Extended Survival with Pancreatic Carcinosarcoma: A Case Report and Literature Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
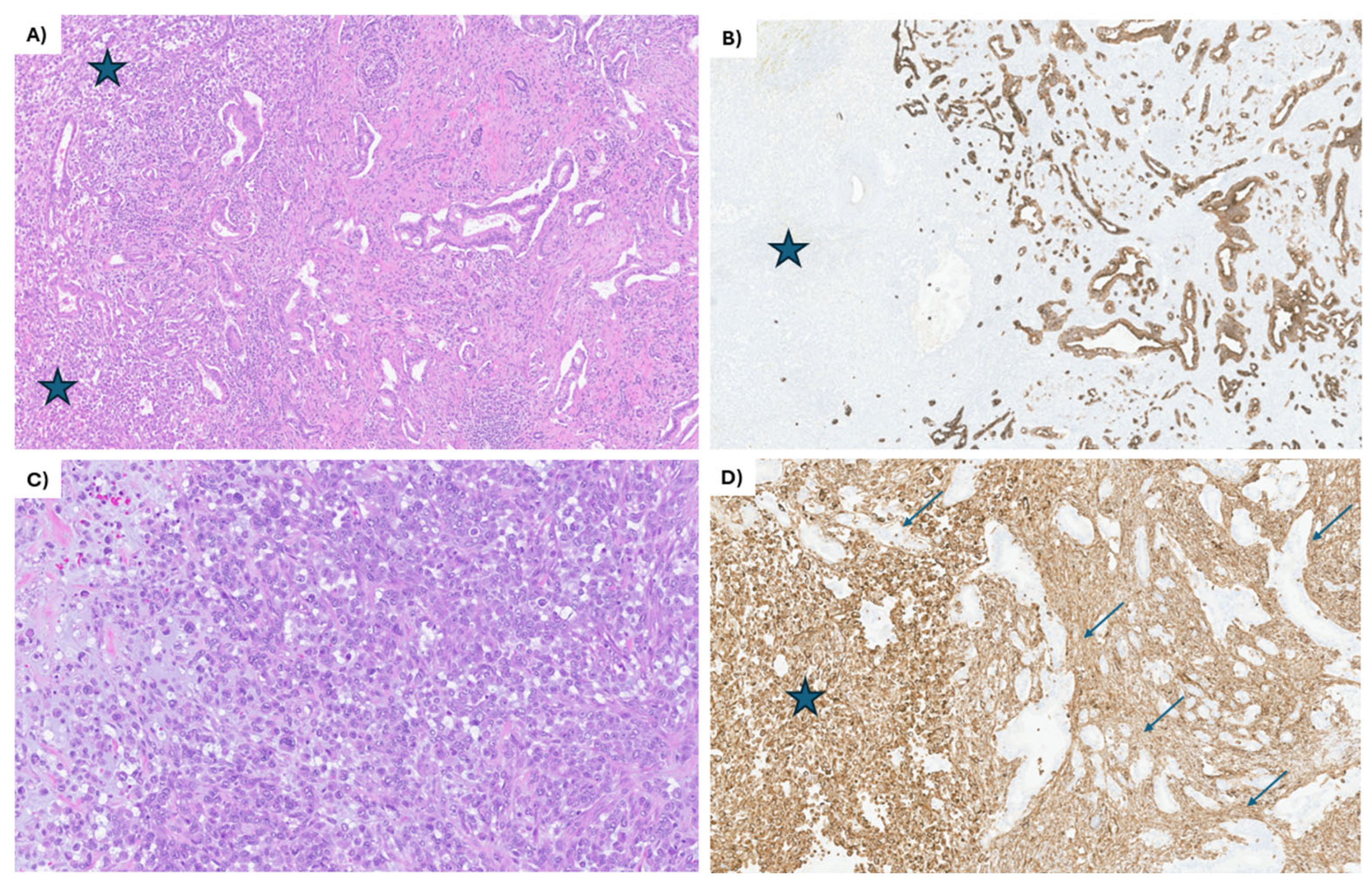
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| ctDNA | circulating tumor DNA |
| EMT | epithelial–mesenchymal transition |
| EUS | endoscopic ultrasound |
| FOLFIRINOX | leucovorin, 5-fluorouracil, irinotecan, oxaliplatin |
| FOLFOX | leucovorin, 5-fluorouracil, oxaliplatin |
| GTX | gemcitabine, docetaxel, capecitabine |
| IHC | immunohistochemistry |
| MMR | mismatch repair |
| mOS | median overall survival |
| MSI-H | high microsatellite instability |
| NGS | next-generation sequencing |
| PDAC | pancreatic ductal adenocarcinoma |
| PET | positron emission tomography |
| SABR | stereotactic ablative radiotherapy |
| SMA | superior mesenteric artery |
| RT | radiation |
| WHO | World Health Organization |
References
- Alhatem, A.; Quinn, P.L.; Xia, W.; Chokshi, R.J. Pancreatic Carcinosarcoma Clinical Outcome Analysis of the National Cancer Institute Database. J. Surg. Res. 2021, 259, 62–70. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Dann, A.M.; Elliott, I.A.; Baba, H.; Kim, S.; Sedarat, A.; Muthusamy, V.R.; Girgis, M.D.; Hines, O.J.; Reber, H.A.; et al. A Comprehensive Assessment of Accurate Lymph Node Staging and Preoperative Detection in Resected Pancreatic Cancer. J. Gastrointest. Surg. 2018, 22, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.; Hoppe, A.; Bayat, A.; Kogelschatz, B.; Mbanugo, C.; Chung, M.; Wolf, A.; Assifi, M.M.; Wright, G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery 2021, 169, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Ruess, D.A.; Kayser, C.; Neubauer, J.; Fichtner-Feigl, S.; Hopt, U.T.; Wittel, U.A. Carcinosarcoma of the Pancreas: Case Report With Comprehensive Literature Review. Pancreas 2017, 46, 1225. [Google Scholar] [CrossRef]
- Khan, J.; Cheng, L.; House, M.G.; Guo, S. Carcinosarcoma, a Rare Malignant Neoplasm of the Pancreas. Curr. Oncol. 2021, 28, 5295–5303. [Google Scholar] [CrossRef]
- Gkountakos, A.; Simbolo, M.; Bariani, E.; Scarpa, A.; Luchini, C. Undifferentiated Sarcomatoid Carcinoma of the Pancreas: From Histology and Molecular Pathology to Precision Oncology. Int. J. Mol. Sci. 2022, 23, 1283. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Zhang, H.; Mugaanyi, J.; Hu, Y.; Wu, S.; Lu, C.; Mao, S.; Wang, K. Sarcomatoid carcinoma of the pancreas (Review). Oncol. Lett. 2024, 28, 477. [Google Scholar] [CrossRef]
- Zhou, P.; Li, B.; Liu, F.; Zhang, M.; Wang, Q.; Liu, Y.; Yao, Y.; Li, D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer 2017, 16, 52. [Google Scholar] [CrossRef]
- Miyauchi, J.; Ogura, M.; Sato, M.; Matsui, J. Esophageal carcinosarcoma comprised of minimally invasive squamous cell carcinoma and undifferentiated pleomorphic sarcoma: A collision cancer? Pathol. Int. 2018, 68, 479–484. [Google Scholar] [CrossRef]
