Predicting Tumor Recurrence with Early 18F-FDG PET-CT After Thermal and Non-Thermal Ablation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
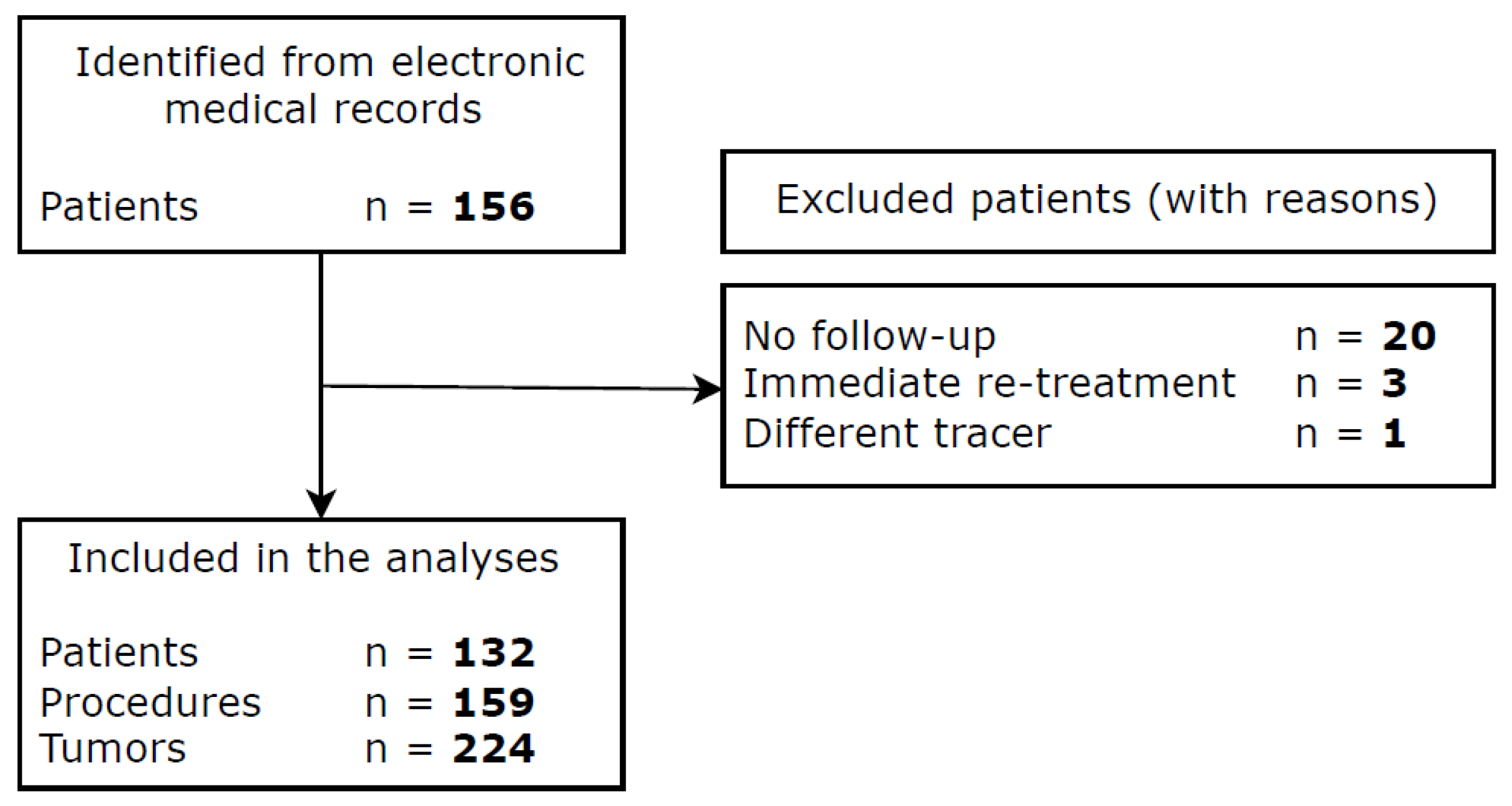
2.1. Patient Selection and Data Collection
2.2. Ablation Procedures
2.3. Imaging and Follow-Up
2.4. Outcome Measures and Statistical Analysis
3. Results
3.1. Baseline Characteristics
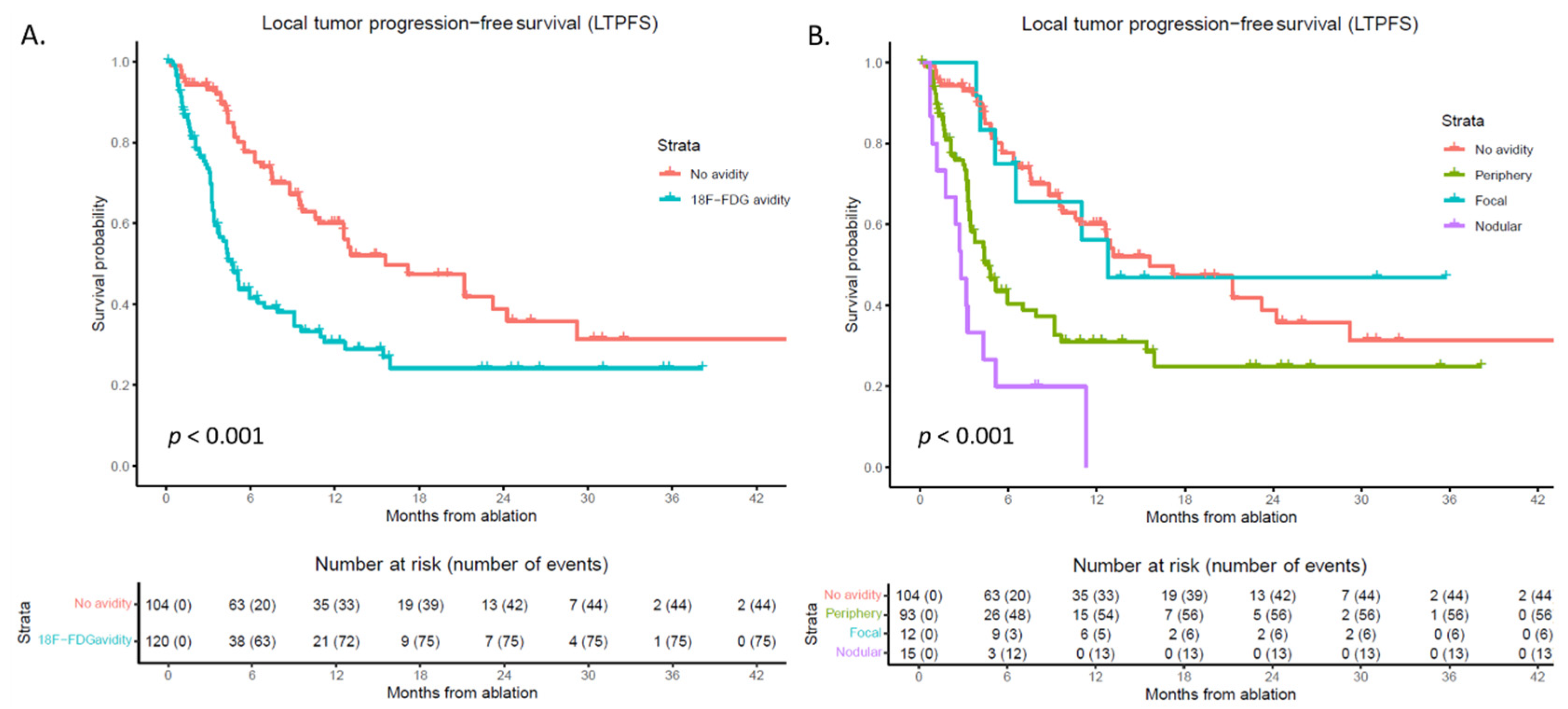
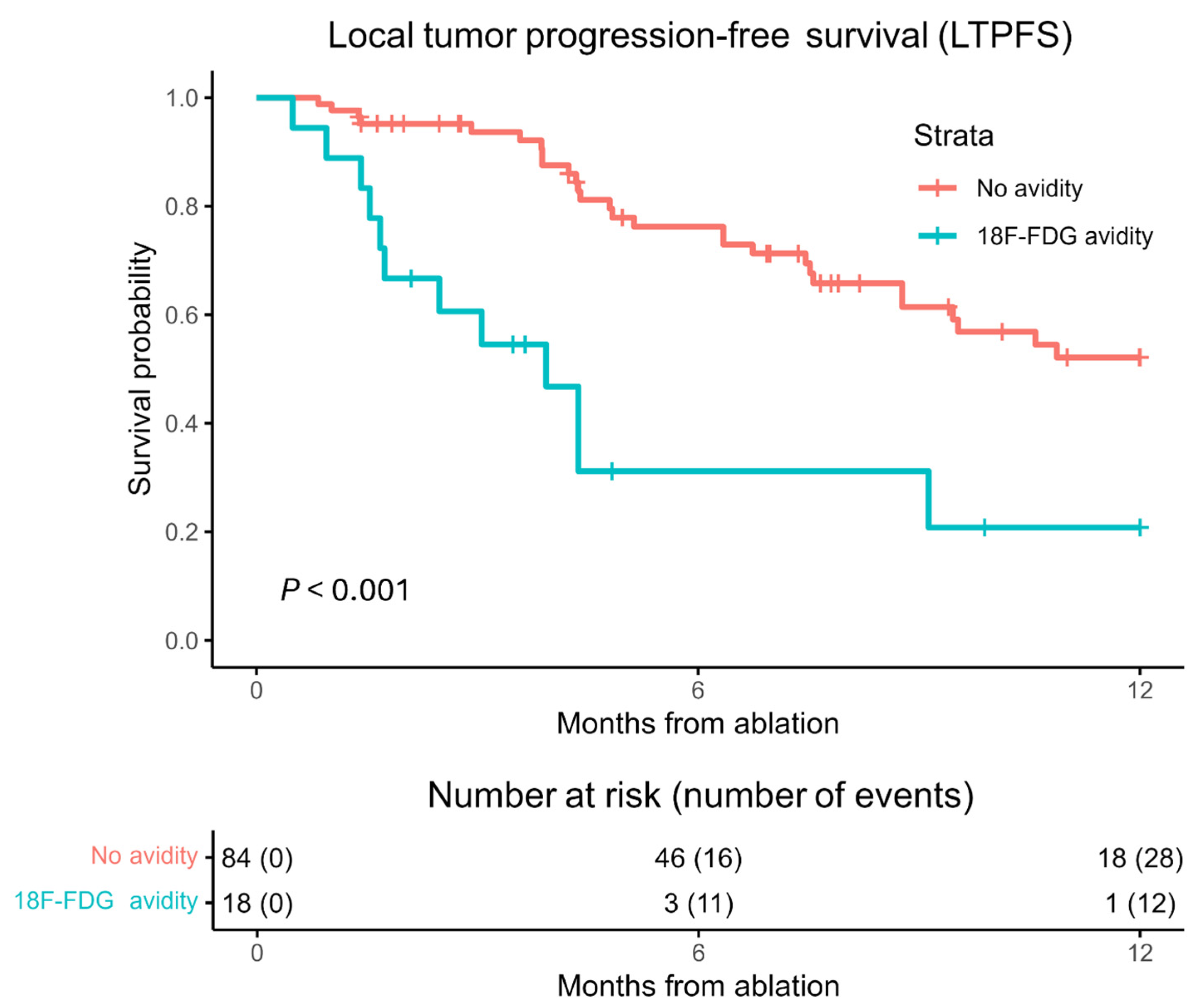
3.2. Local Tumor Progression-Free Survival (LTPFS)
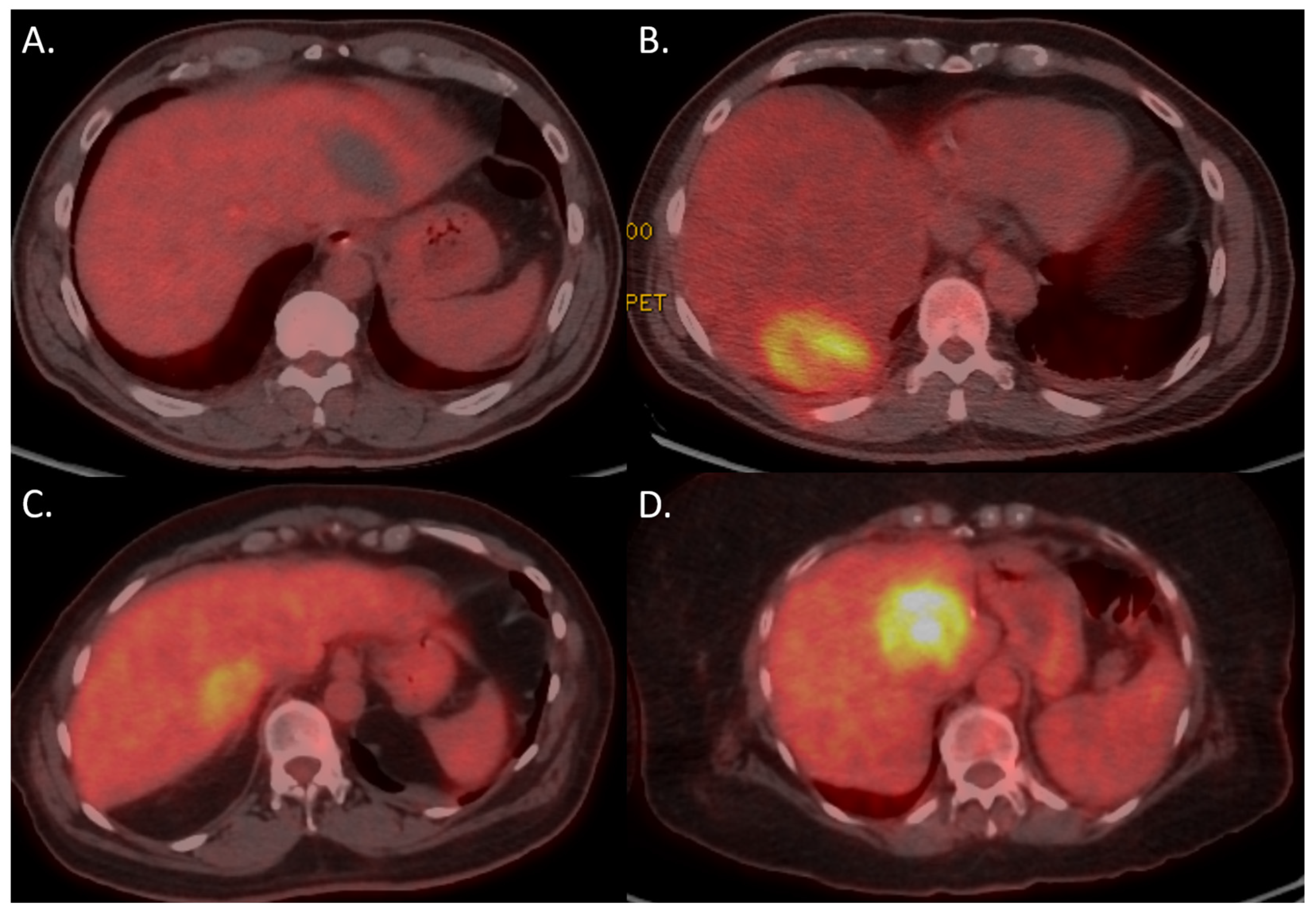
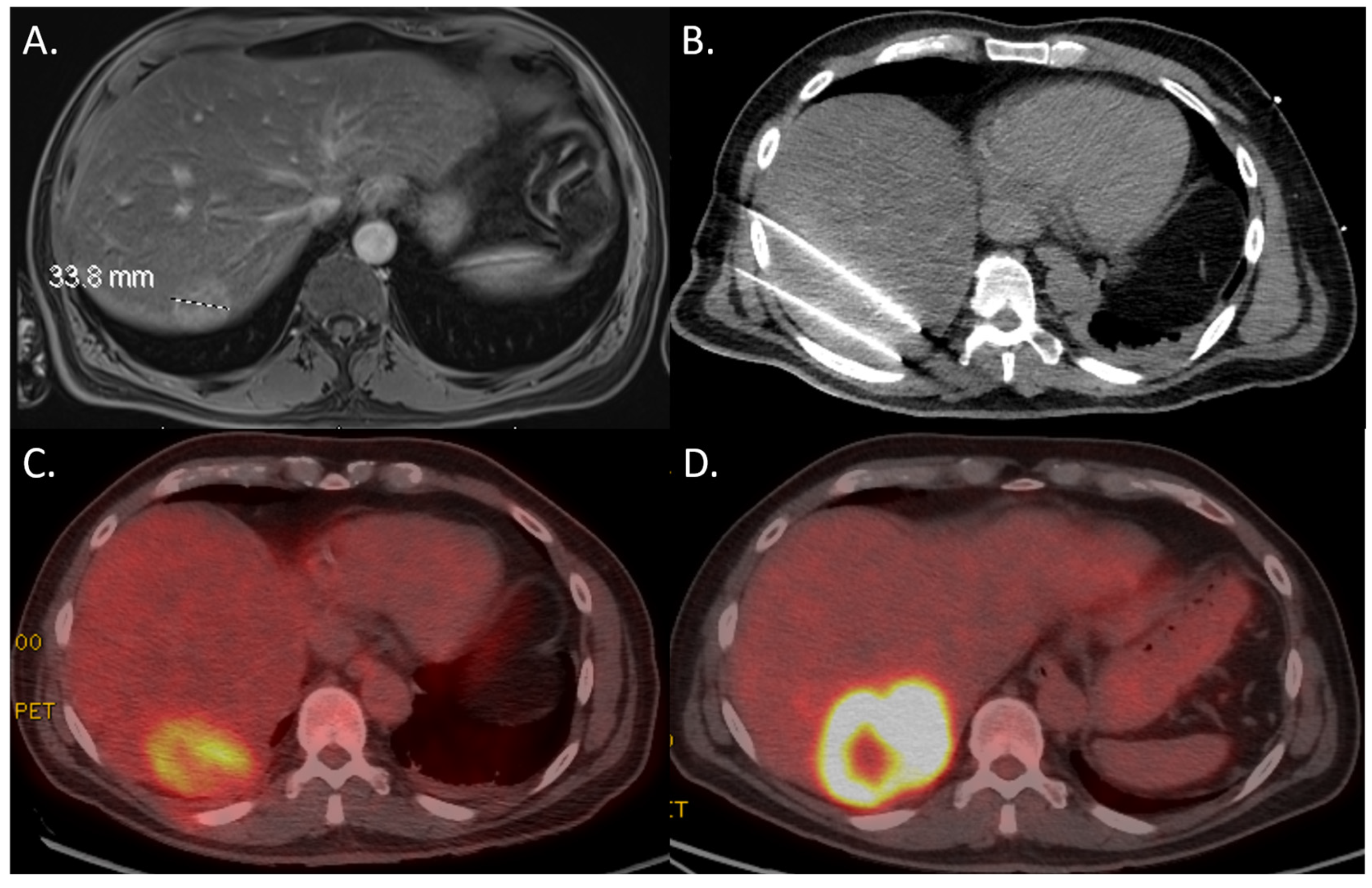
3.3. 18F-FDG PET-CT
3.4. Multivariable Analysis
3.5. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18F-FDG | 18-Fluorodeoxyglucose |
| Ce | Contrast Enhancement |
| CRC | Colorectal Cancer |
| CT | Computed Tomography |
| HCC | Hepatocellular Carcinoma |
| HR | Hazard Ratio |
| IRE | Irreversible Electroporation |
| LTP | Local Tumor Progression |
| LTPFS | Local Tumor Progression-Free Survival |
| mCRC | Metastatic Colorectal Cancer |
| MRI | Magnetic Resonance Imaging |
| MWA | Microwave Ablation |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| RCC | Renal Cell Carcinoma |
| RFA | Radiofrequency Ablation |
| STROBE | Strengthening the Reporting of Observational studies in Epidemiology |
| TACE | Transarterial Chemoembolization |
| US | Ultrasound |
References
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baere, T.; Dodd, G.D., 3rd; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Lee, E.W.; Thai, S.; Kee, S.T. Irreversible electroporation: A novel image-guided cancer therapy. Gut Liver 2010, 4 (Suppl. S1), S99–S104. