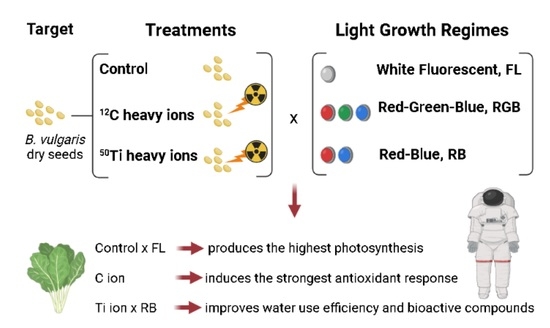
Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space
Abstract
:1. Introduction
2. Results
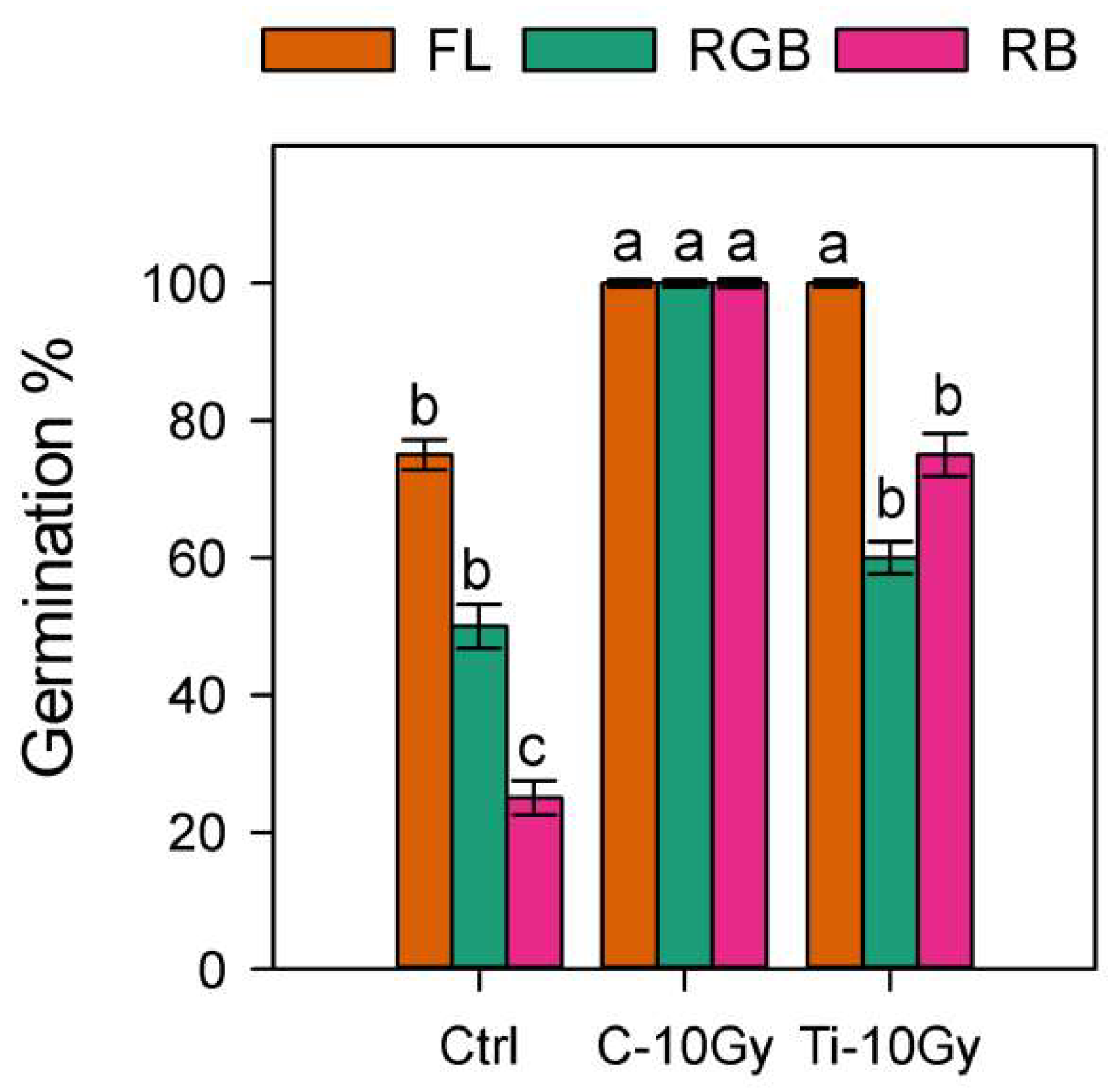
2.1. Germination and Plant Biomass
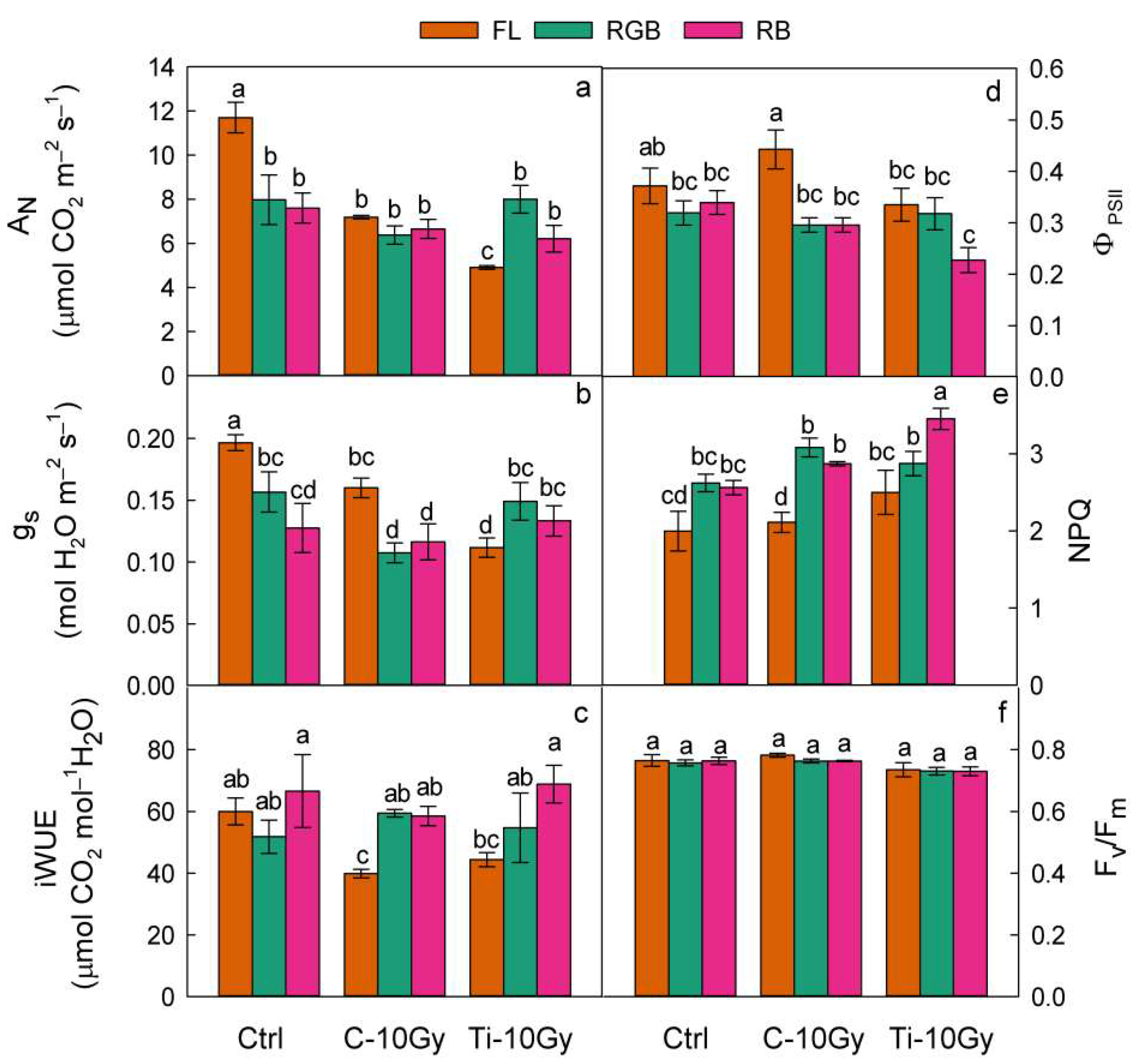
2.2. Gas Exchanges and Chlorophyll Fluorescence Emission Measurements
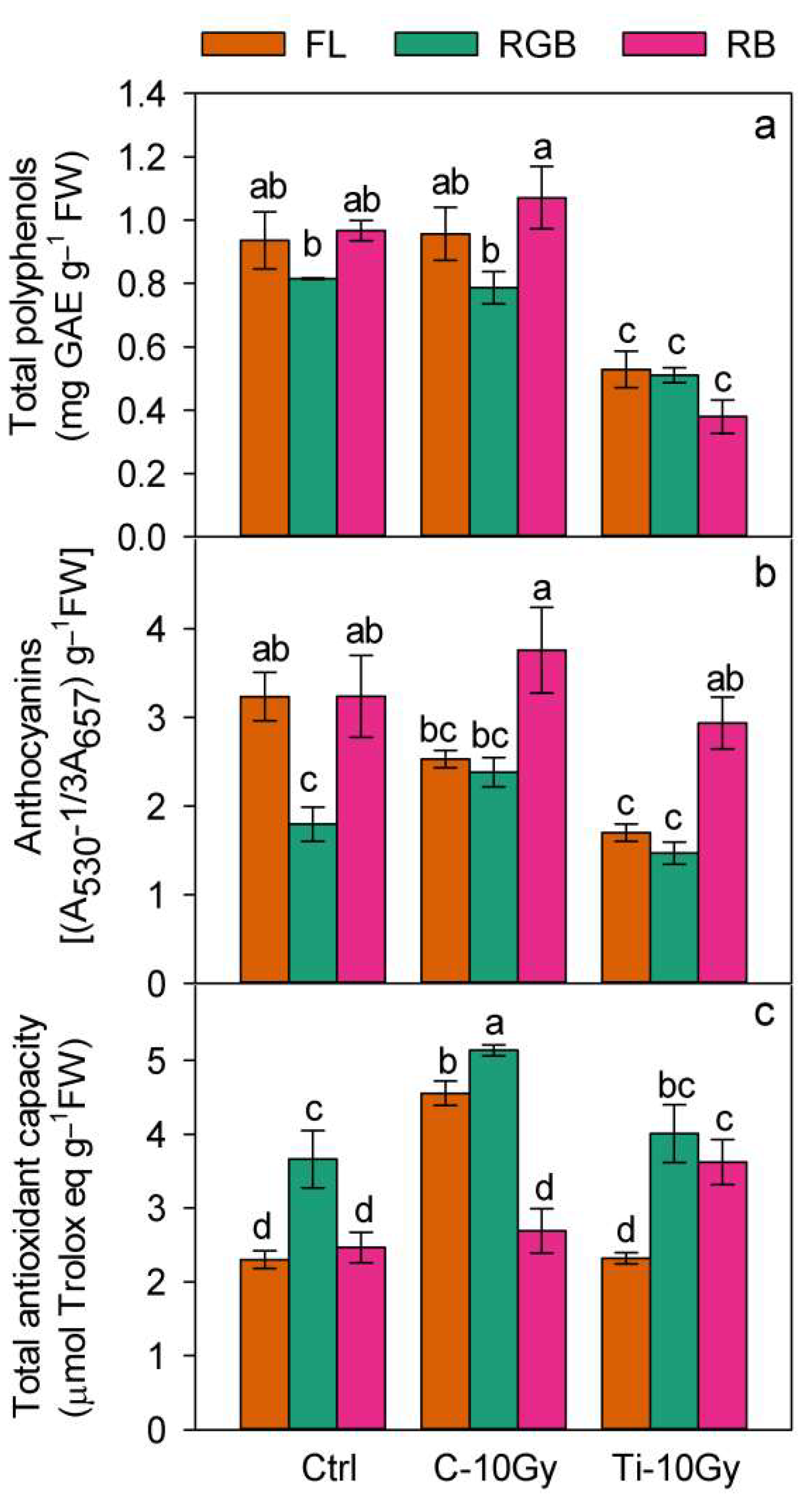
2.3. Plants Nutritional Traits and Bioactive Compounds
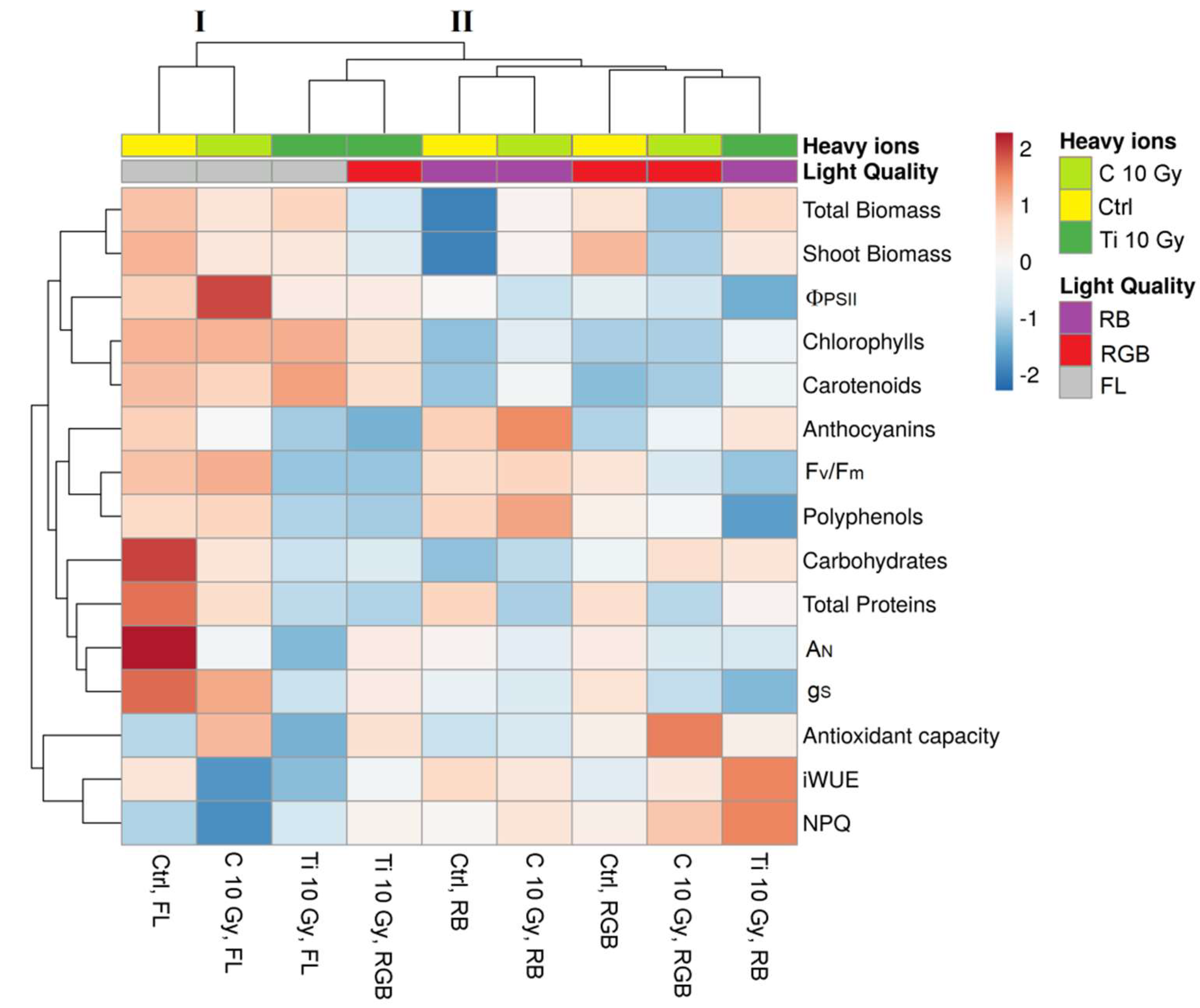
2.4. Heatmap Analysis
3. Discussion
4. Materials and Methods
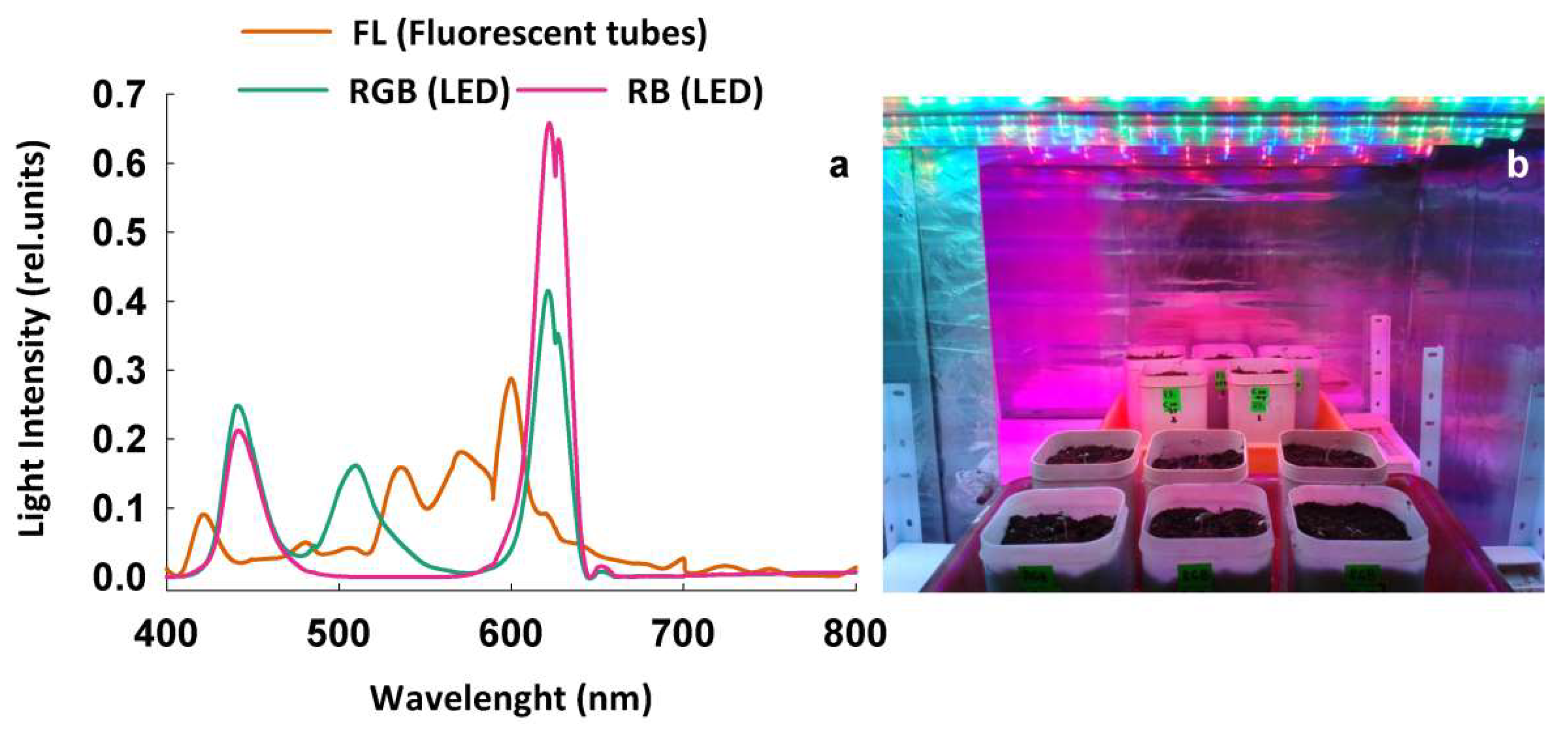
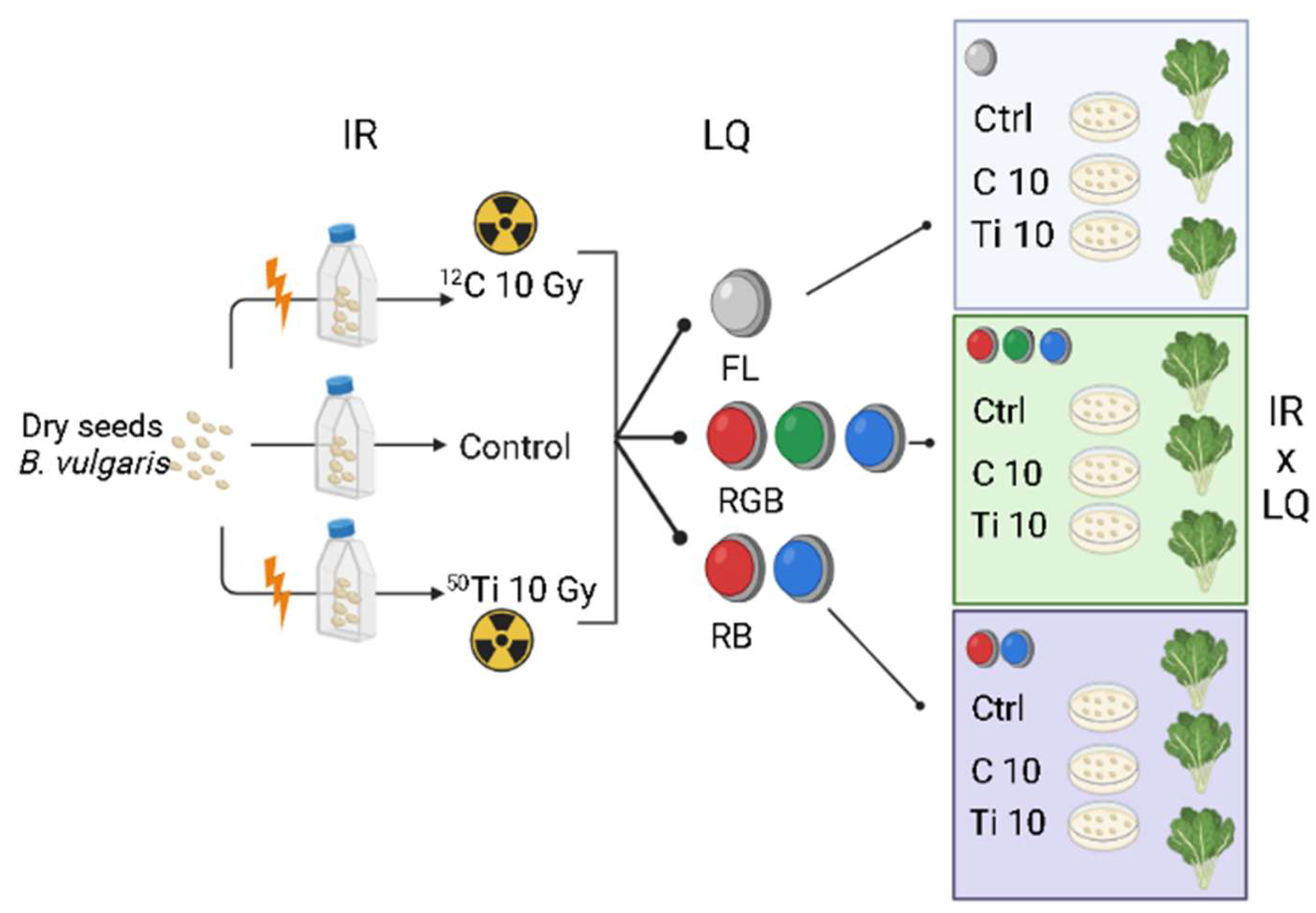
4.1. Plant Material, Irradiation Procedure, and Experimental Design
4.2. Germination and Biometrical Measurements
4.3. Leaf Gas Exchange and Chlorophyll aFluorescence Emission Measurements
4.4. Determinationof Photosynthetic Pigments and Antioxidants
4.5. Total Soluble Carbohydrate Content and Protein Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolff, S.A.; Coelho, L.H.; Zabrodina, M.; Brinckmann, E.; Kittang, A.I. Plant mineral nutrition, gas exchanges and photosynthesis in space: A review. Adv. Space Res. 2013, 51, 465–475. [Google Scholar] [CrossRef]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Zabel, P. Designing a Closed Ecological Life Support System for Plants, Overview. In Handbook of Life Support Systems for Spacecraft and Extraterrestrial Habits; Seedhouse, E., Shayler, D., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Arena, C.; Vitale, E.; Hay Mele, B.; Cataletto, P.R.; Turano, M.; Simoniello, P.; De Micco, V. Suitability of Solanum lycopersicum L. ‘Microtom’ to be grown in Bioregenerative Life Support Systems: Exploring the effect of high-LET ionising radiation on photosynthesis, leaf structure and fruit traits. Plant Biol. 2019, 21, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; De Micco, V.; De Maio, A. Growth alteration and leaf biochemical responses in Phaseolus vulgaris exposed to different doses of ionising radiation. Plant Biol. 2014, 161, 194–202. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Hwang, J.E.; Hwang, S.G.; Kim, S.E.; Lee, K.J.; Jang, C.S.; Kim, J.B.; Kim, S.H.; Ha, B.K.; Ahn, J.W.; Kang, S.Y.; et al. Transcriptome profiling in response to different types of ionizing radiation and identification of multiple radio marker genes in rice. Physiol. Plant. 2014, 150, 606–679. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.J.; Kim, D.S.; Jang, C.S. Rice RING E3 ligase may negatively regulate gamma-ray response to mediate the degradation of photosynthesis-related proteins. Planta 2015, 241, 1119–1129. [Google Scholar] [CrossRef]