- Matsumoto, T.; Fujii, H.; Arakawa, A.; Yamasaki, S.; Sonoue, H.; Hattori, K.; Kajiyama, Y.; Hirose, S.; Tsurumaru, M. Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic progression and divergence in esophageal carcinosarcoma. Hum. Pathol. 2004, 35, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, O.; Sugiyama, Y.; Takazawa, Y.; Kato, K.; Tanaka, N.; Omatsu, K.; Takeshima, N.; Nomura, H.; Hasegawa, K.; Fujiwara, K.; et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat. Commun. 2019, 10, 4965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, T.; Zhang, J.; Wei, S.; Chen, Q.; Chen, B.W.; Zhou, Y.; Wen, L.; Qin, H.; Bai, X.; et al. Carcinosarcoma of the pancreas: Comprehensive clinicopathological and molecular characterization. HPB 2020, 22, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Joo, S.H.; Yang, D.M.; Lee, S.H.; Choi, S.H.; Lim, S.J. Carcinosarcoma of the Pancreas: A Unique Case with Emphasis on Metaplastic Transformation and the Presence of Undifferentiated Pleomorphic High-Grade Sarcoma. J. Gastrointestin. Liver Dis. 2011, 20, 197–200. [Google Scholar]
- Bai, Q.; Zhang, X.; Zhu, X.; Wang, L.; Huang, D.; Cai, X.; Zhou, X.; Wang, J.; Sheng, W. Pancreatic carcinosarcoma with the same KRAS gene mutation in both carcinomatous and sarcomatous components: Molecular evidence for monoclonal origin of the tumour. Histopathology 2016, 69, 393–405. [Google Scholar] [CrossRef]
- Zalewski, A.; Chlebicka, I.; Szepietowski, J.C. Collision tumours: Our recent experience. Postępy Dermatol. Alergol. 2024, 41, 622–625. [Google Scholar] [CrossRef]
- Petersson, F. Mixed tumors and collision tumors: A unifying concept with relevance to diagnosis and classification. Path. Int. 2015, 65, 459–466. [Google Scholar]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Shi, H.-Y.; Xie, J.; Miao, F. Pancreatic carcinosarcoma: First literature report on computed tomography imaging. World J. Gastroenterol. 2015, 21, 1357–1361. [Google Scholar] [CrossRef]
- Lim, H.J.; Kang, H.S.; Lee, J.E.; Min, J.H.; Shin, K.S.; You, S.K.; Kim, K.H. Sarcomatoid carcinoma of the pancreas—Multimodality imaging findings with serial imaging follow-up: A case report and review of literature. World J. Clin. Cases 2021, 9, 3102–3113. [Google Scholar] [CrossRef]
- Zhao, S.; Su, W.; Deng, L.; Chen, Y.; Zuo, C.; Shao, C.; Ren, F. Pancreatic sarcomatoid carcinoma: CT, MRI, and 18F-FDG PET/CT features. Clin. Radiol. 2020, 75, 397.e7–397.e14. [Google Scholar] [CrossRef]
- Mencel, J.; Feber, A.; Begum, R.; Carter, P.; Smalley, M.; Bourmpaki, E.; Shur, J.; Zar, S.; Kohoutova, D.; Popat, S.; et al. Liquid biopsy for diagnosis in patients with suspected pancreatic and biliary tract cancers: PREVAIL ctDNA pilot trial. J. Clin. Oncol. 2022, 40, 522. [Google Scholar] [CrossRef]