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21, S187–S191. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Petre, E.N.; Gonen, M.; Brown, K.T.; Solomon, S.B.; Covey, A.M.; Alago, W.; Brody, L.A.; Thornton, R.H.; D’Angelica, M.; et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J. Vasc. Interv. Radiol. 2011, 22, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Vasiniotis Kamarinos, N.; Gonen, M.; Sotirchos, V.; Kaye, E.; Petre, E.N.; Solomon, S.B.; Erinjeri, J.P.; Ziv, E.; Kirov, A.; Sofocleous, C.T. 3D margin assessment predicts local tumor progression after ablation of colorectal cancer liver metastases. Int. J. Hyperth. 2022, 39, 880–887. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Chang, Z.H.; Lu, Z.M.; Guo, Q.Y. Early PET/CT after radiofrequency ablation in colorectal cancer liver metastases: Is it useful? Chin. Med. J. 2010, 123, 1690–1694. [Google Scholar] [CrossRef]
- Asagi, A.; Ohta, K.; Nasu, J.; Tanada, M.; Nadano, S.; Nishimura, R.; Teramoto, N.; Yamamoto, K.; Inoue, T.; Iguchi, H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: Impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013, 42, 11–19. [Google Scholar] [CrossRef]
- Endo, K.; Oriuchi, N.; Higuchi, T.; Iida, Y.; Hanaoka, H.; Miyakubo, M.; Ishikita, T.; Koyama, K. PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int. J. Clin. Oncol. 2006, 11, 286–296. [Google Scholar] [CrossRef]
