Renal Cell Carcinoma: Prognosis in the Era of Targeted Therapy
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Outcome Measures
2.2. Statistical Analyses
3. Results
3.1. Overall Demographic and Disease Characteristics
3.2. Trends in the Patient Population
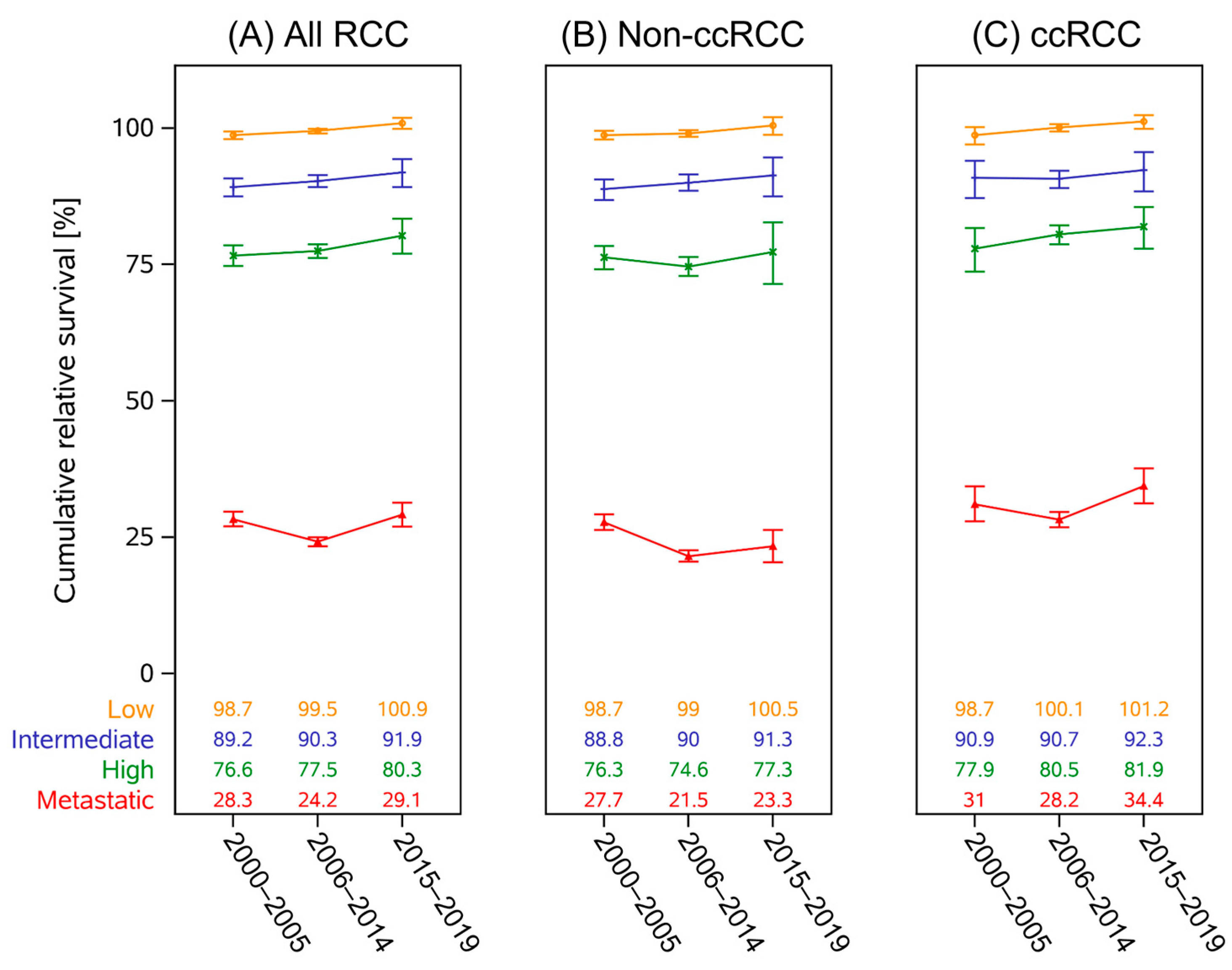
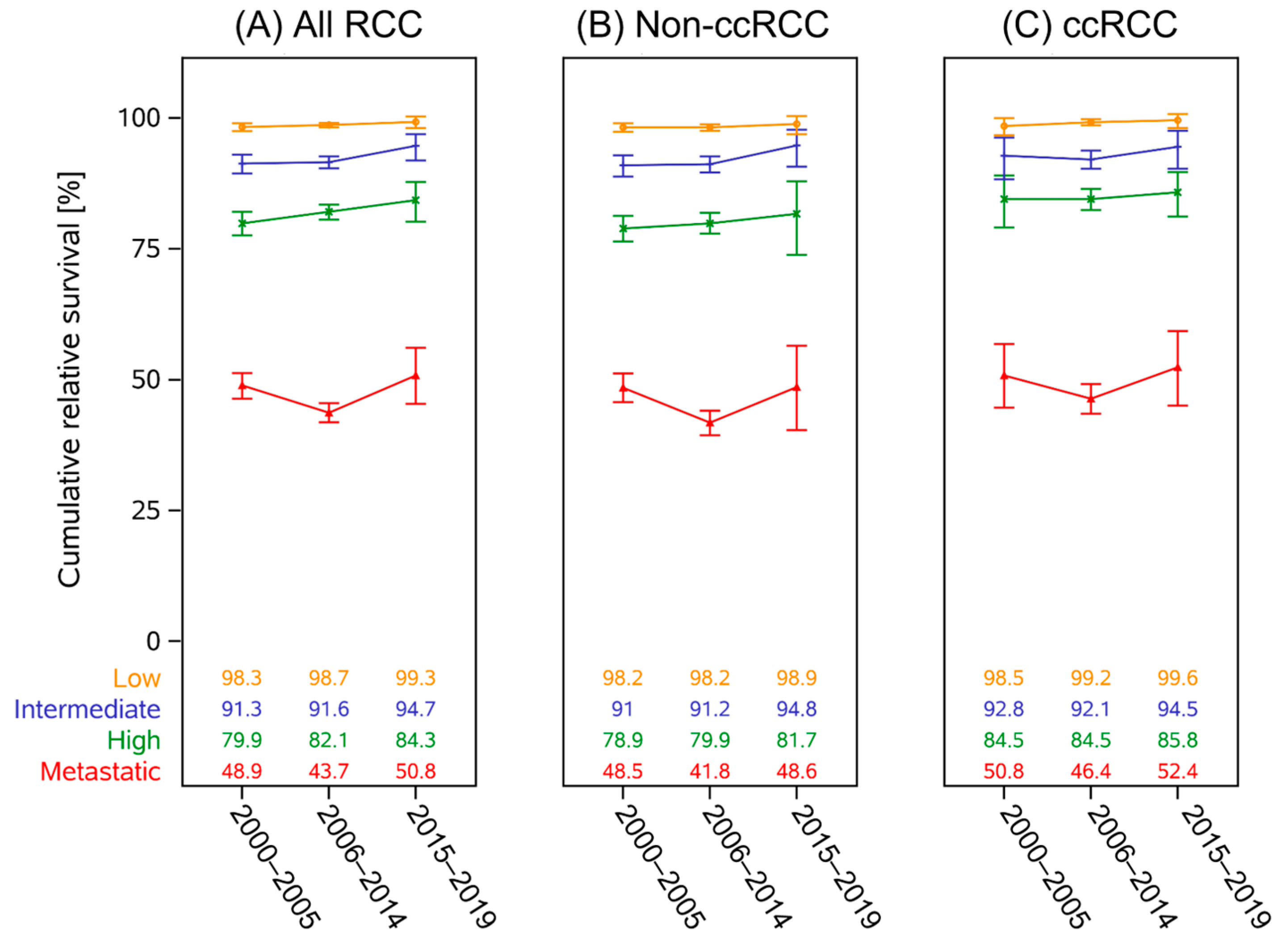
3.3. Survival Trends
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ccRCC | clear cell renal carcinoma |
| DCO | death certificate only |
| IQ-range | interquartile range (Q1–Q3) |
| mRCC | metastatic renal cell carcinoma |
| NET | neuroendocrine tumor |
| Non-ccRCC | non-clear cell renal carcinoma |
| RCC | renal cell carcinoma |
| RER | relative excess risk |
| TKI | tyrosine kinase inhibitor |
| UISS | University of California Los Angeles integrated staging system |
References
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Wilson, M.; Stewart, G.D.; Adeyoju, A.; Cartledge, J.; Kimuli, M.; Datta, S.; Hanbury, D.; Hrouda, D.; Oades, G.; et al. Challenges of early renal cancer detection: Symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ Open 2020, 10, e035938. [Google Scholar] [CrossRef]
- Krebs in Deutschland für 2019/2020. Robert Koch-Institut (Hrsg) und die Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Available online: https://edoc.rki.de/handle/176904/11438 (accessed on 1 February 2025).
