Focal Therapy for Localized Prostate Cancer: A Case Series with Cost Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
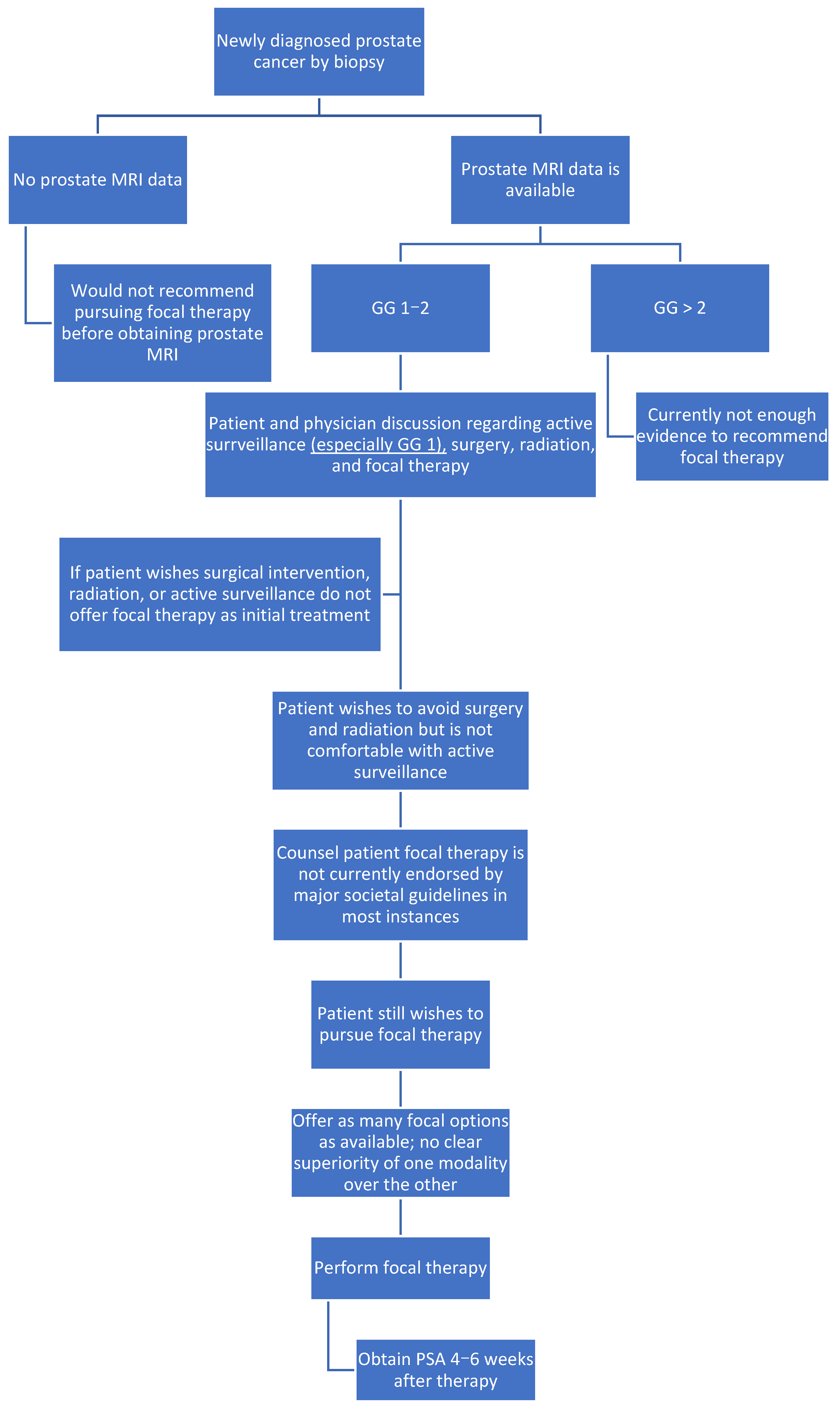
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCa | Prostate cancer |
| USA | United States of America |
| RP | Radical prostatectomy |
| ED | Erectile dysfunction |
| MRI | Magnetic resonance imaging |
| HIFU | High-intensity focused ultrasound |
| IRE | Irreversible electroporation |
| OS | Overall survival |
| MFS | Metastasis-free survival |
| LUTS | Lower urinary tract symptoms |
| IIEF | International Index of Erectile Function |
| IPSS | International Prostatic Symptom Score |
| GG | Grade group |
| NCCN | National Comprehensive Cancer Network |
| FALCOS | FocAL therapy CONsensus |
| TURP | Transurethral resection of prostate |
| CSS | Cancer-specific survival |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Anandadas, C.N.; Clarke, N.W.; Davidson, S.E.; O’Reilly, P.H.; Logue, J.P.; Gilmore, L.; Swindell, R.; Brough, R.J.; Wemyss-Holden, G.D.; Lau, M.W.; et al. Early prostate cancer—Which treatment do men prefer and why? BJU Int. 2011, 107, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Kaps, B.; Leapman, M.; An, Y. Trends in prostatectomy utilization: Increasing upfront prostatectomy and postprostatectomy radiotherapy for high-risk prostate cancer. Cancer Med. 2020, 9, 8754–8764. [Google Scholar] [CrossRef]
- Diven, M.A.; Tshering, L.; Ma, X.; Hu, J.C.; Barbieri, C.; McClure, T.; Nagar, H. Trends in Active Surveillance for Men with Intermediate-Risk Prostate Cancer. JAMA Netw. Open 2024, 7, e2429760. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, D.T.; Flum, A.S.; Lai, J.D.; Meeks, J.J. The effect of minimally invasive prostatectomy on practice patterns of American urologists. Urol. Oncol. 2016, 34, 255.e1–255.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Tan, H.-J.; Fang, R.; Mbassa, R.; Matulewicz, R. Primary Question: How Has the Average Number of Radical Prostatectomies Performed by Urologists Changed Over Time? AUA NEWS, 25 October 2023. Available online: https://auanews.net/issues/articles/2023/october-extra-2023/primary-question-how-has-the-average-number-of-radical-prostatectomies-performed-by-urologists-changed-over-time (accessed on 19 June 2025).
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Muise, A.; Pan, M.M.; Rose, B.; Buckley, J.C. Functional outcomes after prostate cancer treatment: A comparison between single and multiple modalities. Urol. Oncol. 2023, 41, 104.e1–104.e9. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.H.; Ollendorf, D.A.; Pearson, S.D.; Barry, M.J.; Kantoff, P.W.; Stewart, S.T.; Bhatnagar, V.; Sweeney, C.J.; Stahl, J.E.; McMahon, P.M. Active surveillance compared with initial treatment for men with low-risk prostate cancer: A decision analysis. JAMA 2010, 304, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Meeks, W.; Fang, R.; Gaylis, F.D.; Catalona, W.J.; Makarov, D.V. Time Trends and Variation in the Use of Active Surveillance for Management of Low-risk Prostate Cancer in the US. JAMA Netw. Open 2023, 6, e231439. [Google Scholar] [CrossRef] [PubMed]