- Amin, A.; Sinha, V.; Sullivan, T.; Mehta, N.; Doshi, M.; Kuker, R.; Lencioni, R.; Narayanan, G. 3:27 PM Abstract No. 184 Using FDG PET/CT to predict response to IRE in nonresectable pancreatic cancer: A retrospective analysis of 50 patients. J. Vasc. Interv. Radiol. 2018, 29, S81. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kutsenko, O.N.G.; Gentile, N.; Gandhi, R. Robotics in Interventional Oncology: The Next Frontier in Image-Guided Interventions. Endovasc. Today 2023, 22, 121. [Google Scholar]
- de Baere, T.; Roux, C.; Deschamps, F.; Tselikas, L.; Guiu, B. Evaluation of a New CT-Guided Robotic System for Percutaneous Needle Insertion for Thermal Ablation of Liver Tumors: A Prospective Pilot Study. Cardiovasc. Intervent. Radiol. 2022, 45, 1701–1709. [Google Scholar] [CrossRef]
- Crocetti, L.; de Baere, T.; Lencioni, R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Intervent. Radiol. 2010, 33, 11–17. [Google Scholar] [CrossRef]
- IBM Corp. IBM® SPSS® Statistics for Windows; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, R for Windows version 4.2.1.; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2018, 29, 268–275.e261. [Google Scholar] [CrossRef]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.P.; Petre, E.N.; Gonen, M.; Do, K.G.; Brown, K.T.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc. Intervent. Radiol. 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, W.J.; Rhim, H.; Lim, H.K.; Choi, D.; Lee, J.Y. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. Am. J. Roentgenol. 2010, 195, 758–765. [Google Scholar] [CrossRef]
