Ten-Year Real-World Outcomes and Clinicopathologic Predictors of Recurrence in Adult Granulosa Cell Tumors: A Turkish Single-Center Experience †
Simple Summary
Abstract
1. Introduction
2. Methods
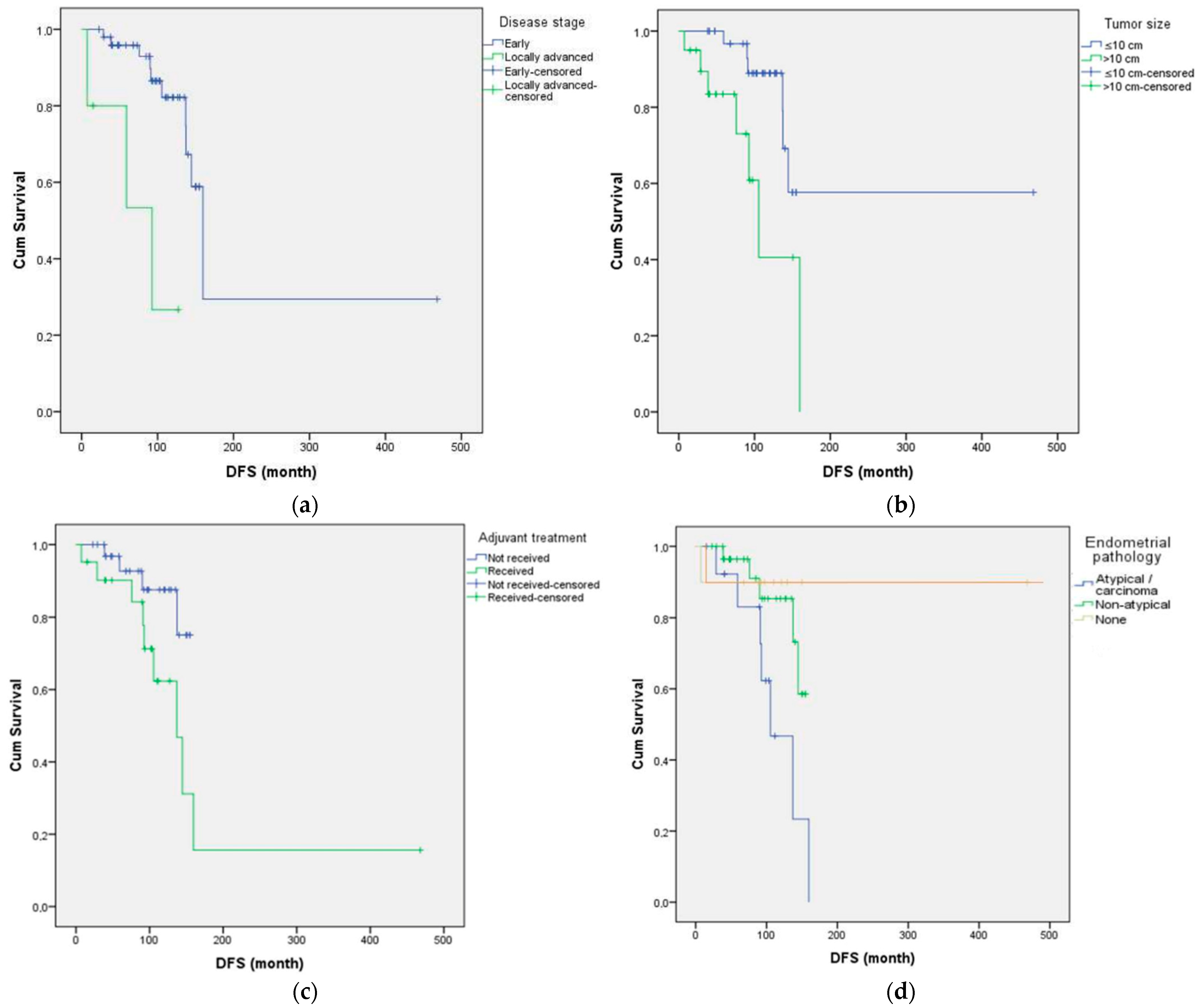
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Comparison with Existing Literature
4.3. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Wang, X.; Liu, J.; Zhang, H.; Chen, Y.; Zhou, L.; Li, F.; Zhao, R.; Sun, P.; Wang, Y.; et al. Clinical characteristics and oncological outcomes of recurrent adult granulosa cell tumor of ovary: A retrospective study of seventy patients. Acta Obstet. Gynecol. Scand. 2023, 102, 782–790. [Google Scholar] [CrossRef]
- Bryk, S.; Pukkala, E.; Unkila-Kallio, L.; Haltia, U.; Heikinheimo, O.; Leminen, A.; Lehtovirta, P.; Maenpaa, J.; Laurema, A.; Bützow, R.; et al. Characteristics and outcome of recurrence in molecu-larly defined adult-type ovarian granulosa cell tumors. Gynecol. Oncol. 2016, 143, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Mangili, G.; Ottolina, J.; Gadducci, A.; Giorda, G.; Breda, E.; Savarese, A.; Candiani, M.; Frigerio, L.; Scarfone, G.; Cormio, G.; et al. Long-term follow-up is crucial after treatment for granulosa cell tu-mours of the ovary. Br. J. Cancer 2013, 109, 29–34. [Google Scholar] [CrossRef]
- Likic Ladjevic, I.; Milosevic-Stevanovic, J.; Vuckovic, B.; Mitrovic, S.; Arsenovic, N.; Lukic, D.; Jo-vanovic, M.; Vukomanovic, P.; Milosevic, J.; Vojvodic, D.; et al. Fertility-sparing surgery for non-epithelial ovarian malignancies: Ten-year retrospective study of oncological and reproductive out-comes. Cancers 2025, 17, 1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, J.; Sun, H.; Zhao, Q.; Li, Y.; Chen, L.; Yang, F.; Liu, Y.; Xu, R.; Zhou, W.; et al. Role of adjuvant chemotherapy in stage IC ovarian granulosa cell tumors: A systematic review and meta-analysis. J. Gynecol. Oncol. 2025, 36, e45. [Google Scholar] [CrossRef]
- Ray, L.J.; Jones, K.; Chen, H.; Patel, S.; Kumar, A.; Davis, R.; Thompson, M.; Lee, Y.; Nguyen, L.; Martin, D.; et al. Adult granulosa cell tumors of the ovary with tubular differentiation: A report of 80 examples of an underemphasized feature with clinicopathologic and genomic differences from other sex cord-stromal tumors. Am. J. Surg. Pathol. 2025, 49, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Rottenstreich, M.; Katorza, E.; Gershuni, V.; Cohen, Y.; Aviram, A.; Rottenstreich, A.; Rosenblum, N.; Shapira, M.; Matanes, E.; et al. Granulosa cell tumor of ovary: A systematic review of recent evidence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 57–61. [Google Scholar] [CrossRef]