- Arena, C.; Turano, M.; Hay Mele, B.; Cataletto, P.R.; Furia, M.; Pugliese, M.; De Micco, V. Anatomy, photochemical activity, and DNA polymorphism in leaves of dwarf tomato irradiated with X-rays. Biol. Plant. 2017, 61, 205–314. [Google Scholar] [CrossRef]
- Zaka, R.; Vandecasteele, C.M.; Misset, T.M. Effect of flow chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipacapillata (Poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef]
- Esnault, M.A.; Legue, F.; Chenal, C. Ionizing radiation: Advances in plant response. Environ. Exp. Bot. 2010, 68, 23–237. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, Y.R.; Lee, M.H.; Kim, J.H.; Wi, S.G.; Park, B.J.; Kim, C.S.; Chung, B.Y. Photosynthetic capacity of Arabidopsis Plants at the reproductive stage tolerates γ-irradiation. J. Radiat. Res. 2011, 52, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, S.; Parween, T.; Siddiqqi, T.O.; Mahmooduzzafar. Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Schuy, C.; Weber, U.; Durante, M. Hybrid active-passive space radiation simulation concept for GSI and the future FAIR facility. Front. Phys. 2020, 8, 337. [Google Scholar] [CrossRef]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef]
- Jangiam, W.; Tungjai, M.; Rithidech, K.N. Induction of chronic oxidative stress, chronic inflammation and aberrant patterns of DNA methylation in the liver of titanium exposed CBA/Caj mice. Int. J. Radiat. Biol. 2015, 91, 389–398. [Google Scholar] [CrossRef]
- Rithidech, K.N.; Jangiam, W.; Tungjai, M.; Gordon, C.; Honikei, L.; Whorton, E.B. Induction of chronic inflammation and altered levels of DNA Hydroxymethylation in Somatic and Germinal tissue of CBA/CaJ Mice exposed to 48Ti ions. Front. Oncol. 2016, 6, 155. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jella, K.K.; Jaafar, L.; Li, S.; Park, S.; Story, M.D.; Wang, H.; Wang, Y.; Dynan, W.S. Exposure to galactic cosmic radiation compromises DNA repair and increases the potential for oncogenic chromosomal. Sci. Rep. 2018, 8, 11038. [Google Scholar] [CrossRef]
- De Micco, V.; De Francesco, S.; Amitrano, C.; Arena, C. Comparative Analysis of the Effect of Carbon- and Titanium-Ions Irradiation on Morpho-Anatomical and Biochemical Traits of Dolichosmelanophthalmus DC. Seedlings Aimed to Space Exploration. Plants 2021, 10, 2272. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottesen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Liu, X.Y.; Guo, S.R.; Xu, Z.G.; Jiao, X.L.; Takafumi, T. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by light-emitting diodes. HortScience 2011, 45, 217–221. [Google Scholar]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sust. Energ. Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef]
- Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LED) with and without supplemental blue light. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanusorientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the red-light syndrome in cucumber. Environ. Exp. Bot. 2016, 121, 12175–12182. [Google Scholar] [CrossRef]
- Izzo, L.G.; Hay Mele, B.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, V.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Amitrano, C.; Vitale, E.; De Micco, V.; Arena, C. Light fertilization affects growth and photosynthesis in mung bean (Vigna radiata) plants. J. Environ. Account. Manag. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Sams, C.E.; Kopsell, D.; Morrow, R.C. Light quality impacts on growth, flowering, mineral uptake and petal pigmentation of marigold. Proc. VIII Int. Symp. Light Hortic. 2016, 8, 139–145. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Tuteja, N.; Gill, S.S.; Tuteja, R. Plant responses to abiotic stresses: Shedding light on salt, drought, cold and heavy metal stress. In Omics and Plant Abiotic Stress Tolerance; Tuteja, N., Singh, S.G., Tuteja, R., Eds.; Bentham Science Publisher Ltd.: Sharjah, United Arab Emirates, 2011; Volume 3, pp. 39–64. [Google Scholar]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Gosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 12, 1420. [Google Scholar] [CrossRef] [Green Version]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A.; Cary, A. Plant productivity in response to LED lighting. Hortic. Sci. 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Avercheva, O.; Berkovich, Y.A.; Smolyanina, S.; Bassarskaya, E.; Pogosyan, S.; Ptushenko, V.; Erokhin, A.; Zhigalova, T. Biochemical, photosynthetic and productive parameters of Chinese cabbage grown under blue–red LED assembly designed for space agriculture. Adv. Space Res. 2014, 53, 1574–1581. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. Hortic. Rev. 2015, 43, 1–87. [Google Scholar]
- Gómez, C.; Izzo, L.G. Increasing efficiency of crop production with LEDs. AIMS Agric. Food 2018, 3, 135–153. [Google Scholar] [CrossRef]