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current Status of Circulating Tumor DNA Liquid Biopsy in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Min, L.; Zhou, Y.; Tang, F.; Luo, Y.; Zhang, W.; Duan, H.; Tu, C. The efficacy and safety of apatinib in metastatic alveolar soft part sarcoma: A case series of six patients in one institution. Cancer Manag. Res. 2019, 11, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Olivier, M.; Bergemann, T.L.; Hainaut, P. Sarcomas in TP53 germline mutation carriers: A review of the IARC TP53 database. Cancer 2012, 118, 1387–1396. [Google Scholar] [CrossRef]
- Xu, D.; Yin, S.; Shu, Y. NF2: An underestimated player in cancer metabolic reprogramming and tumor immunity. NPJ Precis. Oncol. 2024, 8, 133. [Google Scholar] [CrossRef]
- Irie, T.; Iida, Y.; Hamada, Y.; Matsushima, J.; Iizuka, M.; Takakura, S. Primary carcinosarcoma of the uterine cervix with somatic mutations of the ATM and NF2 genes: A case report. Int. Cancer Conf. J. 2025, 14, 302–310. [Google Scholar] [CrossRef]
- Azizian, A.; Rühlmann, F.; Krause, T.; Bernhardt, M.; Jo, P.; König, A.; Kleiß, M.; Leha, A.; Ghadimi, M.; Gaedcke, J. CA19-9 for detecting recurrence of pancreatic cancer. Sci. Rep. 2020, 10, 1332. [Google Scholar] [CrossRef]
- Wlodarczyk, B.; Durko, L.; Wlodarczyk, P.; Talar-Wojnarowska, R.; Malecka-Wojciesko, E. CA 19-9 but Not IGF-1/IGFBP-2 Is a Useful Biomarker for Pancreatic Ductal Adenocarcinoma (PDAC) and Chronic Pancreatitis (CP) Differentiation. J. Clin. Med. 2023, 12, 4050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, B.; Chen, F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, T.; Miyamoto, A.; Maeda, S.; Tsujie, M.; Hama, N.; Yamamoto, K.; Miyake, M.; Haraguchi, N.; Nishikawa, K.; Hirao, M.; et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology 2016, 16, 434–440. [Google Scholar] [CrossRef]
- Takagi, C.; Kikuchi, Y.; Shirakawa, H.; Hoshimoto, S.; Tomikawa, M.; Ozawa, I.; Hishinuma, S.; Ogata, Y. Predictive Factors for Elevated Postoperative Carbohydrate Antigen 19-9 Levels in Patients With Resected Pancreatic Cancer. Anticancer Res. 2019, 39, 3177–3183. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, X.H.; Zhang, Y.; Jing, W.; Zheng, L.Q.; Liu, Y.P.; Qu, X.J. Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: A pilot study. World J. Surg. Oncol. 2014, 12, 171. [Google Scholar] [CrossRef]
- Bergquist, J.R.; Puig, C.A.; Shubert, C.R.; Groeschl, R.T.; Habermann, E.B.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Farnell, M.B.; Truty, M.J. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J. Am. Coll. Surg. 2016, 223, 52–65. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.I.; Jeong, M.; Seo, J.H.; Kim, I.K.; Cheung, D.Y.; Kim, T.J.; Kang, C.S. Pancreatic adenocarcinosarcoma of monoclonal origin: A case report. World J. Gastroenterol. 2014, 20, 12682–12686. [Google Scholar] [CrossRef]
- Lee, M.; Cho, Y.J.; Jung, H.S.; Yun, W.G.; Han, Y.; Kwon, W.; Jang, J.Y. Collective review of pancreatic carcinosarcoma, a very rare pancreatic malignancy. Ann. Hepatobiliary Pancreat. Surg. 2023, 27, 141–150. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Wu, L.M.; Hu, J.N.; Hua, J.; Liu, M.J.; Chen, J.; Xu, J.R. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: A meta-analysis using a hierarchical regression model. J. Gastroenterol. Hepatol. 2012, 27, 1027–1035. [Google Scholar] [CrossRef]
