Primary Retroperitoneal Mucinous Cystadenocarcinoma in a Male Patient: A Case Report
Simple Summary
Abstract
1. Introduction
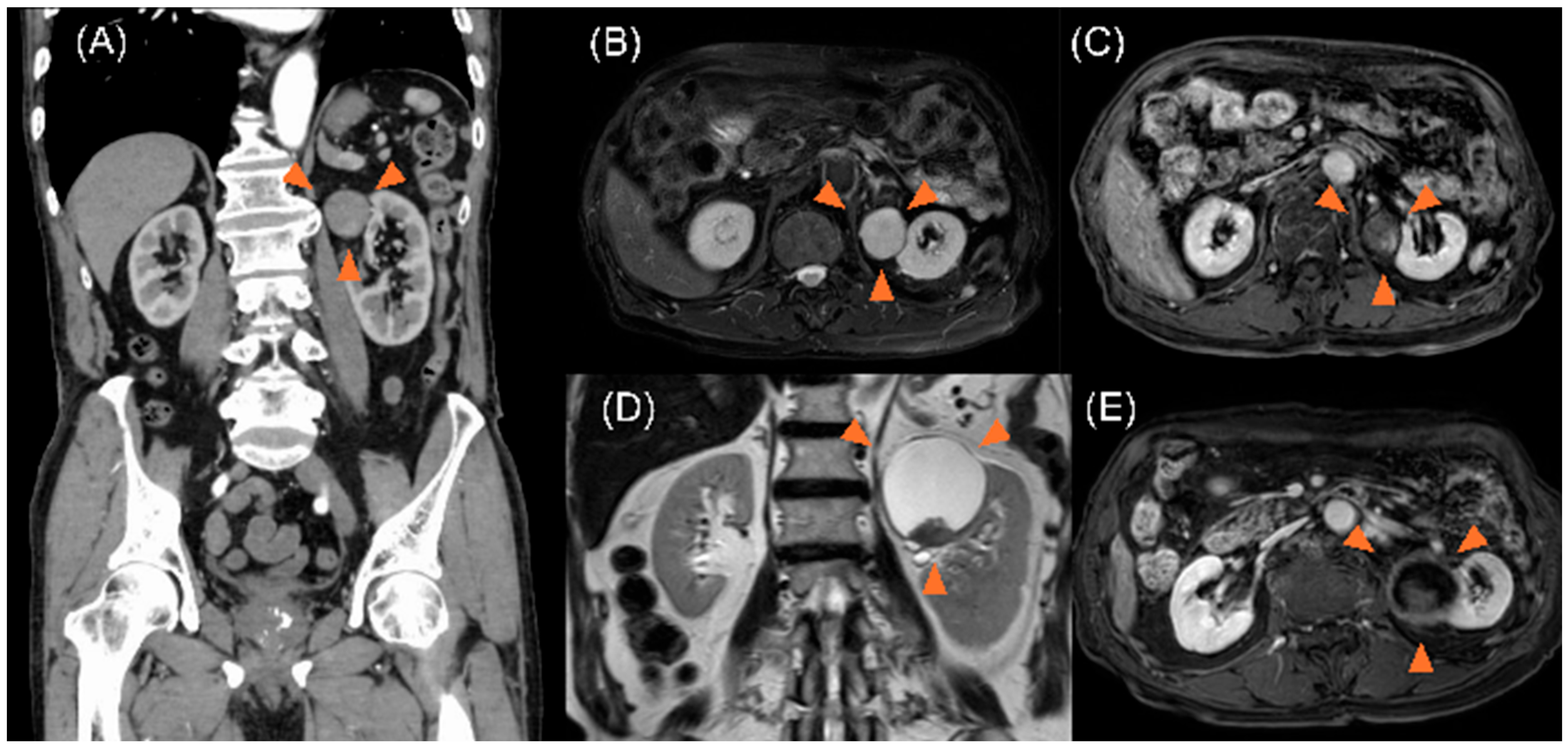
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRMC | Primary retroperitoneal mucinous adenocarcinoma |
| CT | Computed tomography |
| CEA | Carcinoembryonic antigen |
| CA | Carbohydrate antigen 19-9 |
| CK | Cytokeratin |
References
- Myriokefalitaki, E.; Luqman, I.; Potdar, N.; Brown, L.; Steward, W.; Moss, E.L. Primary retroperitoneal mucinous cystadenocarcinoma (PRMCa): A systematic review of the literature and meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Bruner, B.C.; Tang, W.W.; Orihuela, E. Retroperitoneal mucinous cystadenocarcinoma in a man: Case report and review of the literature. Urol. Oncol. 2007, 25, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Ehrlich, C.E. Mucinous cystadenocarcinoma of the retroperitoneum. Obstet. Gynecol. 1977, 49, 486–488. [Google Scholar] [PubMed]
- Storch, M.P.; Raghavan, U. Mucinous cystadenocarcinoma of retroperitoneum. Conn. Med. 1980, 44, 140–141. [Google Scholar] [PubMed]
- Thamboo, T.P.; Sim, R.; Tan, S.Y.; Yap, W.M. Primary retroperitoneal mucinous cystadenocarcinoma in a male patient. J. Clin. Pathol. 2006, 59, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Hrora, A.; Reggoug, S.; Jallal, H.; Sabbah, F.; Benamer, A.; Alaoui, M.; Raiss, M.; Ahallat, M. Primary retroperitoneal mu-cinous cystadenocarcinoma in a male patient: A case report. Cases J. 2009, 2, 7196. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.P.; Wu, C.T.; Chin, C.C.; Chuang, C.K. Long-term survival after hand-assisted laparoscopic approach of primary retroperitoneal mucinous cystadenocarcinoma in male: Case report and review of literature. Eur. Surg. 2013, 45, 106–109. [Google Scholar] [CrossRef]
- Feng, J.; Liu, H.; Chen, D. Primary retroperitoneal mucinous cystadenocarcinoma in a male patient: A rare case report. Hippokratia 2013, 17, 271–273. [Google Scholar] [PubMed]
- Tokai, H.; Nagata, Y.; Taniguchi, K.; Matsumura, N.; Kitasato, A.; Tokunaga, T.; Takeshita, H.; Kuroki, T.; Maeda, S.; Ito, M.; et al. The long-term survival in primary retroperitoneal mucinous cystadenocarcinoma: A case report. Surg. Case Rep. 2017, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, M.T.; Aragone, L.; Rebdza, V.S.; Nardi, W.; Toscano, M.; Quildrian, S. Primary retroperitoneal tumor: Mucinous cystoadenocarcinoma. Medicina 2024, 84, 750–755. [Google Scholar]