- De Micco, V.; Amitrano, C.; Vitaglione, P.; Ferracane, C.; Pugliese, M.; Arena, C. Effect of light quality and ionising radiation on morphological and nutraceutical traits of sprouts for astronauts’ diet. Acta Astronaut. 2021, 185, 188–197. [Google Scholar] [CrossRef]
- Vitale, E.; Vitale, L.; Costanzo, G.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation. Plants 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, L.; Milašević, I.; Topalović, A.; Durović, D.; Mugoša, B.; Knežević, M.; Vrvić, M. Nutritional and phytochemical content of Swiss chard from Montenegro, under different fertilization and irrigation treatments. Br. Food J. 2018, 121, 411–425. [Google Scholar] [CrossRef]
- Sjahril, R.; Riadi, M.; Raffiundin, S.T.; Toriyama, K.; Abe, T.; Trisnawaty, A.R. Effect of heavy ion beam irradiation on germination of local Toraja rice seed (M1-M2) mutant generation. IOP Conference Series. Earth Environ. Sci. 2018, 157, 012046. [Google Scholar] [CrossRef]
- Komai, F.; Shikazono, N.; Tanaka, A. Sexual modification of female spinach seeds (Spinacia oleracea L.) by irradiation with ion particles. Plant Cell Rep. 2003, 21, 713–717. [Google Scholar] [CrossRef]
- Hammond, E.C.; Bridgers, K.; Berry, F.D. Germination, growth rates, and electron microscope analysis of tomato seeds flown on the LDEF. Radiat. Meas. 1996, 26, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A Role for Barley Cryptochrome1 in Light Regulation of Grain Dormancy and Germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A-and B- specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [Green Version]
- Thiede, M.E.; Link, S.O.; Fellows, R.J.; Beedlow, P.A. Effect of gamma radiation on stem diameter growth, carbon gain and biomass partitioning in Helianthus annus. Environ. Exp. Bot. 1995, 35, 33–41. [Google Scholar] [CrossRef]
- Honda, I.; Kikuchi, K.; Matsuo, S.; Fukuda, M.; Saito, H.; Ryuto, H.; Fukunishi, N.; Abe, T. Heavy-ion-induced mutants in sweet pepper isolated by M1 plant selection. Euphytica 2006, 152, 61–66. [Google Scholar] [CrossRef]
- De Micco, V.; Paradiso, R.; Aronne, G.; De Pascale, S.; Quarto, M.; Arena, C. Leaf Anatomy and Photochemical Behaviour of Solanum lycopersicum L. Plants from Seeds Irradiated with Low-LET Ionising Radiation. Sci. World J. 2014, 2014, 428141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.D.; Kim, S.H.; Hwang, J.E.; Kim, Y.S.; Kang, H.S.; Kim, S.W.; Kwon, S.J.; Ryu, J.; Kim, J.B.; Kang, S.Y. Construction of mutation populations by Gamma-ray and Carbon beam irradiated in Chili pepper (Capsicum annuum L.). Hortic. Environ. Biotchnol. 2016, 57, 606–614. [Google Scholar] [CrossRef]