- Spellman, A.A.; Golla, V.; Lin, L.; Katz, A.; Chen, R.C.; Zullig, L.L. Long-Term Trends in Decisional Regret Among Men with Localized Prostate Cancer. JU Open Plus 2024, 2, e00027. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Zhao, Z.; Huang, L.-C.; Penson, D.F.; Koyama, T.; Kaplan, S.H.; Greenfield, S.; Luckenbaugh, A.N.; Klaassen, Z.; Conwill, R.; et al. Association of Treatment Modality, Functional Outcomes, and Baseline Characteristics With Treatment-Related Regret Among Men With Localized Prostate Cancer. JAMA Oncol. 2022, 8, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.; Soon-Sutton, W.; Skelton, W. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gordetsky, J.B.; Saylor, B.; Bae, S.; Nix, J.W.; Rais-Bahrami, S. Prostate cancer management choices in patients undergoing multiparametric magnetic resonance imaging/ultrasound fusion biopsy compared to systematic biopsy. Urol. Oncol. 2018, 36, 241.e7–241.e13. [Google Scholar] [CrossRef]
- Dix, D.B.; McDonald, A.M.; Gordetsky, J.B.; Nix, J.W.; Thomas, J.V.; Rais-Bahrami, S. How Would MRI-targeted Prostate Biopsy Alter Radiation Therapy Approaches in Treating Prostate Cancer? Urology 2018, 122, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gordetsky, J.B.; Schultz, L.; Porter, K.K.; Nix, J.W.; Thomas, J.V.; Del Carmen Rodriguez Pena, M.; Rais-Bahrami, S. Defining the optimal method for reporting prostate cancer grade and tumor extent on magnetic resonance/ultrasound fusion-targeted biopsies. Hum. Pathol. 2018, 76, 68–75. [Google Scholar] [CrossRef]
- Sankineni, S.; Wood, B.J.; Rais-Bahrami, S.; Walton Diaz, A.; Hoang, A.N.; Pinto, P.A.; Choyke, P.L.; Türkbey, B. Image-guided focal therapy for prostate cancer. Diagn. Interv. Radiol. Ank. Turk. 2014, 20, 492–497. [Google Scholar] [CrossRef]
- Deivasigamani, S.; Kotamarti, S.; Rastinehad, A.R.; Salas, R.S.; de la Rosette, J.J.M.C.H.; Lepor, H.; Pinto, P.; Ahmed, H.U.; Gill, I.; Klotz, L.; et al. Primary Whole-gland Ablation for the Treatment of Clinically Localized Prostate Cancer: A Focal Therapy Society Best Practice Statement. Eur. Urol. 2023, 84, 547–560. [Google Scholar] [CrossRef]
- Ayerra Perez, H.; Barba Abad, J.F.; Extramiana Cameno, J. An Update on Focal Therapy for Prostate Cancer. Clin. Genitourin. Cancer 2023, 21, 712.e1–712.e8. [Google Scholar] [CrossRef] [PubMed]
