: identification of dominant (288 b.p.) and recessive (414 b.p.) alleles of Ppd-D1 gene (a), dominant (715+624 b.p.) and recessive (484 b.p.) alleles of Vrn-A1 gene (b), dominant (1124 b.p.) allele of Vrn-B1 gene (c), and dominant (1671 b.p.) allele of Vrn-D1 gene (d) in wheat varieties by PCR with allele-specific markers. Wheat varieties: 1- AFI-177, 2- AFI-91, 3- Leningradskaya rannayay, 4- Sonora-64, M-DNA ladder; Figure S2, effect of inoculation with Bacillus sp. V2026 on wheat seedlings of early-maturing genotypes.
Author Contributions
Conceptualization, G.V.M. and V.N.P.; methodology, G.V.M., Y.V.K., E.P.C., N.A.R. and V.N.P.; software, G.V.M. and N.A.R.; formal analysis G.V.M. and V.N.P.; investigation, G.V.M., V.N.P., Y.V.K., E.P.C., N.A.R., V.K.C. and V.E.V.; resources, G.V.M., V.N.P. and Y.V.C.; writing—original draft preparation, G.V.M. and V.N.P.; writing—review and editing, G.V.M., V.N.P., Y.V.K., V.K.C., E.P.C. and Y.V.C.; visualization, G.V.M. and N.A.R.; project administration, G.V.M., Y.V.C. and V.N.P.; funding acquisition, Y.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We are thankful to E.V. Zuev (Federal Research Center N. I. Vavilov, All-Russian Institute of Plant Genetic Resources) for providing us with the seeds of cv. Leningradskaya rannayay and Sonora 64.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef]
- Rigin, B.V.; Zuev, E.V.; Tyunin, V.A.; Shrejder, E.R.; Pyzhenkova, Z.S.; Matvienko, I.I. Breeding and genetic aspects of creating productive forms of fast-developing spring bread wheat. Proc. Appl. Bot. Genet. Breed. 2018, 179, 194–202. [Google Scholar] [CrossRef]
- Chumanova, E.V.; Efremova, T.T.; Kruchinina, Y.V. The effect of different dominant VRN alleles and their combinations on the duration of developmental phases and productivity in common wheat lines. Russ. J. Genet. 2020, 56, 822–834. [Google Scholar] [CrossRef]
- Rehman, H.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.M.A.; Reynolds, M. Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants 2021, 10, 455. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef]
- Trevaskis, B.; Hemming, M.N.; Dennis, E.S.; Peacock, W.J. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 2007, 12, 352–357. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef]
- Stelmakh, A.F. Genetic effects of Vrn genes on heading date and agronomic traits in bread wheat. Euphytica 1993, 65, 53–60. [Google Scholar] [CrossRef]
- Cockram, J.; Jones, H.; Leigh, F.J.; O’Sullivan, D.; Powell, W.; Laurie, D.A.; Greenland, A.J. Control of flowering time in temperate cereals: Genes, domestication, and sustainable productivity. J. Exp. Bot. 2007, 58, 1231–1244. [Google Scholar] [CrossRef]
- Zhang, H.; Turner, N.C.; Poole, M.L. Increasing the harvest index of wheat in the high rainfall zones of southern Australia. Field Crops Res. 2012, 129, 111–123. [Google Scholar] [CrossRef]
- Mecha, B.; Alamerew, S.; Assefa, A.; Dutamo, D.; Assefa, E. Correlation and path coefficient studies of yield and yield associated traits in bread wheat (Triticum aestivum L.) genotypes. Adv. Plants Agric. Res. 2017, 6, 00226. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Trujillo-Negrellos, E.; Molero, G.; Reynolds, M.P.; Sylvester-Bradley, R.; Foulkes, M.J. Optimizing dry-matter partitioning for increased spike growth, grain number and harvest index in spring wheat. Field Crops Res. 2019, 240, 154–167. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Shcherbakov, A.V.; Maslennikova, S.N.; Zaplatkin, A.N.; Kanarsky, A.V.; Zavalin, A.A. Endophytic bacteria of woody plants as the basis of complex microbial preparations for agriculture and forestry. Russ. Agric. Sci. 2016, 42, 339–342. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2014, 3, 121–128. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Seifikalhor, M.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant growth-promoting bacteria: Biotic strategy to cope with abiotic stresses in wheat. In Wheat Production in Changing Environments; Hasanuzzaman, M., Nahar, K., Hossain, M., Eds.; Springer: Singapore, 2019; pp. 579–614. [Google Scholar] [CrossRef]