- Kim, H.H.; Wheeler, R.M.; Sager, J.C.; Gains, G.D.; Naikane, J.H. Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment—A review of research at Kennedy Space Center. V International Symposium on Artificial Lighting in Horticulture. Acta Hortic. 2006, 711, 111–120. [Google Scholar] [CrossRef]
- Ursino, D.J.; Schefski, H.; McCabe, J. Radiation-induced changes in rate of photosynthetic CO2 uptake in soybean plants. Environ. Exp. Bot. 1977, 17, 27–34. [Google Scholar] [CrossRef]
- Jia, C.F.; Li, A.L. Effect of gamma radiation on mutant induction of Fagopyrum dibotrys Hara. Photosynthetica 2008, 46, 363–369. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Maheran, A.A.; Philip, E. Effects of Acute Gamma Irradiation on Physiological Traits and Flavonoid accumulation of Centella asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Shi, M.; Huang, J.Z.; Xu, J.; Wang, Z.D.; Guo, D.P. Regulation of photosynthetic performance and antioxidant capacity by 60Co γ-irradiation in Zizania latifolia plants. J. Environ. Radioact. 2014, 129, 33–42. [Google Scholar] [CrossRef]
- Jones, H. What is water use efficiency. In Water Use Efficiency in Plant Biology? Bacon, M., Ed.; Blackwell Publishing: Hoboken, NJ, USA; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Sun, J.; Nishio, J.N.; Vofelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, U. Leaf Functional Anatomy in Relation to Photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Van-Labeke, M.C. Long-term effects of Red-and Blue-Light Emitting Diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akoyunoglou, G.; Anni, H. Blue light effect on chloroplast development in higher plants. In Blue Light Effects in Biological Systems; Senger, H., Ed.; Springer: Berlin, Germany, 1984; pp. 397–406. [Google Scholar]
- Sæbø, A.; Krekling, T.; Applegren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Org. Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.Y.; Wang, L.T.; Ma, J.H.; Ma, E.D.; Li, J.Y.; Gong, M. Effect of light quality on growth and development, photosynthetic characteristics and content of carbohydrates in tobacco (Nicotiana tabacum L.) plants. Photosynthetica 2017, 55, 467–477. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Garab, G.; Adams, W.W.; Govindjee, U. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee, U.; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rise (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef]