- Downs, T.M.; Schultzel, M.; Shi, H.; Sanders, C.; Tahir, Z.; Sadler, G.R. Renal cell carcinoma: Risk assessment and prognostic factors for newly diagnosed patients. Crit. Rev. Oncol. Hematol. 2009, 70, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020; posted to the SEER web site. [Google Scholar]
- Surveillance Research Program, National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. 2 July 2025. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 5 February 2024).
- SEER Incidence Data, November 2024 Submission (1975-2022), SEER 21 Registries Munich Cancer Registry. Available online: http://www.tumorregister-muenchen.de/en/facts/specific_analysis.php (accessed on 6 June 2023).
- Barata, P.C.; Rini, B.I. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Larkin, J.; Oya, M.; Thistlethwaite, F.; Martignoni, M.; Nathan, P.; Powles, T.; McDermott, D.; Robbins, P.B.; Chism, D.D.; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018, 19, 451–460. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulieres, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.H.; Lee, J.L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.E.; Kim, S.E.; Master, V.; McDermott, D.F.; Harshman, L.C.; Cole, S.M.; Drake, C.G.; Signoretti, S.; Akgul, M.; Baniak, N.; et al. Perioperative nivolumab versus observation in patients with renal cell carcinoma undergoing nephrectomy (PROSPER ECOG-ACRIN EA8143): An open-label, randomised, phase 3 study. Lancet Oncol. 2024, 25, 1038–1052. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Alekseev, B.Y.; Lee, J.L.; Suarez, C.; Stroyakovskiy, D.; De Giorgi, U.; et al. Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma. JAMA Oncol. 2022, 8, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grunwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthelemy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef]
- RKI. Zentrum für Krebsregisterdaten (ZfKD). Available online: https://www.krebsdaten.de/Krebs/SiteGlobals/Forms/Datenbankabfrage/datenbankabfrage_stufe2_form.html (accessed on 1 February 2025).
- Wittekind, C.; Wiley, V.C.H. TNM Klassifikation Maligner Tumoren, Achte Auflage, Korrigierter Nachdruck 2020 Mit Allen Ergängzungen der UICC aus den Jahren 2017 bis 2019 Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020. [Google Scholar]
- Moch, H.; Amin, M.B.; Berney, D.M.; Comperat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Dorey, F.; Said, J.W.; Shvarts, O.; Quintana, D.; Gitlitz, B.J.; deKernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Improved prognostication of renal cell carcinoma using an integrated staging system. J. Clin. Oncol. 2001, 19, 1649–1657. [Google Scholar] [CrossRef]
- Ederer, F.; Axtell, L.M.; Cutler, S.J. The relative survival rate: A statistical methodology. Natl. Cancer Inst. Monogr. 1961, 6, 101–121. [Google Scholar]
- Dickman, P.W.; Sloggett, A.; Hills, M.; Hakulinen, T. Regression models for relative survival. Stat. Med. 2004, 23, 51–64. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.; Abdallah, N.; Mouchati, C.; Jani, R.; Sharma, R.; Bhatt, P.; Hanbury, G.; Salciccioli, J.; Singh, H.; Shalhoub, J.; et al. Trends of kidney cancer burden from 1990 to 2019 in European Union 15 + countries and World Health Organization regions. Sci. Rep. 2022, 12, 22368. [Google Scholar] [CrossRef]
- Hemminki, K.; Forsti, A.; Hemminki, A.; Ljungberg, B.; Hemminki, O. Progress in survival in renal cell carcinoma through 50 years evaluated in Finland and Sweden. PLoS ONE 2021, 16, e0253236. [Google Scholar] [CrossRef]
- Azawi, N.H.; Joergensen, S.M.; Jensen, N.V.; Clark, P.E.; Lund, L.; Academy of Geriatric Cancer Research. Trends in kidney cancer among the elderly in Denmark, 1980–2012. Acta Oncol. 2016, 55 (Suppl. 1), 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ahrenfeldt, J.; Jespersen, J.; Lyngstrand, J.E.; Iisager, L.; Keller, A.K.; Fristrup, N.; Laurberg, T.; Lyskjaer, I. Trends in kidney cancer: Exploring the impact of sex and age on stage of disease, and prognosis during the past three decades in Denmark-a DaRenCa study. Eur. J. Epidemiol. 2025, 40, 527–536. [Google Scholar] [CrossRef]
- Santoni, M.; Buti, S.; Myint, Z.W.; Maruzzo, M.; Iacovelli, R.; Pichler, M.; Kopecky, J.; Kucharz, J.; Rizzo, M.; Galli, L.; et al. Real-world Outcome of Patients with Advanced Renal Cell Carcinoma and Intermediate- or Poor-risk International Metastatic Renal Cell Carcinoma Database Consortium Criteria Treated by Immune-oncology Combinations: Differential Effectiveness by Risk Group? Eur. Urol. Oncol. 2024, 7, 102–111. [Google Scholar] [CrossRef]
- Deutsche Diabetes Gesellschaft (DDG) und Diabetesde–Deutsche Diabetes-Hilfe. Deutscher Gesundheitsbericht: Diabetes 2024-Die Bestandsaufnahme. Available online: https://www.ddg.info/fileadmin/user_upload/Gesundheitsbericht_2024_Endversion.pdf (accessed on 1 February 2025).