- Neville, A.; Herts, B.R. CT characteristics of primary retroperitoneal neoplasms. Crit. Rev. Comput. Tomogr. 2004, 45, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Mukherjee, U.; Singaravel, S. Primary retroperitoneal mucinous cystadenocarcinoma in a male patient. Indian J. Pathol. Microbiol. 2016, 59, 229. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.C.; Lam, V.; P’ng, C.H. A rare case of primary retroperitoneal mucinous neoplasm in a male patient. Pathology 2015, 47, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Morihisa, Y.; Urai, S.; Iwagami, H.; Shimoyama, M.; Ogino, S.; Terashita, T.; Morimura, H.; Akamatsu, T.; Uenoyama, Y.; Yamashita, Y. Primary retroperitoneal mucinous cystadenocarcinoma during long-term administration of infliximab for the treatment of Crohn’s disease. Intern. Med. 2023, 62, 3619–3624. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.D.; Kavoussi, L.; Thomas, R.; Savant, D. Primary retroperitoneal mucinous cystadenocarcinoma: A case report. Cureus 2023, 15, e39983. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, F.P.; Nelson, A.W.; Colquhoun, A.J.; Lobo, N. Utility of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Detecting Lymph Node Involvement in Comparison to Conventional Imaging in Patients with Bladder Cancer with Variant Histology. Eur. Urol. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Mehta, D.R.; Holla, D.R. Retroperitoneal mucinous adenocarcinoma in a young male-diagnostic dilemma: A case report. J. Case Rep. Sci. Images 2022, 4, 9–10. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Tang, J.; Yang, P.; Sun, D. Case report: The first known case of male retroperitoneal mesonephric-like adenocarcinoma. Front. Oncol. 2024, 14, 1433563. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, F.; Morje, M.; Jamall, H.; Polson, A.; Deo, N. Primary Retroperitoneal Mucinous Tumours Diagnosed in Pregnancy: A Case Report and Literature Review. Int. J. Womens Health 2019, 11, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Woo, C.G.; Yun, S.J.; Lee, O.J. Primary retroperitoneal mucinous cystic neoplasm of borderline malignancy with KRAS and GNAS co-mutation: A case report. J. Int. Med. Res. 2023, 51, 3000605231172469. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Age (Years) | Tumor Size (cm) | Symptoms | Management | Outcome |
|---|---|---|---|---|---|