- Solbiati, M.; Muglia, R.; Goldberg, S.N.; Ierace, T.; Rotilio, A.; Passera, K.M.; Marre, I.; Solbiati, L. A novel software platform for volumetric assessment of ablation completeness. Int. J. Hyperth. 2019, 36, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.A.; Cornelis, F.H.; Petre, E.N.; Tyagi, N.; Shady, W.; Shi, W.; Zhang, Z.; Solomon, S.B.; Sofocleous, C.T.; Durack, J.C. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur. Radiol. 2019, 29, 2698–2705. [Google Scholar] [CrossRef]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 31, 6489–6499. [Google Scholar] [CrossRef]
- Puijk, R.S.; Nieuwenhuizen, S.; van den Bemd, B.A.T.; Ruarus, A.H.; Geboers, B.; Vroomen, L.; Muglia, R.; de Jong, M.C.; de Vries, J.J.J.; Scheffer, H.J.; et al. Transcatheter CT Hepatic Arteriography Compared with Conventional CT Fluoroscopy Guidance in Percutaneous Thermal Ablation to Treat Colorectal Liver Metastases: A Single-Center Comparative Analysis of 2 Historical Cohorts. J. Vasc. Interv. Radiol. 2020, 31, 1772–1783. [Google Scholar] [CrossRef]
- Puijk, R.S.; Dijkstra, M.; van der Lei, S.; Schulz, H.H.; Vos, D.J.W.; Timmer, F.E.F.; Geboers, B.; Scheffer, H.J.; de Vries, J.J.J.; Smits, M.L.J.; et al. The Added Value of Transcatheter CT Hepatic Angiography (CTHA) Image Guidance in Percutaneous Thermal Liver Ablation: An Experts’ Opinion Pictorial Essay. Cancers 2024, 16, 1193. [Google Scholar] [CrossRef]
- van der Lei, S.; Opperman, J.; Dijkstra, M.; Kors, N.; Boon, R.; van den Bemd, B.A.T.; Timmer, F.E.F.; Nota, I.; van den Bergh, J.E.; de Vries, J.J.J.; et al. The Added Diagnostic Value of Transcatheter CT Hepatic Arteriography for Intraprocedural Detection of Previously Unknown Colorectal Liver Metastases During Percutaneous Ablation and Impact on the Definitive Treatment Plan. Cardiovasc. Intervent. Radiol. 2023, 46, 1257–1266. [Google Scholar] [CrossRef]