- Velloso, C.C.V.; Ribeiro, V.P.; de Carvalho, C.G.; de Oliveira Christiane, A.U.; de Paula Lana, G.; Marriel, I.E.; de Sousa, S.M.; Gomes, E.A. Tropical endophytic Bacillus species enhance plant growth and nutrient uptake in cereals. In Endophytes: Mineral Nutrient Management, Volume 3; Springer: Cham, Switzerland, 2021; pp. 157–180. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuz’mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin pro ducing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Gupta, G.; Panwar, J.; Jha, P.N. Natural occurrence of Pseudomonas aeruginosa, a dominant cultivable diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L.) R. Br. Appl. Soil Ecol. 2013, 64, 252–261. [Google Scholar] [CrossRef]
- Amna; Ud Din, B.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Sharma, M. Significance of inoculation with Bacillus subtilis to alleviate drought stress in wheat (Triticum aestivum L.). Vegetos 2020, 33, 782–792. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2021, 41, 549–569. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; You, Y.H.; Kim, J.G.; Kamran, M.; Lee, I.J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.Y.; Melent’ev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Comparison of effects of bacterial strains differing in their ability to synthesize cytokinins on growth and cytokinin content in wheat plants. Russ. J. Plant Physiol. 2006, 53, 507–513. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.M.; Yun, B.-W.; Lee, I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Pandey, P.K.; Singh, M.C.; Singh, S.; Singh, A.K.; Kumar, M.; Pathak, M.; Shakywar, R.C.; Pandey, A.K. Inside the plants: Endophytic bacteria and their functional attributes for plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 11–21. [Google Scholar] [CrossRef]
- Kuramshina, Z.M.; Smirnova, Y.V.; Khairullin, R.M. Increasing Triticum aestivum tolerance to cadmium stress through endophytic strains of Bacillus subtilis. Russ. J. Plant Physiol. 2016, 63, 636–644. [Google Scholar] [CrossRef]
- Pishchik, V.N.; Filippova, P.S.; Mirskaya, G.V.; Khomyakov, Y.V.; Vertebny, V.E.; Dubovitskaya, V.I.; Ostankova, Y.V.; Semenov, A.V.; Chakrabarty, D.; Zuev, E.V.; et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve wheat growth and antioxidant status under Ni stress. Plants 2021, 10, 2334. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Baig, K.S.; Arshad, M.; Shaharoona, B.; Khalid, A.; Ahmed, I. Comparative effectiveness of Bacillus spp. possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L.). Ann. Microbiol. 2012, 62, 1109–1119. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef]
- Schwachtje, J.; Karojet, S.; Thormählen, I.; Bernholz, C.; Kunz, S.; Brouwer, S.; Schwochow, M.; Köhl, K.; van Dongen, J.T. A naturally associated rhizobacterium of Arabidopsis thaliana induces a starvation-like transcriptional response while promoting growth. PLoS ONE 2011, 6, e29382. [Google Scholar] [CrossRef]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar] [CrossRef]
- Wang, B.; Seiler, J.R.; Mei, C. Burkholderia phytofirmans strain PsJN advanced development and altered leaf level physiology of switchgrass. Biomass Bioenergy 2015, 83, 493–500. [Google Scholar] [CrossRef]
- Gagné-Bourque, F.; Mayer, B.F.; Charron, J.-B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Shi, Y. Synthesis and characterization of cellulose/silica hybrid materials with chemical crosslinking. Carbohydr. Polym. 2009, 78, 799–805. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM 196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef]
- Kuramshina, Z.M.; Khairullin, R.M.; Smirnova, Y.V. Responsiveness of Triticum aestivum L. cultivars to inoculation with cells of endophytic Bacillus subtilis strains. Russ. Agric. Sci. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Santos, M.F.; Freitas, I.A.S.; Pereira, V.L.G.; Pires, W.K.L. Effect of gibberellin on growth and development of Spondias tuberosa seedlings. Rev. Caatinga 2020, 33, 1124–1130. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Tan, G.F. Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Bawa, G.; Feng, L.; Chen, G.; Chen, H.; Hu, Y.; Pu, T.; Cheng, Y.; Shi, J.; Xiao, T.; Zhou, W.; et al. Gibberellins and auxin regulate soybean hypocotyl elongation under low light and high-temperature interaction. Physiol. Plant. 2020, 170, 345–356. [Google Scholar] [CrossRef]