- Schienkiewitz, A.; Kuhnert, R.; Blume, M.; Mensink, G.B.M. Overweight and obesity among adults in Germany-Results from GEDA 2019/2020-EHIS. J. Health Monit. 2022, 7, 21–28. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC); Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
| Period of Diagnosis | Total | |||||
|---|---|---|---|---|---|---|
| 2000–2005 | 2006–2014 | 2015–2019 | ||||
| N (%) | N (%) | N (%) | p-value (effect size) | N (%) | ||
| 41,290 (19.6) | 106,644 (50.7) | 62,484 (29.7) | 210,418 | |||
| Sex | Male | 25,991 (63.0) | 68,063 (63.8) | 41,172 (65.9) | <0.0001 a (0.0231) | 135,226 (64.3) |
| Female | 15,299 (37.0) | 38,581 (36.2) | 21,312 (34.1) | 75,192 (35.7) | ||
| Age (median, q1–q3) | 66 (59–73) | 69 (59–75) | 68 (59–76) | <0.0001 b (0.0051) | 68 (59–75) | |
| Age groups | ≤44 | 2000 (4.8) | 4259 (4.0) | 2258 (3.6) | <0.0001 c (0.0752) | 8517 (4.0) |
| 45–54 | 5245 (12.7) | 12,650 (11.9) | 6998 (11.2) | 24,893 (11.8) | ||
| 55–64 | 10,998 (26.6) | 23,689 (22.2) | 15,038 (24.1) | 49,725 (23.6) | ||
| 65–74 | 15,098 (36.6) | 36,773 (34.5) | 18,220 (29.2) | 70,091 (33.3) | ||
| >75 | 7949 (19.2) | 29,273 (27.4) | 19,970 (32.0) | 57,192 (27.2) | ||
| c/pT | T1 | 21,585 (61.0) | 60,982 (65.2) | 37,622 (68.0) | <0.0001 c (0.0377) | 120,189 (65.3) |
| T2 | 4128 (11.7) | 8850 (9.5) | 5107 (9.2) | 18,085 (9.8) | ||
| T3 | 9045 (25.6) | 22,081 (23.6) | 11,630 (21.0) | 42,756 (23.2) | ||
| T4 | 621 (1.8) | 1556 (1.7) | 962 (1.7) | 3139 (1.7) | ||
| Missing | 5911 (14.3) | 13,175 (12.3) | 7163 (11.5) | 26,249 (12.5) | ||
| c/pN | N0 | 22,016 (91.7) | 49,747 (91.0) | 33,943 (92.1) | 0.0084 a (0.0173) | 105,706 (91.5) |
| N+ | 1992 (8.3) | 4895 (9.0) | 2901 (7.9) | 9788(8.5) | ||
| NX/Missing | 17,282 (41.9) | 52,002 (48.8) | 25,640 (41.0) | 94,924 (45.1) | ||
| c/pM | M0 | 19,471 (81.4) | 52,420 (83.5) | 38,511 (86.1) | <0.0001 a (0.0465) | 110,402 (84.0) |
| M1a–c | 4458 (18.6) | 10,356 (16.5) | 6207 (13.9) | 21,021 (16.0) | ||
| Missing | 17,361 (42.0) | 43,868 (41.1) | 17,766 (28.4) | 78,995 (37.5) | ||
| Histology | Clear cell | 9879 (23.9) | 50,535 (47.4) | 35,438 (56.7) | <0.0001 c (0.2402) | 95,852 (45.5) |
| Papillary | 1202 (2.9) | 10,254 (9.6) | 8677 (13.9) | 20,133 (9.6) | ||
| Chromophobe | 882 (2.1) | 4678 (4.4) | 3662 (5.9) | 9222 (4.4) | ||
| NOS/Other | 29,129 (70.5) | 40,543 (38.0) | 14,338 (23.0) | 84,010 (39.9) | ||
| Collecting duct | 90 (0.2) | 370 (0.3) | 183 (0.3) | 643 (0.3) | ||
| Transitional cell | 106 (0.3) | 260 (0.2) | 174 (0.3) | 540 (0.3) | ||
| Molecular defined | 2 (0.0) | 4 (0.0) | 12 (0.0) | 18 (0.0) | ||
| Grade | G1 | 7452 (20.7) | 18,674 (19.6) | 12,302 (23.3) | <0.0001 c (0.0480) | 38,428 (20.9) |