- van Tilborg, A.A.; Scheffer, H.J.; van der Meijs, B.B.; van Werkum, M.H.; Melenhorst, M.C.; van den Tol, P.M.; Meijerink, M.R. Transcatheter CT hepatic arteriography-guided percutaneous ablation to treat ablation site recurrences of colorectal liver metastases: The incomplete ring sign. J. Vasc. Interv. Radiol. 2015, 26, 583–587.e581. [Google Scholar] [CrossRef]
- Cornelis, F.; Sotirchos, V.; Violari, E.; Sofocleous, C.T.; Schoder, H.; Durack, J.C.; Siegelbaum, R.H.; Maybody, M.; Humm, J.; Solomon, S.B. 18F-FDG PET/CT Is an Immediate Imaging Biomarker of Treatment Success After Liver Metastasis Ablation. J. Nucl. Med. 2016, 57, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- McGhana, J.P.; Dodd, G.D., 3rd. Radiofrequency ablation of the liver: Current status. Am. J. Roentgenol. 2001, 176, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Veit, P.; Antoch, G.; Stergar, H.; Bockisch, A.; Forsting, M.; Kuehl, H. Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: Initial results. Eur. Radiol. 2006, 16, 80–87. [Google Scholar] [CrossRef]
- Romanato, J.; Menezes, M.R.; Santos, A.O.; Bezerra, R.O.F.; Lima, M.C.L.; Etchebehere, E. (18)F-FDG PET/CT performed immediately after percutaneous ablation to evaluate outcomes of the procedure: Preliminary results. Radiol. Bras. 2019, 52, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, F.; Vandemeulebroucke, J.; Ilsen, B.; Verdries, D.; Belsack, D.; Everaert, H.; Buls, N.; Ros, P.R.; de Mey, J. Predictive value of pattern classification 24 hours after radiofrequency ablation of liver metastases on CT and positron emission tomography/CT. J. Vasc. Interv. Radiol. 2014, 25, 1240–1249. [Google Scholar] [CrossRef]