- Oktar, O.; Gultekin, M.; Kucukgoz Gulec, U.; Uzunoglu, H.; Ayhan, A.; Caglar, H.O.; Kose, M.F.; Aytac, R.; Kucukali, T.; Cakar, A.; et al. Prognostic factors of adult granulosa cell tumors of the ova-ry: A Turkish retrospective multicenter study. J. Gynecol. Oncol. 2024, 35, e39. [Google Scholar] [CrossRef]
- Rizzo, S.; Avesani, G.; Panico, C.; Manganaro, L.; Gui, B.; Lakhman, Y.; Andrieu, P.C.; Bharwani, N.; Rockall, A.; Thomassin-Naggara, I.; et al. Ovarian cancer staging and follow-up: Updated guidelines from the European Society of Urogenital Radiology female pelvic imaging working group. Eur. Radiol. 2025, 35, 4029–4039. [Google Scholar] [CrossRef]
- Babarović, E.; Franković, L.; Culić, V.; Vrdoljak, E.; Crljenko, A.; Capkun, V.; Peharec, P.; Sasso, A.; Butorac, D.; Lukšić, I.; et al. Adult granulosa cell tumors of the ovary: A retrospective study of 36 FIGO stage I cases with emphasis on prognostic pathohistological features. Anal. Cell. Pathol. 2018, 9148124. [Google Scholar] [CrossRef]
- Thomakos, N.; Biliatis, I.; Koutroumpa, I.; Rodolakis, A.; Zacharakis, D.; Sotiropoulou, M.; Hai-dopoulos, D.; Mavromatis, C.; Vorgias, G.; Papadimitriou, C.; et al. Prognostic factors for recur-rence in early stage adult granulosa cell tumor of the ovary. Arch. Gynecol. Obstet. 2016, 294, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Fukuda, J.; Hasegawa, K.; Morimune, A.; Kamada, N.; Sugiyama, T.; Tanaka, T.; Sasa-no, H.; Terakawa, N. Histopathological prognostic factors of adult granulosa cell tumors of the ova-ry. Acta Obstet. Gynecol. Scand. 2001, 80, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Lee, S.J.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H.; Lee, C.; Park, C.; Lee, H. A long-term follow-up study of 91 cases with ovarian granulosa cell tumors. Anticancer Res. 2014, 34, 1001–1010. [Google Scholar]
- Inzani, F.; Angelico, G.; Arciuolo, D.; Cianfrini, F.; Santoro, A.; Spadola, S.; Cagnacci, R.; Gallo, D.; Scambia, G.; Zannoni, G.F. Prognostic predictors in recurrent adult granulosa cell tumors of the ovary: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2022, 306, 315–321. [Google Scholar] [CrossRef]
- Iyibozkurt, A.C.; Topuz, S.; Gungor, F.; E Akhan, S.; Demirci, F.; Salihoglu, Y.; Berkman, S.; Bengisu, E. Factors affecting recurrence and disease-free survival in granulosa cell tumors of the ovary. Eur. J. Gynaec. Oncol. 2010, 31, 667–671. [Google Scholar]