- Ma, H.Y.; Zhao, D.D.; Ning, Q.R.; Wei, J.P.; Li, Y.; Wang, M.M.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef]
- Ayub, Q.; Khan, S.M.; Hussain, I.; Gurmani, A.R.; Naveed, K.; Mehmood, A.; Ali, S.; Ahmad, T.; Haq, N.; Hussain, A. Mitigating the adverse effects of NaCl salinity on pod yield and ionic attributes of okra plants by silicon and gibberellic acid application. Italus Hortus 2021, 28, 59–73. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Vachova, P.; Hajihashemi, S.; Martinkova, J.; Hejnak, V. Effect of gibberellic acid on growing-point development of non-vernalized wheat plants under long-day conditions. Plants 2020, 9, 1735. [Google Scholar] [CrossRef]
- Karnwal, A. Isolation and identification of plant growth promoting rhizobacteria from maize (Zea mays L.) rhizosphere and their plant growth promoting effect on rice (Oryza sativa L.). J. Plant Prot. Res. 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Bhutani, N.; Maheshwari, R.; Negi, M.; Suneja, P. Optimization of IAA production by endophytic Bacillus spp. from Vigna radiata for their potential use as plant growth promoters. Isr. J. Plant Sci. 2018, 65, 83–96. [Google Scholar] [CrossRef]
- Gutierrez-Manero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Likhenko, I.E.; Zyryanova, A.F.; Likhenko, N.I.; Salina, E.A.; Stasyuk, A.I.; Shcherban, A.B. Study of allelic composition of VRN-1 and PPD-1 genes in early–ripening and mid-early varieties of spring soft wheat in Siberia. Russ. J. Genet. Appl. Res. 2015, 5, 198–207. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2017, 64, 574–587. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.V.; Franco, C.M.; Paasricha, N.; Saifi, S.K.; Tuteja, N.; Sharma, A.K. Field performance of bacterial inoculants to alleviate water stress effects in wheat (Triticum aestivum L.). Plant Soil 2019, 441, 261–281. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, N. Microbial amelioration of salinity stress in HD 2967 wheat cultivar by up-regulating antioxidant defense. Commun. Integr. Biol. 2021, 14, 136–150. [Google Scholar] [CrossRef]
- Mäder, P.; Kaiser, F.; Adholeya, A.; Singh, R.; Uppal, H.S.; Sharma, A.K.; Srivastava, R.; Sahai, V.; Aragno, M.; Wiemken, A.; et al. Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biol. Biochem. 2011, 43, 609–619. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Sharif, S.; Kazmi, M.; Sultan, T.; Aslam, M. Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst. 2011, 145, 159–168. [Google Scholar] [CrossRef]
- Turan, M.; Gulluce, M.; Von Wiren, N.; Sahin, F. Yield promotion and phosphorus solubilization by plant growth–promoting rhizobacteria in extensive wheat production in Turkey. J. Plant Nutr. Soil Sci. 2012, 175, 818–826. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Hussain, A.; Hasnain, S. Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J. Microbiol. Biotechnol. 2011, 27, 2645–2654. [Google Scholar] [CrossRef]
- Miyawaki, K.; Matsumoto-Kitano, M.; Kakimoto, T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 2004, 37, 128–138. [Google Scholar] [CrossRef]
- Abbasdokht, H.; Gholami, A. The effect of seed inoculation (Pseudomonas putida + Bacillus lentus) and different levels of fertilizers on yield and yield components of wheat (Triticum aestivum L.) cultivars. Int. J. Agric. Biosyst. Eng. 2010, 4, 678–682. [Google Scholar] [CrossRef]
- Cai, T.; Xu, H.; Peng, D.; Yin, Y.; Yang, W.; Ni, Y.; Chen, X.; Xu, C.; Yang, D.; Cui, Z.; et al. Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crops Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Y.; Tang, H.; Sui, N.; Zhang, X.; Wang, F. Genetic, hormonal, and environmental control of tillering in wheat. Crop J. 2021, 9, 986–991. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Сhеbotаr, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Villaume, S.; Rabenoelina, F.; Crouzet, J.; Clément, C.; Vaillant-Gaveau, N.; Dhondt-Cordelier, S. Different Arabidopsis thaliana photosynthetic and defense responses to hemibiotrophic pathogen induced by local or distal inoculation of Burkholderia phytofirmans. Photosynth. Res. 2017, 134, 201–214. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Kangas, A.; Salo, Y.; Jauhiainen, L. Grain number dominates grain weight in temperate cereal yield determination: Evidence based on 30 years of multi-location trials. Field Crops Res. 2007, 100, 179–188. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crops Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Fischer, R.A. Wheat physiology: A review of recent developments. Crop Pasture Sci. 2011, 62, 95–114. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- González, F.G.; Miralles, D.J.; Slafer, G.A. Wheat floret survival as related to pre-anthesis spike growth. J. Exp. Bot. 2011, 62, 4889–4901. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Savin, R.; Slafer, G.A. Is floret primordia death triggered by floret development in durum wheat? J. Exp. Bot. 2013, 64, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Terrile, I.I.; Miralles, D.J.; González, F.G. Fruiting efficiency in wheat (Triticum aestivum L): Trait response to different growing conditions and its relation to spike dry weight at anthesis and grain weight at harvest. Field Crops Res. 2017, 201, 86–96. [Google Scholar] [CrossRef]
- Abeledo, L.G.; Alvarez Prado, S.; Puhl, L.E.; Zhou, Y.; Costa, J.M.; Miralles, D.J. Phenotypic and genetic analysis to identify secondary physiological traits for improving grain yield in wheat considering anthesis time variability. Euphytica 2019, 215, 171. [Google Scholar] [CrossRef]
- Pishchik, V.N.; Ktitorova, I.N.; Skobeleva, O.V.; Mirskaya, G.V.; Vorobyov, N.I. Method of instrumental assessment of plant-bacterial interaction in agricultural system in forecasting the yield. In EIITA/WCCA, Proceedings of the 8th European Federation of Information Technology in Agriculture, Food and the Environment/World Congress on Computers in Agriculture, Prague, Czech Republic; Czech Centre for Science and Society: Praha, Czech Republic, 2011; pp. 597–605. [Google Scholar]
- Pishchik, V.N.; Vorobyev, N.I.; Moiseev, K.G.; Sviridova, O.V.; Surin, V.G. Influence of Bacillus subtilis on the physiological state of wheat and the microbial community of the soil under different rates of nitrogen fertilizers. Eurasian Soil Sci. 2015, 48, 77–84. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Baltensperger, D.D.; Santra, D.K.; Hergert, G.W.; Knox, S. Gibberellic acid promotes early growth of winter wheat and rye. Am. J. Plant Sci. 2014, 5, 50149. [Google Scholar] [CrossRef]
- Pearce, S.; Vanzetti, L.S.; Dubcovsky, J. Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1. Plant Physiol. 2013, 163, 1433–1445. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, M.; Liu, L.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.-C. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Egorshina, A.A.; Khairullin, R.M.; Sakhabutdinova, A.R.; Luk’yantsev, M.A. Involvement of phytohormones in the development of interaction between wheat seedlings and endophytic Bacillus subtilis strain 11BM. Russ. J. Plant Physiol. 2012, 59, 134–140. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Chen, Y.H.; Martin, S.; Alhusaini, N.; Green, R.; Coller, J. The DEAD-box protein Dhh1p couples mRNA decay and translation by monitoring codon optimality. Cell 2016, 167, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Joo, G.J.; Kim, Y.M.; Lee, I.J.; Song, K.S.; Rhee, I.K. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol. Lett. 2004, 26, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hasnain, S. Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol. J. Microbiol. 2014, 63, 261. [Google Scholar] [CrossRef] [PubMed]
- Retzer, K.; Butt, H.; Korbei, B.; Luschnig, C. The far side of auxin signaling: Fundamental cellular activities and their contribution to a defined growth response in plants. Protoplasma 2014, 251, 731–746. [Google Scholar] [CrossRef]
- Ruzza, V.; Sessa, G.; Sassi, M.; Morelli, G.; Ruberti, I. Auxin coordinates shoot and root development during shade avoidance response. In Auxin and Its Role in Plant Development; Zažímalová, E., Petrášek, J., Benková, E., Eds.; Springer: Vienna, Austria, 2014; pp. 389–412. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Laskowski, M. Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. J. Exp. Bot. 2013, 64, 2609–2617. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Vernoux, T. Auxin, Chief Architect of the Shoot Apex. In Auxin and Its Role in Plant Development; Zažímalová, E., Petrášek, J., Benková, E., Eds.; Springer: Vienna, Austria, 2014; pp. 191–212. [Google Scholar] [CrossRef]