- Guillaumier, S.; Peters, M.; Arya, M.; Afzal, N.; Charman, S.; Dudderidge, T.; Hosking-Jervis, F.; Hindley, R.G.; Lewi, H.; McCartan, N.; et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur. Urol. 2018, 74, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E.; Klein, N.; Zapf, S.; Weil, S.; Schlosser, C.; Rubinsky, B.; Stehling, M.K. Prostate cancer treatment with Irreversible Electroporation (IRE): Safety, efficacy and clinical experience in 471 treatments. PLoS ONE 2019, 14, e0215093. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Tracey, A.T.; Nogueira, L.M.; Alvim, R.G.; Coleman, J.A.; Murray, K.S. Focal therapy for primary and salvage prostate cancer treatment: A narrative review. Transl. Androl. Urol. 2021, 10, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Bomers, J.G.R.; Overduin, C.G.; Jenniskens, S.F.M.; Cornel, E.B.; van Lin, E.N.J.T.; Sedelaar, J.P.M.; Fütterer, J.J. Focal Salvage MR Imaging-Guided Cryoablation for Localized Prostate Cancer Recurrence after Radiotherapy: 12-Month Follow-up. J. Vasc. Interv. Radiol. JVIR 2020, 31, 35–41. [Google Scholar] [CrossRef]
- Kanthabalan, A.; Peters, M.; Van Vulpen, M.; McCartan, N.; Hindley, R.G.; Emara, A.; Moore, C.M.; Arya, M.; Emberton, M.; Ahmed, H.U. Focal salvage high-intensity focused ultrasound in radiorecurrent prostate cancer. BJU Int. 2017, 120, 246–256. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 1067–1096. [Google Scholar] [CrossRef] [PubMed]
- Javier-DesLoges, J.; Dall’Era, M.A.; Brisbane, W.; Chamie, K.; Washington, S.L.; Chandrasekar, T.; Marks, L.S.; Nguyen, H.; Daneshvar, M.; Gin, G.; et al. The state of focal therapy in the treatment of prostate cancer: The university of California collaborative (UC-Squared) consensus statement. Prostate Cancer Prostatic Dis. 2024, 27, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Chen, K.; Grummet, J.; Yaxley, J.; Scheltema, M.J.; Stricker, P.; Tay, K.J.; Lawrentschuk, N. Guidelines of guidelines: Focal therapy for prostate cancer, is it time for consensus? BJU Int. 2023, 131, 20–31. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, L.; Reiter, R.; Rodríguez, A.; Emberton, M.; de Reijke, T.; Compérat, E.M.; Bossi, A.; Sanchez-Salas, R. The FocAL therapy CONsensus (FALCON): Enhancing partial gland ablation for localised prostate cancer. BJU Int. 2024, 134, 50–53. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, L.; Cathelineau, X.; de Reijke, T.M.; Stricker, P.; Emberton, M.; Lantz, A.; Miñana López, B.; Dominguez-Escrig, J.L.; Bianco, F.J.; Salomon, G.; et al. Refining partial gland ablation for localised prostate cancer: The FALCON project. BJU Int. 2025, 135, 1000–1009. [Google Scholar] [CrossRef]
- Scheltema, M.J.; Tay, K.J.; Postema, A.W.; de Bruin, D.M.; Feller, J.; Futterer, J.J.; George, A.K.; Gupta, R.T.; Kahmann, F.; Kastner, C.; et al. Utilization of multiparametric prostate magnetic resonance imaging in clinical practice and focal therapy: Report from a Delphi consensus project. World J. Urol. 2017, 35, 695–701. [Google Scholar] [CrossRef]
- Gordetsky, J.; Rais-Bahrami, S.; Epstein, J.I. Pathological Findings in Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion-guided Biopsy: Relation to Prostate Cancer Focal Therapy. Urology 2017, 105, 18–23. [Google Scholar] [CrossRef]