- Sehouli, J.; Drescher, F.S.; Mustea, A.; Elling, D.; Kelling, K.; Lichtenegger, W.; Woelber, L. Granu-losa cell tumor of the ovary: 10 years follow-up data of 65 patients. Anticancer Res. 2004, 24, 1223–1229. [Google Scholar] [PubMed]
- Le, D.T.; Bui, D.C.; Tran, H.T.; Nguyen, T.H.; Pham, T.H.; Vu, T.H.; Do, T.T.; Hoang, M.H.; Le, H.T.; Dang, T.M.; et al. Clinical and paraclinical features, outcome, and prognosis of ovarian granulosa cell tumor: A retrospective study of 28 Vietnamese women. Rare Tumors 2022, 14, 20363613221148547. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, T.H.; Kim, J.-W.; Kim, S.Y.; Kim, H.S.; Lee, T.S.; Chung, H.H.; Kim, Y.-B.; Park, N.H.; Song, Y.S. Improvements to the FIGO staging for ovarian cancer: Reconsideration of lymphatic spread and intraoperative tumor rupture. J. Gynecol. Oncol. 2013, 24, 352–358. [Google Scholar] [CrossRef]
- Alhusaini, H.; Al-Badawi, I.; Alsubhi, S.; Alturki, A.; Tulbah, A.; Balaraj, K.; AlDakhiel, S.; Alhejaily, A.; Alzahrani, H.; Almutlaq, H.; et al. Adult-type ovarian granulosa cell tumour: Treatment outcomes from a single-institution experience. Cureus 2022, 14, e31045. [Google Scholar] [CrossRef]
- ahin, M.; Yıldız, Ş.; Demirkiran, F.; Gultekin, M.; Kose, F.; Akin, F.; Cakar, A.; Saygili, H.; Gungor, T.; Koc, S.; et al. The experıance of tertıary center for adult granulosa cell tumor: Which factors predict survival? J. Ovarian Res. 2024, 17, 127. [Google Scholar] [CrossRef]
- Wilson, M.K.; Fong, P.; Mesnage, S.; Chrystal, K.; Shelling, A.; Payne, K.; Mackay, H.; Wang, L.; Laframboise, S.; Rouzbahman, M.; et al. Stage I granulosa cell tumours: A management conundrum? Results of long-term follow up. Gynecol. Oncol. 2015, 138, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Temtanakitpaisan, A.; Kleebkaow, P.; Aue-Aungkul, A. Adult-type Granulosa Cell Ovarian Tumor: Clinicopathological Features, Outcomes, and Prognostic Factors. Asian Pac. J. Cancer Care 2019, 4, 33–37. [Google Scholar] [CrossRef]
- Brink, G.J.; Piek, J.M.J.; van Meurs, H.S.; Piek, J.; van der Aa, M.; Schagen van Leeuwen, J.H.; van der Aa, M.; de Hullu, J.A.; Massuger, L.; de Hullu, J.A.; et al. Is it time to abandon staging surgery and prolonged follow-up in patients with primary adult-type granulosa cell tumor? J. Ovarian Res. 2025, 18, 37. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Jiang, W. Effects of different surgical extents on prognosis of patients with malignant ovarian sex cord-stromal tumors: A retrospective cohort study. Sci. Rep. 2024, 14, 22630. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.-L.L.; Ann, P.; Butler, S.; Shahabi, S. Ovarian granulosa cell tumor: A National Cancer Database study. Gynecol. Oncol. 2017, 146, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, W.; Sun, H.; Zhao, J.; Liu, C.; Wang, X.; Yang, Y.; Li, F.; Chen, L.; Han, S.; et al. Can adjuvant chemotherapy improve the prognosis of adult ovarian granulosa cell tumors? A narrative review. Medicine 2022, 101, e29351. [Google Scholar] [CrossRef]
- Tokalioglu, A.A.; Altintas, A.; Kuru, O.; Yildirim, N.; Gultekin, M.; Kose, F.; Yildiz, S.; Demirkiran, F.; Usubutun, A.; Saygili, H.; et al. Defining the relationship between ovarian adult granulosa cell tumors and synchronous endometrial pathology: Does ovarian tumor size correlate with endometrial cancer? J. Obstet. Gynaecol. Res. 2024, 50, 655–662. [Google Scholar] [CrossRef]
- Sun, H.-D.; Lin, H.; Jao, M.-S.; Wang, K.-L.; Liou, W.-S.; Hung, Y.-C.; Chiang, Y.-C.; Lu, C.-H.; Lai, H.C.; Yu, M.-H. A long-term follow-up study of 176 cases with adult-type ovarian granulosa cell tumors. Gynecol. Oncol. 2012, 124, 244–249. [Google Scholar] [CrossRef]
- Yaacoub, S.; Hajj, L.; Khairallah, A. A clinicopathological study about the epidemiology of granulosa cell tumors in Lebanon. BMC Cancer 2024, 24, 309. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, H. The Prognostic Significance of Adjuvant Chemotherapy in Adult Ovarian Granulosa Cell Tumors: A Systematic Review and Meta-analysis. Cancer Control. 2023, 30, 10732748231215165. [Google Scholar] [CrossRef]
- Plett, H.; Werner, H.M.J.; Concin, N.; Kommoss, S.; Denschlag, D.; Gockley, A.; Kommoss, F.; Papon, L.; Wilson, M.K.; Okamoto, A.; et al. Adult ovarian granulosa cell tumors: Analysis of outcomes and risk factors for recurrence. Int. J. Gynecol. Cancer 2023, 33, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, A.; Cormio, G.; Ferrandina, G.; Lorusso, D.; Giorda, G.; Scarfone, G.; Bocciolone, L.; Raspagliesi, F.; Tateo, S.; Cassani, C.; et al. Conservative surgery in stage I adult type granulosa cells tumors of the ovary: Results from the MITO-9 study. Gynecol. Oncol. 2019, 154, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Michálková, R.; Pospíšilová, H.; Hejtmánková, D.; Králíčková, P.; Křížová, K.; Dvořáková, S.; Zikán, M.; Dundr, P.; Žaloudík, J.; Matějovský, Z.; et al. The molecular landscape of 227 adult granulosa cell tumors of the ovary: Insights into the progression from primary to recurrence. Lab. Invest. 2025, 105, 102201. [Google Scholar] [CrossRef]