- Demeulenaere, M.J.F.; Beeckman, T. The interplay between auxin and the cell cycle during plant development. In Auxin and Its Role in Plant Development; Zažímalová, E., Petrášek, J., Benková, E., Eds.; Springer: Vienna, Austria, 2014; pp. 119–141. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2015; p. 390. [Google Scholar] [CrossRef]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates theexpression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.Y.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L.(wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhang, X.; Lv, G.; Pan, Y.; Chen, F. Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat. BMC Plant Biol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef]
- Moon, J.; Suh, S.-S.; Lee, H.; Choi, K.-R.; Hong, C.B.; Paek, N.-C.; Kim, S.-G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Shitsukawa, N.; Ikari, C.; Mitsuya, T.; Sakiyama, T.; Ishikawa, A.; Takumi, S.; Murai, K. Wheat SOC1 functions independently of WAP1/VRN1, an integrator of vernalization and photoperiod flowering promotion pathways. Physiol. Plant. 2007, 130, 627–636. [Google Scholar] [CrossRef]
- Rudakova, A.S.; Rudakov, S.V.; Davydova, N.V.; Mirskaya, G.V.; Zhuravleva, E.V.; Chesnokov, Y.V. Isoenzyme analysis of esterases in mature seeds of hexaploid soft wheat (Triticum aestivum L.). Agric. Biol. 2016, 51, 327–334. [Google Scholar] [CrossRef]
- Graner, A.; Jahoor, A.; Schondelmaier, J.; Siedler, H.; Pillen, K.; Fischbeck, G.; Wenzel, G.; Herrmann, R.G. Construction of an RFLP map of barley. Theor. Appl. Genet. 1991, 83, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Helguera, M.; Kato, K.; Fukuyama, S.; Sherman, J.; Dubcovsky, J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 2004, 109, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Szűcs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; Von Zitzewitz, J.; Hayes, P.M.; Dubcovsky, J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Normand, P.; Cournoyer, B.; Simonet, P.; Nazaret, S. Analysis of a ribosomal RNA operon in the actinomycete Frankia. Gene 1992, 111, 119–124. [Google Scholar] [CrossRef]
- Onishchuk, O.P.; Chizhevskaya, E.P.; Kurchak, O.N.; Andronov, E.E.; Simarov, B.V. Identification of new genes of nodule bacteria Sinorhizobium meliloti involved in the control of efficiency of symbiosis with alfalfa Medicago sativa. Russ. J. Genet. Appl. Res. 2015, 5, 126–131. [Google Scholar] [CrossRef]
- Hugh, R.; Leifson, F. The taxonomic significance of fermentative oxydation metabolism of carbohydrates by various Gram-negative bacteria. J. Bacteriol. 1953, 66, 24–26. [Google Scholar] [CrossRef]
- Microbiological Methods, 5th ed.; Collins, C.H., Lyne, P.M., Eds.; Butterworth & Co, Ltd.: London, UK, 1985; p. 450. [Google Scholar]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Joshi, M.; Prasanna, R.; Kumar, K.; Nain, L. Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann. Microbiol. 2011, 61, 893–900. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis Strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chesnokov, Y.V.; Mirskaya, G.V.; Kanash, E.V.; Kocherina, N.V.; Lohwasser, U.; Börner, A. QTL mapping of bread wheat (Triticum aestivum L.) grown under controlled conditions of an agroecobiological testing ground. Russ. J. Plant Physiol. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Kochetov, A.A.; Mirskaya, G.V.; Sinyavina, N.G.; Egorova, K.V. Transgressive breeding: A methodology for accelerated creating of new forms of plants with a predictable complex of economically valuable traits. Russ. Agric. Sci. 2021, 47, S40–S50. [Google Scholar] [CrossRef]
- Ermakov, A.I.; Arasimovich, V.V.; Yarosh, N.P.; Peruansky, Y.V.; Lukovnikova, G.A.; Ikonnikova, M.I. Methods of biochemical analysis of plants. Leningr. Agropromizdat 1987, 3, 430. (In Russian) [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).