- Flegar, L.; Zacharis, A.; Aksoy, C.; Heers, H.; Derigs, M.; Eisenmenger, N.; Borkowetz, A.; Groeben, C.; Huber, J. Alternative- and focal therapy trends for prostate cancer: A total population analysis of in-patient treatments in Germany from 2006 to 2019. World J. Urol. 2022, 40, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, G.; Colin, P.; Nevoux, P.; Villers, A.; Mordon, S.; Betrouni, N. Focal Therapy of prostate cancer: Energies and procedures. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mearini, L.; Porena, M. Pros and cons of focal therapy for localised prostate cancer. Prostate Cancer 2011, 2011, 584784. [Google Scholar] [CrossRef]
- Tay, K.J.; Fong, K.Y.; Stabile, A.; Dominguez-Escrig, J.L.; Ukimura, O.; Rodriguez-Sanchez, L.; Blana, A.; Becher, E.; Laguna, M.P. Established focal therapy-HIFU, IRE, or cryotherapy-where are we now?—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, R.; Aoun, F.; Marcelis, Q.; Albisinni, S.; Zanaty, M.; Lemort, M.; Peltier, A.; Limani, K. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Arnouil, N.; Gelet, A.; Matillon, X.; Rouviere, O.; Colombel, M.; Ruffion, A.; Mège-Lechevallier, F.; Subtil, F.; Badet, L.; Crouzet, S. [Focal HIFU vs robot-assisted total prostatectomy: Functionnal and oncologic outcomes at one year]. Prog. Urol. 2018, 28, 603–610. [Google Scholar] [CrossRef]
- Nahar, B.; Bhat, A.; Reis, I.M.; Soodana-Prakash, N.; Becerra, M.F.; Lopategui, D.; Venkatramani, V.; Patel, R.; Madhusoodanan, V.; Kryvenko, O.N.; et al. Prospective Evaluation of Focal High Intensity Focused Ultrasound for Localized Prostate Cancer. J. Urol. 2020, 204, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.H.; Passoni, N.M.; Pow-Sang, J.; Jones, J.S.; Polascik, T.J. Comparison of Outcomes Between Preoperatively Potent Men Treated with Focal Versus Whole Gland Cryotherapy in a Matched Population. J. Endourol. 2015, 29, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.G.; Law, Y.M.; Ngo, N.T.; Khor, L.Y.; Tan, P.H.; Ong, E.H.W.; Yuen, J.S.P.; Ho, H.S.S.; Tuan, J.K.L.; Kanesvaran, R.; et al. Patient-reported functional outcomes and oncological control after primary focal cryotherapy for clinically significant prostate cancer: A Phase II mandatory biopsy-monitored study. Prostate 2023, 83, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.; Deivasigamani, S.; Schwartz, M.J.; Ward, J.F.; George, A.; Sidana, A.; Becher, E.; Katz, A.E.; Sanchez-Salas, R.; Polascik, T.J. MP25-16 FOCAL CRYOTHERAPY FOR LOCALIZED PROSTATE CANCER: INITIAL REPORT FROM THE INTERNATIONAL FOCAL THERAPY SOCIETY (FTS) REGISTRY. J. Urol. 2024, 211, e410. [Google Scholar] [CrossRef]
- Xia, Z.-Y.; Yang, J.; Xiao, F.; Xu, J.-Z.; Zhong, X.-Y.; Wang, S.-G.; Xia, Q.-D. A preliminary follow-up study on irreversible electroporation therapy in older patients with prostate cancer. Discov. Oncol. 2025, 16, 278. [Google Scholar] [CrossRef]