- Fan, X. Antioxidant capacity of fresh-cut vegetables exposed to ionizing radiation. J. Sci. Food Agric. 2005, 85, 995–1000. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Fang, S.; Zhou, M.; Qin, J. Responses of Morphology, Gas Exchange, Photochemical Activity of Photosystem II, and Antioxidant Balance in Cyclocaryapaliurus to Light Spectra. Front. Plant Sci. 2018, 9, 1704. [Google Scholar] [CrossRef] [Green Version]
- Stajner, D.; Milosevic, M.; Popovic, B.M. Irradiation effects on phenolic content, lipid and protein oxidation and scavenger ability of soybean seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological Responses of Orthosiphon stamineus Plantles to Gamma Irradiation. Am. Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]
- Hammeed, A.; Shah, T.M.; Atta, B.M.; Haq, M.A.; Sayed, H. Gamma Irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in Desi and Kabuli Chickpea. Pak. J. Bot. 2008, 40, 1033–1041. [Google Scholar]
- Chen, Q.F.; Ya, H.Y.; Feng, Y.R.; Jiao, Z. Expression of the Key Genes Involved in ABA Biosynthesis in Rice Implanted by Ion Beam. Appl. Biochem. Biotechnol. 2014, 173, 239–247. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Zhang, Z.; Yang, R.; Yang, X. Dynamic effects of different light qualities on pea sprouts quality. North. Hortic. 2010, 8, 4–7. [Google Scholar]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of Different Light Sources on the Growth of Non-heading Chinese Cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.; Kroon, P.A.; Quideau, S. Plant phenolics—Secondary metabolites with diverse functions. In Recent Advances in Polyphenol Research; Daay, F., Lattanzio, V., Eds.; Wiley-Blackwell: Oxford, UK, 2008; pp. 1–35. [Google Scholar]
- De Micco, V.; Arena, C.; Aronne, G. Anatomical alterations of Phaseolus vulgaris L. mature leaves irradiated with X-rays. Plant Biol. 2014, 16, 187–193. [Google Scholar] [CrossRef]
- He, J.; Dong, L.; Lixia, Y.; Wenjian, L. Pigment analysis of color-leaf mutant in Wanderig Jew (Tradescantiafluminensis) irradiated by carbon ions. Nucl. Sci. Techol. 2011, 22, 77–83. [Google Scholar]
- Arena, C.; De Micco, V.; Aronne, G.; Pugliese, M.; Virzo, A.; De Maio, A. Response of Phaseolus vulgaris L. plants to low-LET ionisingradiation: Growth and oxidative stress. Acta Astronaut. 2013, 91, 107–114. [Google Scholar] [CrossRef]