| Thamboo [5], 2006 | 64 | 24 × 20 × 16 | Acute abdominal discomfort | Complete tumor excision (laparotomy) | No recurrence at 18 months |
| Green [2], 2007 | 83 | 26 × 20 × 16 | Abdominal discomfort and cachexia | Complete tumor excision (open surgery) | No recurrence at 6 months |
| Hrora [6], 2009 | 42 | 5 × 4 × 3 | Abdominal discomfort and distension | Two-stage surgical removal of all cysts | No recurrence at 6 months |
| Shiau [7], 2013 | 59 | 7.5 × 7 × 3 | Sudden onset of left flank pain | Complete tumor excision (laparotomy) | No recurrence at 79 months |
| Feng [8], 2013 | 63 | 4 × 3 × 3 | Chronic lower back pain | Complete tumor excision (laparotomy) | No recurrence at 13 months |
| Vargas [13], 2015 | 68 | 16 × 14.5 × 11 | Self-detected palpable right abdominal mass | Complete tumor excision (laparotomy) | Not reported |
| Khurana [12], 2016 | 57 | 21 × 18 × 7 | Weight loss, poor appetite, and palpable fixed mass | Complete tumor excision (laparotomy) | No recurrence at 15 months |
| Morihisa [14], 2023 | 60 | 20 × 8.8 × 4.7 | Lower back pain | Systemic chemotherapy with carboplatin and paclitaxel | Bone metastasis at diagnosis |
| Present case | 86 | 58 × 60 × 59 | Asymptomatic | Complete tumor excision (laparotomy) | No recurrence at 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomioka, M.; Nakane, K.; Iinuma, K.; Kawase, K.; Taniguchi, T.; Tobisawa, Y.; Muto, A.; Kanayama, T.; Miyazaki, T.; Koie, T. Primary Retroperitoneal Mucinous Cystadenocarcinoma in a Male Patient: A Case Report. Curr. Oncol. 2025, 32, 500. https://doi.org/10.3390/curroncol32090500
Tomioka M, Nakane K, Iinuma K, Kawase K, Taniguchi T, Tobisawa Y, Muto A, Kanayama T, Miyazaki T, Koie T. Primary Retroperitoneal Mucinous Cystadenocarcinoma in a Male Patient: A Case Report. Current Oncology. 2025; 32(9):500. https://doi.org/10.3390/curroncol32090500
Chicago/Turabian StyleTomioka, Masayuki, Keita Nakane, Koji Iinuma, Kota Kawase, Tomoki Taniguchi, Yuki Tobisawa, Aoi Muto, Tomohiro Kanayama, Tatsuhiko Miyazaki, and Takuya Koie. 2025. "Primary Retroperitoneal Mucinous Cystadenocarcinoma in a Male Patient: A Case Report" Current Oncology 32, no. 9: 500. https://doi.org/10.3390/curroncol32090500
APA StyleTomioka, M., Nakane, K., Iinuma, K., Kawase, K., Taniguchi, T., Tobisawa, Y., Muto, A., Kanayama, T., Miyazaki, T., & Koie, T. (2025). Primary Retroperitoneal Mucinous Cystadenocarcinoma in a Male Patient: A Case Report. Current Oncology, 32(9), 500. https://doi.org/10.3390/curroncol32090500