- Zirakchian Zadeh, M.; Yeh, R.; Kunin, H.S.; Kirov, A.S.; Petre, E.N.; Gonen, M.; Silk, M.; Cornelis, F.H.; Soares, K.C.; Ziv, E.; et al. Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection. Cancers 2022, 14, 6253. [Google Scholar] [CrossRef]
| Patient Characteristics | Value (N = 132) | |
|---|---|---|
| Sex | Male | 55 (41.7%) |
| Female | 77 (58.3%) | |
| Age in years (mean, range) | 65 (32–92) | |
| Primary cancer origin * | CRC and anal | 61 (46.2%) |
| HCC | 2 (1.5%) | |
| Pancreatic | 18 (13.6%) | |
| Biliary | 5 (3.8%) | |
| Breast | 12 (9.1%) | |
| Lung | 9 (6.8%) | |
| RCC and urothelial | 13 (9.8%) | |
| Gynecological | 4 (3.0%) | |
| Other | 9 (6.8%) | |
| Number of procedures per patient | 1 | 111 (84.1%) |
| 2 | 16 (12.1%) | |
| 3 | 4 (3.0%) | |
| 4 | 1 (0.8%) | |
| Procedure characteristics | Value (N = 159) | |
| Number of tumors per procedure | 1 | 113 (71.1%) |
| 2 | 31 (19.5%) | |
| 3 | 12 (7.5%) | |
| 4 | 2 (1.3%) | |
| 5 | 1 (0.6%) | |
| Tumor characteristics | Value (N = 224) | |
| Tumor setting | Primary | 25 (11.2%) |
| Metastatic | 199 (88.8%) | |
| Tumor location | Lymph node | 15 (6.7%) |
| Liver | 156 (69.6%) | |
| Lung | 9 (4.0%) | |
| Pancreas | 10 (4.5%) | |
| Kidney or adrenal | 10 (4.5%) | |
| Bone | 2 (0.9%) | |
| Soft tissue | 22 (9.8%) | |
| Ablation modality | MWA | 126 (56.3%) |
| Cryoablation | 9 (4.0%) | |
| IRE | 89 (39.7%) | |
| Tumor size in mm (median, range) | 18 (5–92) | |
| Local Tumor Progression | Yes | No | p-Value |
|---|---|---|---|
| N = 120 | N = 104 | ||
| 18F-FDG avidity | |||