- Brink, G.J.; Zikan, M.; Piek, J.M.J.; van Meurs, H.S.; Trum, J.W.; Massuger, L.; van der Aa, M.; de Hullu, J.A.; Piek, J.; van der Aa, M.; et al. The prognostic value of FOXL2 mutant circulating tumor DNA in adult granulosa cell tumor patients. Cancers 2025, 17, 3125. [Google Scholar] [CrossRef] [PubMed]

| Variable | Category | n (%) |
|---|---|---|
| Menstrual Status | Premenopausal | 21 (38.2%) |
| Perimenopausal | 5 (9.1%) | |
| Postmenopausal | 29 (52.7%) | |
| Comorbidity Count | None | 25 (45.5%) |
| One | 11 (20.0%) | |
| ≥2 | 19 (34.5%) | |
| Recurrence | Absent | 42 (76.4%) |
| Present | 13 (23.6%) | |
| Recurrence Site | Local recurrence | 4 (7.3%) |
| Visceral metastasis | 3 (5.5%) | |
| Peritoneal | 6 (10.9%) | |
| Endometrial Pathology | None | 28 (50.9%) |
| Non-atypical hyperplasia | 17 (30.9%) | |
| Atypical hyperplasia | 7 (12.7%) | |
| Carcinoma | 3 (5.4%) | |
| Estrogen Receptor Status | Positive | 18 (32.7%) |
| Negative | 10 (18.2%) | |
| Unknown | 27 (49.1%) | |
| Inhibin Status | Negative | 9 (16.4%) |
| Positive | 46 (83.6%) | |
| Adjuvant Treatment | None | 34 (61.8%) |
| BEP/EP | 16 (29.1%) | |
| Carboplatin–paclitaxel | 5 (9.1%) | |
| Parity | Unknown | 6 (10.9%) |
| ≤2 | 33 (60.0%) | |
| ≥3 | 16 (29.1%) | |
| Symptoms | Menstrual irregularity | 8 (14.5%) |
| Abdominal distension | 21 (38.2%) | |
| Postmenopausal bleeding | 18 (32.7%) | |
| Vaginal bleeding | 5 (9.1%) | |
| Acute abdomen | 1 (1.8%) | |
| Incidental | 2 (3.6%) | |
| Surgery Type | Primary staging | 51 (92.7%) |
| Other | 4 (7.2%) | |
| Tumor Size | ≤10 cm | 35 (63.6%) |
| >10 cm | 20 (36.4%) | |
| Tumor Location | Right | 25 (45.5%) |
| Left | 30 (54.5%) | |
| Pathological Stage | Stage 1 | 49 (89.1%) |
| Stage 2 | 1 (1.8%) | |
| Stage 3 | 5 (9.1%) | |
| Mitotic Index | ≤4 | 19 (34.5%) |
| >4 | 36 (65.5%) | |
| Vital Status | Alive | 50 (90.9%) |
| Deceased | 5 (9.1%) |
| Variable | Category Comparison | Chi-Square (χ2) | p-Value |
|---|---|---|---|
| Endometrial pathology | None vs. Non-atypical vs. Atypical/Carcinoma | 7.389 | 0.025 |
| Adjuvant chemotherapy | No vs. BEP/EP or Carboplatin–Paclitaxel | 6.953 | 0.008 |
| Stage | Stage I–II vs. Stage III | 4.029 | 0.045 |
| Age group | ≤65 vs. >65 | 0.101 | 0.751 |
| Menopausal status | Pre vs Peri/Post | 0.458 | 0.498 |
| Tumor size | ≤10 cm vs. >10 cm | 2.248 | 0.134 |
| Mitotic index | ≤4 vs. >4 | 0.99 | 0.32 |