- Wang, L.; Dong, P.; Wang, W.G.; Tian, B.L. Positron emission tomography modalities prevent futile radical resection of pancreatic cancer: A meta-analysis. Int. J. Surg. 2017, 46, 119–125. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Y.; Wang, J.; Yang, X.; Lu, L. The Clinical Diagnostic Value of F-FDG PET/CT Combined with MRI in Pancreatic Cancer. Contrast Media Mol. Imaging 2022, 2022, 1479416. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, G.; Zuo, C.; Jia, N.; Wang, H. 18F- FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer 2017, 17, 695. [Google Scholar] [CrossRef] [PubMed]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Citterio, C.; Anselmi, E.; Cavanna, L.; Vecchia, S. FOLFIRINOX or Gemcitabine Plus Nab-paclitaxel as First Line Treatment in Pancreatic Cancer: A Real-World Comparison. Cancer Diagn. Progn. 2024, 4, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Gurruchaga, S.I.; Gómez-Mateo, M.C.; Ortega Izquierdo, M.E.; Martínez-Trufero, J. Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data. Cancers 2024, 16, 267. [Google Scholar] [CrossRef]
- Imaoka, H.; Ikeda, M.; Maehara, K.; Umemoto, K.; Ozaka, M.; Kobayashi, S.; Terashima, T.; Inoue, H.; Sakaguchi, C.; Tsuji, K.; et al. Clinical outcomes of chemotherapy in patients with undifferentiated carcinoma of the pancreas: A retrospective multicenter cohort study. BMC Cancer 2020, 20, 946. [Google Scholar] [CrossRef]
- Mayrhofer, K. Pembrolizumab in MSI-high Pancreatic Sarcomatoid Carcinoma. Ann. Hematol. Oncol. 2021, 8, 1327. [Google Scholar] [CrossRef]
- Quinn, P.L.; Ohioma, D.; Jones, A.M.K.; Ahlawat, S.K.; Chokshi, R.J. Treatment of Rare and Aggressive Pancreatic Carcinosarcoma. ACG Case Rep. 2020, 7, e00435. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.; Ibrahim, M.A.; Mujeeb, Q.; Khir, I. Pancreatic carcinosarcoma: A rare type of pancreatic neoplasia with long-term survival. BMJ Case Rep. 2025, 18, e262648. [Google Scholar] [CrossRef] [PubMed]
- Gelos, M.; Behringer, D.; Philippou, S.; Mann, B. Pancreatic carcinosarcoma. Case report of multimodal therapy and review of the literature. J. Pancreas 2008, 9, 50–55. [Google Scholar]
- Zhu, W.Y.; Liu, T.G.; Zhu, H. Long-term recurrence-free survival in a patient with pancreatic carcinosarcoma: A case report with a literature review. Med. Oncol. 2012, 29, 140–143. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, K.; Huang, R.; Zhou, X.; Jiang, L. Pancreatic carcinosarcoma with rare long-term survival: Case report and review of the literature. Medicine 2017, 96, e5966. [Google Scholar] [CrossRef]
- Salibay, C.J.; Rewerska, J.; Gupta, S.; Ree, N. Primary Carcinosarcoma of the Pancreas With CD10-Positive Sarcoma Component. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617740906. [Google Scholar] [CrossRef]