| Nodular | 13 (10.8%) | 2 (1.9%) | |
| Focal | 6 (5.0%) | 6 (5.8%) | |
| Periphery | 56 (46.7%) | 37 (35.6%) | |
| No | 45 (37.5%) | 59 (56.7%) | 0.005 * |
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| 18F-FDG avidity | No | Reference | <0.001 | Reference | <0.001 |
| Yes | 2.203 (1.515–3.204) | 2.355 (1.614–3.436) | |||
| Patient-related characteristics | |||||
| Sex | Male | Reference | 0.011 | Reference | 0.003 |
| Female | 1.638 (1.120–2.396) | 1.805 (1.230–2.647) | |||
| Age | 0.997 (0.984–1.010) | 0.663 | |||
| Primary cancer origin | CRC and anal | Reference | 0.683 | ||
| HCC | 1.362 (0.332–5.585) | ||||
| Pancreatic | 1.841 (1.009–3.359) | ||||
| Biliary | 1.101 (0.433–2.797) | ||||
| Breast | 1.176 (0.633–2.187) | ||||
| Lung | 1.411 (0.565–3.521) | ||||
| RCC and urothelial | 1.518 (0.834–2.764) | ||||
| Gynecological | 1.750 (0.425–7.198) | ||||
| Other | 1.022 (0.441–2.365) | ||||
| Number of procedures per patient | 1 | Reference | 0.290 | ||
| 2 | 1.118 (0.690–1.813) | ||||
| 3 | 1.140 (0.655–1.983) | ||||
| 4 | 2.679 (0.970–7.400) | ||||
| Procedure-related characteristics | |||||
| Number of tumors per procedure | 1 | Reference | 0.239 | ||
| 2 | 1.169 (0.780–1.753) | ||||
| 3 | 0.589 (0.339–1.024) | ||||
| 4 | 0.812 (0.324–2.033) | ||||
| 5 | NA | ||||
| Tumor-related characteristics | |||||
| Tumor setting | Primary | Reference | 0.359 | ||
| Metastatic | 0.781 (0.461–1.324) | ||||
| Tumor location | Lymph node | Reference | 0.312 | ||
| Liver | 0.902 (0.472–1.752) | ||||
| Lung | 1.414 (0.517–3.872) | ||||
| Pancreas | 1.958 (0.778–4.930) | ||||