| Ki-67 index | ≤10% vs. >10% | 0.103 | 0.749 |
| Estrogen receptor status | Negative vs. Positive vs Unknown | 0.408 | 0.815 |
| Inhibin expression | Negative vs Positive | 0.561 | 0.454 |
| Variable | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value |
|---|---|---|---|---|
| Stage (Stage III vs. I–II) | 7.14 (1.78–28.73) | 0.006 | 4.45 (1.03–19.27) | 0.046 |
| Tumor size (>10 cm vs. ≤10 cm) | 3.59 (1.18–10.95) | 0.025 | 2.13 (0.60–7.49) | 0.241 |
| Endometrial pathology (Absent) | 0.343 (0.138–0.858) | 0.022 | 0.51 (0.18–1.42) | 0.197 |
| Adjuvant chemotherapy (Yes vs. No) | 3.21 (0.96–10.69) | 0.058 | - | - |
| Mitotic index (>4 vs. ≤4) | 1.40 (0.54–3.63) | 0.488 | - | - |
| Menopausal status | 0.866 (0.501–1.495) | 0.605 | - | - |
| Ki-67 (>10% vs. ≤10%) | 1.01 (0.40–2.58) | 0.984 | - | - |
| Estrogen receptor positivity (Yes vs. No) | 1.17 (0.45–3.06) | 0.746 | - | - |
| Tumor laterality (Unilateral vs. Bilateral) | 1.23 (0.47–3.25) | 0.675 | - | - |
| Parity (≥3 vs. ≤2) | 0.85 (0.33–2.21) | 0.740 | - | - |
| Inhibin positivity (Yes vs. No) | 0.77 (0.27–2.23) | 0.620 | - | - |
| Comorbidity (Yes vs. No) | 1.11 (0.43–2.86) | 0.820 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geçgel, A.; Özcan, O.; Peker, P.; Serin, G.; Karaca Yayla, B.; Göker, E.; Şanlı, U.A. Ten-Year Real-World Outcomes and Clinicopathologic Predictors of Recurrence in Adult Granulosa Cell Tumors: A Turkish Single-Center Experience. Curr. Oncol. 2025, 32, 504. https://doi.org/10.3390/curroncol32090504
Geçgel A, Özcan O, Peker P, Serin G, Karaca Yayla B, Göker E, Şanlı UA. Ten-Year Real-World Outcomes and Clinicopathologic Predictors of Recurrence in Adult Granulosa Cell Tumors: A Turkish Single-Center Experience. Current Oncology. 2025; 32(9):504. https://doi.org/10.3390/curroncol32090504
Chicago/Turabian StyleGeçgel, Aslı, Oğuzcan Özcan, Pınar Peker, Gürdeniz Serin, Burçak Karaca Yayla, Erdem Göker, and Ulus Ali Şanlı. 2025. "Ten-Year Real-World Outcomes and Clinicopathologic Predictors of Recurrence in Adult Granulosa Cell Tumors: A Turkish Single-Center Experience" Current Oncology 32, no. 9: 504. https://doi.org/10.3390/curroncol32090504
APA StyleGeçgel, A., Özcan, O., Peker, P., Serin, G., Karaca Yayla, B., Göker, E., & Şanlı, U. A. (2025). Ten-Year Real-World Outcomes and Clinicopathologic Predictors of Recurrence in Adult Granulosa Cell Tumors: A Turkish Single-Center Experience. Current Oncology, 32(9), 504. https://doi.org/10.3390/curroncol32090504