| G2 | 23,152 (64.2) | 60,119 (63.2) | 30,890 (58.5) | 114,161 (62.0) | ||
| G3 | 5211 (14.4) | 15,200 (16.0) | 8430 (16.0) | 28,841 (15.7) | ||
| Anaplastic | 247 (0.7) | 1118 (1.2) | 1177 (2.2) | 2542 (1.4) | ||
| Missing | 5228 (12.7) | 11,533 (10.8) | 9685 (15.5) | 26,446 (12.6) | ||
| UISS-risk | Low | 11,569 (49.2) | 32,269 (52.7) | 22,531 (55.1) | <0.0001 c (0.0315) | 66,369 (52.8) |
| Intermediate | 3103 (13.2) | 7559 (12.3) | 5544 (13.5) | 16,206 (12.9) | ||
| High | 3344 (14.2) | 8704 (14.2) | 5468 (13.4) | 17,516 (13.9) | ||
| mRCC | 5482 (23.3) | 12,709 (20.7) | 7377 (18.0) | 25,568 (20.3) | ||
| Missing | 17,792 (43.1) | 45,403 (42.6) | 21,564 (34.5) | 84,759 (40.3) | ||
| UISS-Risk Category | mRCC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | ||||||||||
| Factor | RER | 95% CI | p-Value | RER | 95% CI | p-Value | RER | 95% CI | p-Value | RER | 95% CI | p-Value |
| Sex | 0.6577 | 0.8019 | 0.0430 | 0.0008 | ||||||||
| Female | ref | - | ref | - | ref | - | ref | - | ||||
| Male | 1.077 | 0.775–1.498 | 1.021 | 0.868–1.200 | 0.909 | 0.829–0.997 | 0.942 | 0.909–0.975 | ||||
| Age at diagnosis | 0.0739 | 0.0018 | 0.0286 | <0.0001 | ||||||||
| <65 | ref | - | ref | - | ref | - | ref | - | ||||
| ≥65 | 0.663 | 0.422–1.040 | 1.289 | 1.099–1.511 | 1.106 | 1.010–1.211 | 1.224 | 1.183–1.266 | ||||
| Period of diagnosis | 0.0549 | 0.1772 | 0.0009 | <0.0001 | ||||||||
| 2000–2005 | ref | - | ref | - | ref | - | ref | - | ||||
| 2006–2014 | 0.934 | 0.647–1.341 | 0.7021 | 0.895 | 0.745–1.075 | 0.2357 | 0.933 | 0.841–1.035 | 0.1886 | 1.099 | 1.055–1.145 | <0.0001 |
| 2015–2019 | 0.482 | 0.229–1.015 | 0.0549 | 0.796 | 0.624–1.016 | 0.0668 | 0.771 | 0.669–0.888 | 0.0003 | 1.035 | 0.985–1.088 | 0.1694 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halfter, K.; Staehler, M.; Hölzel, D.; Crispin, A.; Schlesinger-Raab, A. Renal Cell Carcinoma: Prognosis in the Era of Targeted Therapy. Curr. Oncol. 2025, 32, 515. https://doi.org/10.3390/curroncol32090515
Halfter K, Staehler M, Hölzel D, Crispin A, Schlesinger-Raab A. Renal Cell Carcinoma: Prognosis in the Era of Targeted Therapy. Current Oncology. 2025; 32(9):515. https://doi.org/10.3390/curroncol32090515
Chicago/Turabian StyleHalfter, Kathrin, Michael Staehler, Dieter Hölzel, Alexander Crispin, and Anne Schlesinger-Raab. 2025. "Renal Cell Carcinoma: Prognosis in the Era of Targeted Therapy" Current Oncology 32, no. 9: 515. https://doi.org/10.3390/curroncol32090515
APA StyleHalfter, K., Staehler, M., Hölzel, D., Crispin, A., & Schlesinger-Raab, A. (2025). Renal Cell Carcinoma: Prognosis in the Era of Targeted Therapy. Current Oncology, 32(9), 515. https://doi.org/10.3390/curroncol32090515