- Lekkham, P.; Srilaong, V.; Pongprasert, N.; Kondo, S. Anthocyanin concentration and antioxidant activity in light-emitting diode (LED)-treated apples in a greenhouse environmental control system. Fruits 2016, 71, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Content in Acyanic and Cyanic OciumBasilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livadariu, O.; Raiciu, D.; Maximilian, C.; Capitanu, E. Studies regarding treatments of LED-s emitted light on sprouting hemp (Cannabis sativa L.). Rom. Biotechnol. Lett. 2018, 24, 485–490. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Effects of radiation processing on phytochemicals and antioxidants in plant procedure. Trends Food Sci. Technol. 2009, 20, 201–212. [Google Scholar] [CrossRef]
- Sallam, E.M.; Anwar, M.M. Antioxidant Activity of some Extracts from Gamma irradiated Purslane (Portulaca oleracea) Plant. Int. J. Agric. Biol. 2017, 19, 48–52. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fukiwara, K.; Kurata, K. Effect of light quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Urbanovičiūtė, A.; Brazaitytė, A.; Šabajevienė, G.; Sakalauskaitė, J.; Duchovskis, P. The impact of LED illumination on antioxidant properties of sprouted seeds. Cent. Eur. J. Biol. 2011, 6, 68–74. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgariscicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Kazama, Y.; Hirano, T.; Saito, H.; Liu, Y.; Ohbu, S.; Hayashi, Y.; Abe, T. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. Plant Biol. 2011, 11, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Temperature dependence of violaxanthin deepoxidation and nonphotochemical fluorescence quenching in intact leaves of Gossypium kirsutum L. and Malva parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef]
- Lichtentaler, H.K. Chlorophylls and Carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Mancinelli, A.L.; Yang, C.P.H.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of Anthocyanin Synthesis. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersicumesculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate chemistry. In Methods in Carbohydrate Chemistry; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; Volume 17. [Google Scholar]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A universal and rapid protocol for protein extraction from recalcitrant plant tissue for proteomic analysis. J. Electrophor. 2006, 27, 2782–2786. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| TB | SB | |
|---|---|---|
| IR | ||
| Ctrl | 25 a | 21 a |
| C-10 | 27 a | 21 a |
| Ti-10 | 27 a | 21 a |
| LQ | ||
| FL | 29 a | 23 a |
| RGB | 24 a | 19 a |
| RB | 26 a | 21 a |
| IR × LQ | ||
| Ctrl-FL | 31 a | 25 a |
| Ctrl-RGB | 28 a | 24 a |
| Ctrl-RB | 17 b | 14 b |