- Ślusarczyk, A.; Gurwin, A.; Barnaś, A.; Ismail, H.; Miszczyk, M.; Zapała, P.; Przydacz, M.; Krajewski, W.; Antczak, A.; Życzkowski, M.; et al. Outcomes of Focal Therapy for Localized Prostate Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Eur. Urol. Oncol. 2025, S2588931125000392. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.; Pellegrino, A.; Mazzone, E.; Cannoletta, D.; de Angelis, M.; Barletta, F.; Scuderi, S.; Cucchiara, V.; Gandaglia, G.; Raggi, D.; et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur. Urol. Oncol. 2022, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Novara, G.; Ficarra, V.; Rosen, R.C.; Artibani, W.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Shariat, S.F.; Stolzenburg, J.-U.; et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 431–452. [Google Scholar] [CrossRef]
- Azzouzi, A.-R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
- Gill, I.S.; Azzouzi, A.-R.; Emberton, M.; Coleman, J.A.; Coeytaux, E.; Scherz, A.; Scardino, P.T. PCM301 Study Group Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J. Urol. 2018, 200, 786–793. [Google Scholar] [CrossRef]
- Labbate, C.V.; Klotz, L.; Morrow, M.; Cooperberg, M.; Esserman, L.; Eggener, S.E. Focal Therapy for Prostate Cancer: Evolutionary Parallels to Breast Cancer Treatment. J. Urol. 2023, 209, 49–57. [Google Scholar] [CrossRef]
- Reddy, D.; van Son, M.; Peters, M.; Bertoncelli Tanaka, M.; Dudderidge, T.; Cullen, E.; Ho, C.L.T.; Hindley, R.G.; Emara, A.; McCracken, S.; et al. Focal therapy versus radical prostatectomy and external beam radiotherapy as primary treatment options for non-metastatic prostate cancer: Results of a cost-effectiveness analysis. J. Med. Econ. 2023, 26, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.A.L.; Lima, A.F.C. Direct costs of treating men with prostate cancer with High Intensity Focused Ultrasound. Rev. Esc. Enferm. USP 2023, 57, e20230132. [Google Scholar] [CrossRef] [PubMed]
| Variable | HIFU | IRE | Cryoablation | Total |
|---|---|---|---|---|
| N | 4 | 21 | 20 | 45 |
| Age | 66.5 (13.3) | 70.5 (7.4) | 70.6 (7.4) | 70.1 (8.2) |
| Caucasian | 3 (75) | 16 (76) | 15 (75) | 34 (76) |
| Black | 1 (25) | 5 (24) | 2 (10) | 8 (18) |
| Other | 0 | 0 | 3 (15) | 3 (7) |
| Charlson Comorbidity | 4 (5.0) | 5.7(1.7) | 5.9 (2.0) | 5.7 (1.9) |
| IIEF preoperative | 19.5 (0.7) | 13.6 (7.3) | 14.7 (8.3) | 14.7 (7.4) |
| ED preoperative | 1 (25) | 12 (57) | 14 (70) | 27 (60) |
| IPSS preoperative | 7.3 (4.6) | 8.1 (3.5) | 8.0 (7.4) | 7.9 (5.2) |
| LUTS preoperative | 2 (50) | 11 (52) | 10 (50) | 23 (51) |
| Focal | 1 (25) | 17 (81) | 12 (60) | 30 (67) |
| Hemi | 3 (75) | 4 (19) | 8 (40) | 15 (33) |
| Prior treatment | ||||
| Brachytherapy | 0 (0) | 0 (0) | 2 (10) | 2 (15) |
| Radiation | 0 (0) | 1 (5) | 3 (15) | 4 (31) |
| Cryoablation | 0 (0) | 2 (10) | 4 (20) | 6 (46) |
| IRE | 0 (0) | 0 (0) | 1 (5) | 1 (8) |
| Preoperative PSA (ng/mL) | 7.2 (2.2) | 7.0 (3.0) | 8.5 (3.8) | 7.7 (3.3) |