- Still, S.A.; Becerra, C.R.; Clement-Kruzel, S.E.; Cavaness, K.M. Locally advanced carcinosarcoma of the pancreas. Bayl. Univ. Med. Cent. Proc. 2018, 31, 210–212. [Google Scholar] [CrossRef]
- Lalonde, C.S.; Wang, L.; Quigley, B.; Patel, P.; Maithel, S.K.; El-Rayes, B.F.; Akce, M. Neoadjuvant treatment of pancreatic carcinosarcoma: A case report and review of literature. Chin. Clin. Oncol. 2022, 11, 8. [Google Scholar] [CrossRef]

| Report | Relevant Case Details | Relevant Pathology Findings | Outcome |
|---|---|---|---|
| Gelos et al., 2008 [54] | Resected. Adjuvant gemcitabine, 6 cycles. | Moderately differentiated adenocarcinoma and poorly differentiated solid tissue. IHC epithelial: strongly positive CK7 and pan-CK, moderate positive CK20. IHC sarcomatoid: strongly positive vimentin; negative for all CK antibodies. | Recurrence 11 months post-operatively, relaparotomy, death 2 days later. |
| Kim et al., 2011 [15] | Resected. Adjuvant gemcitabine, progressed after 3 cycles. | Anaplastic carcinoma and sarcomatoid component in solid lesion. Additional cystic lesion. IHC anaplastic: strongly positive EMA, pan-CK, CK7, CK8/18, and monoclonal/polyclonal CEA; 90% p53 positive. IHC sarcomatoid: strong positive vimentin; negative pan-CK, CK7, CK8/18, alpha-1-antitrypsin, alpha-1-antichymotrypsin, CD34, CD56, CD68, CD117, desmin, estrogen receptor, progesterone receptor, human melanoma black 45 (HMB45), lysozyme, myogenin, S100 and SMA; 80% p53 positive. | Death 4 months post-resection. |
| Zhu et al., 2011 [55] | Resected. Adjuvant gemcitabine–doxorubicin–cisplatin, 5 cycles. | Ductal carcinoma and sarcoma. IHC carcinoma: strongly positive CK18, EMA. IHC sarcomatous: strongly positive SMA; negative CK18, EMA, S-100. | No recurrence 20 months post-op. |
| Jia et al., 2017 [56] | Resected. Adjuvant gemcitabine–raltitrexed, 8 cycles. | Moderately differentiated adenocarcinoma and heterologous mesenchymal osteosarcoma. IHC positive CK7, vimentin. | No recurrence 31 months post-op. |
| Salibay et al., 2017 [57] | Surgical biopsy, no resection. First-line gemcitabine–docetaxel, no response. Second-line doxorubicin–ifosfamide, progressive disease. Plan for palliative RT. | High-grade spindle cell sarcoma and moderately differentiated adenocarcinoma. IHC adenocarcinoma: positive CKAE1/3, CK7, villin, focal CDX2; negative CD10, CK20, desmin, ER, myogenin, TTF1; Ki67 50%. IHC sarcoma: strongly positive CD10; focally positive desmin, SMA; negative CKAE1/3, ER, myogenin, villin, DOG1, CD117; Ki67 90%. | Death 10 months post- diagnosis. |
| Still et al., 2018 [58] | Initial biopsy showed adenocarcinoma. Neoadjuvant FOLFIRINOX, 3 months. Resected. Adjuvant gemcitabine–paclitaxel, metastatic disease identified 1 month post-op. | 60% sarcoma with focal chondrosarcoma and myogenic differentiation, 40% moderately differentiated adenocarcinoma. IHC: patchy positive CK, desmin; nonspecific staining myoglobin; negative myogenin, myo-D1. | Death 13 months post-diagnosis. |