| C 10-FL | 28 a | 22 a |
| C 10-RGB | 25 a | 21 a |
| C 10-RB | 26 a | 21 a |
| Ti 10-FL | 30 a | 22 a |
| Ti 10-RGB | 23 a | 19 a |
| Ti 10-RB | 29 a | 22 a |
| Significance | ||
| IR | NS | NS |
| LQ | NS | NS |
| IR × LQ | * | * |
| AN | gs | iWUE | ϕPSII | NPQ | Fv/Fm | |
|---|---|---|---|---|---|---|
| IR | ||||||
| Ctrl | 9.1 a | 0.16 a | 59 a | 0.34 a | 2.4 b | 0.76 a |
| C-10 | 6.7 b | 0.13 b | 53 a | 0.34 a | 2.7 a | 0.75 a |
| Ti-10 | 6.4 b | 0.13 b | 56 a | 0.29 a | 2.8 a | 0.74 a |
| LQ | ||||||
| FL | 7.9 a | 0.16 a | 48 b | 0.38 a | 2.2 b | 0.75 a |
| RGB | 7.4 b | 0.14 b | 55 b | 0.31 b | 2.8 a | 0.75 a |
| RB | 6.8 c | 0.12 c | 65 a | 0.28 b | 2.9 a | 0.75 a |
| Significance | ||||||
| IR | *** | *** | NS | NS | ** | NS |
| LQ | * | *** | ** | * | *** | NS |
| IR × LQ | *** | *** | NS | NS | *** | NS |
| CHL | CAR | CARB | PROT | POL | ANTH | TAC | |
|---|---|---|---|---|---|---|---|
| IR | |||||||
| Ctrl | 38 b | 5.7 b | 36 a | 480 a | 0.91 a | 2.8 a | 2.8 c |
| C-10 | 41 b | 6.7 b | 33 b | 347 b | 0.94 a | 2.9 a | 4.1 a |
| Ti-10 | 47 a | 8.4 a | 31 b | 321 b | 0.47 b | 2.0 b | 3.3 b |
| LQ | |||||||
| FL | 54 a | 8.8 a | 42 a | 427 a | 0.81 a | 2.5 b | 3.0 b |
| RGB | 37 b | 5.7 b | 33 b | 337 b | 0.70 b | 1.9 c | 4.3 a |
| RB | 36 b | 5.9 b | 26 c | 381 a | 0.80 a | 3.3 a | 2.9 b |
| Significance | |||||||
| IR | *** | *** | * | *** | *** | *** | *** |
| LQ | *** | *** | *** | * | * | *** | *** |
| IR × LQ | *** | *** | *** | ** | ** | NS | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, E.; Izzo, L.G.; Amitrano, C.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space. Plants 2022, 11, 1816. https://doi.org/10.3390/plants11141816
Vitale E, Izzo LG, Amitrano C, Velikova V, Tsonev T, Simoniello P, De Micco V, Arena C. Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space. Plants. 2022; 11(14):1816. https://doi.org/10.3390/plants11141816
Chicago/Turabian StyleVitale, Ermenegilda, Luigi Gennaro Izzo, Chiara Amitrano, Violeta Velikova, Tsonko Tsonev, Palma Simoniello, Veronica De Micco, and Carmen Arena. 2022. "Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space" Plants 11, no. 14: 1816. https://doi.org/10.3390/plants11141816
APA StyleVitale, E., Izzo, L. G., Amitrano, C., Velikova, V., Tsonev, T., Simoniello, P., De Micco, V., & Arena, C. (2022). Light Quality Modulates Photosynthesis and Antioxidant Properties of B. vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space. Plants, 11(14), 1816. https://doi.org/10.3390/plants11141816