| GG preoperative | ||||
| 1 | 0 (0) | 2 (10) | 3 (15) | 5 (11) |
| 2 | 3 (75) | 13 (62) | 14 (70) | 30 (67) |
| 3 | 1 (25) | 5 (24) | 3 (15) | 9 (20) |
| 4 | 0 (0) | 1 (5) | 0 (0) | 1 (2) |
| Prostate size (cc) | 52.8 (17.3) | 51.3 (25.4) | 39.5 (17.0) | 46.1 (21.7) |
| Very low risk | 0 | 2 | 2 | 4 (9) |
| Favorable intermediate risk | 5 | 13 | 17 | 35 (78) |
| Unfavorable intermediate risk | 1 | 5 | 3 | 9 (20) |
| High risk | 0 | 1 | 0 | 1 (2) |
| Operative time (minutes) | 114.3 (20.6) | 43.1 (6.1) | 65.2 (14.0) | 59.2 (23.3) |
| Complication | 1 (25) | 7 (33) | 8 (40) | 16 (36) |
| Clavien | 9 (20) 6 (13) 2 (4) | |||
| I | 0 (0) | 4 (19) | 5 (25) | |
| II | 2 (50) | 3 (14) | 1 (5) | |
| IIIa | 0 (0) | 0 (0) | 2 (10) | |
| IIIb | 0 (0) | 0 (0) | 0 (0) | |
| IV | 0 (0) | 0 (0) | 0 (0) | |
| V | 0 (0) | 0 (0) | 0 (0) | |
| Most recent PSA (ng/mL) | 4.5 (2.4) | 3.5 (2.4) | 2.5 (2.2) | 3.1 (2.3) |
| Biopsy postoperative | 0 (0) | 0 (0) | 3 (15) | 3 (7) |
| Positive biopsy postoperative | 0 (0) | 0 (0) | 3 (15) | 3 (7) |
| GG postoperative | - | - | 3 (100) | 3 (100) |
| IIEF postoperative | - | 14 (67) | 7 | 10.5 (5) |
| ED postoperative | 0 (0) | 4 (19) | 12 (60) | 16 (36) |
| IPSS postoperative | 5 | 0 | 6.3 (6.8) | 6 (5.6) |
| LUTS postoperative | 0 (0) | 7 (33) | 13 (65) | 20 (44) |
| Cost (USD) | - | 3762.3 (2552.3) | 4648.3 | 3804.5 (2495.2) |
| Metastasis | 0 | 0 | 0 | - |
| Dead | 0 | 0 | 0 | - |
| Follow-up (months) | 4 (3–8) | 4 (4–7) | 12 (2.5–17) | 6 (3–12) |
| Therapy | PSA Preoperative (ng/mL) | PSA Postoperative (ng/mL) | p-Value |
|---|---|---|---|
| HIFU | 6.4 (1.9) | 4.5 (2.4) | 0.082 |
| IRE | 7.1 (3.3) | 3.5 (2.4) | 0.001 |
| Cryoablation | 8.0 (3.8) | 2.5 (2.2) | <0.001 |
| Total | 7.5 (3.4) | 3.1 (2.3) | 0.854 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandberg, M.; Thole, D.; Nowatzke, J.; Underwood, G.; Ye, E.; Rais-Bahrami, S.; Davis, R.; Rodriguez, A. Focal Therapy for Localized Prostate Cancer: A Case Series with Cost Analysis. Curr. Oncol. 2025, 32, 476. https://doi.org/10.3390/curroncol32090476
Sandberg M, Thole D, Nowatzke J, Underwood G, Ye E, Rais-Bahrami S, Davis R, Rodriguez A. Focal Therapy for Localized Prostate Cancer: A Case Series with Cost Analysis. Current Oncology. 2025; 32(9):476. https://doi.org/10.3390/curroncol32090476
Chicago/Turabian StyleSandberg, Maxwell, David Thole, Jackson Nowatzke, Gavin Underwood, Emily Ye, Soroush Rais-Bahrami, Ronald Davis, and Alejandro Rodriguez. 2025. "Focal Therapy for Localized Prostate Cancer: A Case Series with Cost Analysis" Current Oncology 32, no. 9: 476. https://doi.org/10.3390/curroncol32090476
APA StyleSandberg, M., Thole, D., Nowatzke, J., Underwood, G., Ye, E., Rais-Bahrami, S., Davis, R., & Rodriguez, A. (2025). Focal Therapy for Localized Prostate Cancer: A Case Series with Cost Analysis. Current Oncology, 32(9), 476. https://doi.org/10.3390/curroncol32090476