| Li et al., 2020 [14] | Resected. Adjuvant gemcitabine–paclitaxel. | Disparate carcinomatous and sarcomatous components. IHC carcinoma: positive EMA and CK7/8; negative vimentin IHC sarcoma: positive vimentin; negative EMA and CK7/8. Sarcoma components had higher Ki67 than carcinoma components. | Recurred 3 months post-op. Death 11 months post-op. |
| Resected. Adjuvant mFOLFIRINOX. | Recurred 13 months post-op. Death 19 months post-op. | ||
| Resected. Adjuvant gemcitabine. | Recurred 10 months post-op. Death 17 months post-op. | ||
| Quinn et al., 2020 [52] | Initial biopsy, inconclusive. Resected. Adjuvant gemcitabine–paclitaxel, 9 cycles total, liver metastases discovered 2 months post-op. Second-line FOLFOX, completed 11 cycles. | Glandular areas and stroma containing atypical spindle cells; labeled mixed mucinous adenocarcinoma and heterologous anaplastic sarcomatous components. IHC epithelial: positive SMA. IHC sarcomatous: positive CD31. | Discontinuation of therapy 16 months post-op and transition to comfort care. |
| Lalonde et al., 2022 [59] | Identified as borderline resectable. Neoadjuvant FOLFIRINOX, 4 months. 2 months concurrent chemoradiotherapy with capecitabine. Resection. | Invasive poorly differentiated carcinosarcoma of the pancreas with both glandular and sarcomatoid components | No recurrence 15 months post-op. |
| Lee et al., 2023 [38] | Initial biopsy. 11 cycles neoadjuvant FOLFIRINOX-pembrolizumab (trial); SBRT 5 fractions; robotic resection. Adjuvant FOLFIRINOX. | Biopsy: adenocarcinoma with irregular glands, poorly differentiated sarcoma. IHC epithelial: strongly positive CK. IHC sarcoma: strongly positive vimentin. | No recurrence 7 months post-op. |
| Gilani et al., 2025 [53] | BRCA1 mutation. Resected. Planned adjuvant FOLFIRINOX. Liver metastases found on imaging 1 month into therapy. | Poorly differentiated carcinosarcoma, grade 2. Specific pathological details not provided. | Long-term complete response in liver with 12 cycles FOLFIRINOX. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, T.; Browne, C.; Black, M.; Marginean, C.; Tsvetkova, E. Extended Survival with Pancreatic Carcinosarcoma: A Case Report and Literature Review. Curr. Oncol. 2025, 32, 470. https://doi.org/10.3390/curroncol32080470
Xiao T, Browne C, Black M, Marginean C, Tsvetkova E. Extended Survival with Pancreatic Carcinosarcoma: A Case Report and Literature Review. Current Oncology. 2025; 32(8):470. https://doi.org/10.3390/curroncol32080470
Chicago/Turabian StyleXiao, Tian, Claire Browne, Morgan Black, Celia Marginean, and Elena Tsvetkova. 2025. "Extended Survival with Pancreatic Carcinosarcoma: A Case Report and Literature Review" Current Oncology 32, no. 8: 470. https://doi.org/10.3390/curroncol32080470
APA StyleXiao, T., Browne, C., Black, M., Marginean, C., & Tsvetkova, E. (2025). Extended Survival with Pancreatic Carcinosarcoma: A Case Report and Literature Review. Current Oncology, 32(8), 470. https://doi.org/10.3390/curroncol32080470