| Kidney or adrenal | 0.397 (0.087–1.808) | ||||
| Bone | 1.021 (0.131–7.982) | ||||
| Soft tissue | 1.178 (0.501–2.768) | ||||
| Ablation modality | MWA | Reference | 0.259 | ||
| Cryoablation | 0.808 (0.294–2.219) | ||||
| IRE | 1.327 (0.918–1.919) | ||||
| Tumor size | 1.017 (1.006–1.029) | 0.003 | 1.011 (0.998–1.024) | 0.099 | |
| LTP | No LTP | |
|---|---|---|
| PET-avidity | 12 (sensitivity 30.0%) | 6 |
| No avidity | 28 | 56 (specificity 90.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, G.; Gentile, N.T.; Schiro, B.J.; Gandhi, R.T.; Peña, C.S.; van der Lei, S.; Dijkstra, M. Predicting Tumor Recurrence with Early 18F-FDG PET-CT After Thermal and Non-Thermal Ablation. Curr. Oncol. 2025, 32, 521. https://doi.org/10.3390/curroncol32090521
Narayanan G, Gentile NT, Schiro BJ, Gandhi RT, Peña CS, van der Lei S, Dijkstra M. Predicting Tumor Recurrence with Early 18F-FDG PET-CT After Thermal and Non-Thermal Ablation. Current Oncology. 2025; 32(9):521. https://doi.org/10.3390/curroncol32090521
Chicago/Turabian StyleNarayanan, Govindarajan, Nicole T. Gentile, Brian J. Schiro, Ripal T. Gandhi, Constantino S. Peña, Susan van der Lei, and Madelon Dijkstra. 2025. "Predicting Tumor Recurrence with Early 18F-FDG PET-CT After Thermal and Non-Thermal Ablation" Current Oncology 32, no. 9: 521. https://doi.org/10.3390/curroncol32090521
APA StyleNarayanan, G., Gentile, N. T., Schiro, B. J., Gandhi, R. T., Peña, C. S., van der Lei, S., & Dijkstra, M. (2025). Predicting Tumor Recurrence with Early 18F-FDG PET-CT After Thermal and Non-Thermal Ablation. Current Oncology, 32(9), 521. https://doi.org/10.3390/curroncol32090521






