A Population-Based Study of Sex Differences in Cardiovascular Disease Mortality Among Adults with Ocular Cancer in the United States, 2000–2021
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
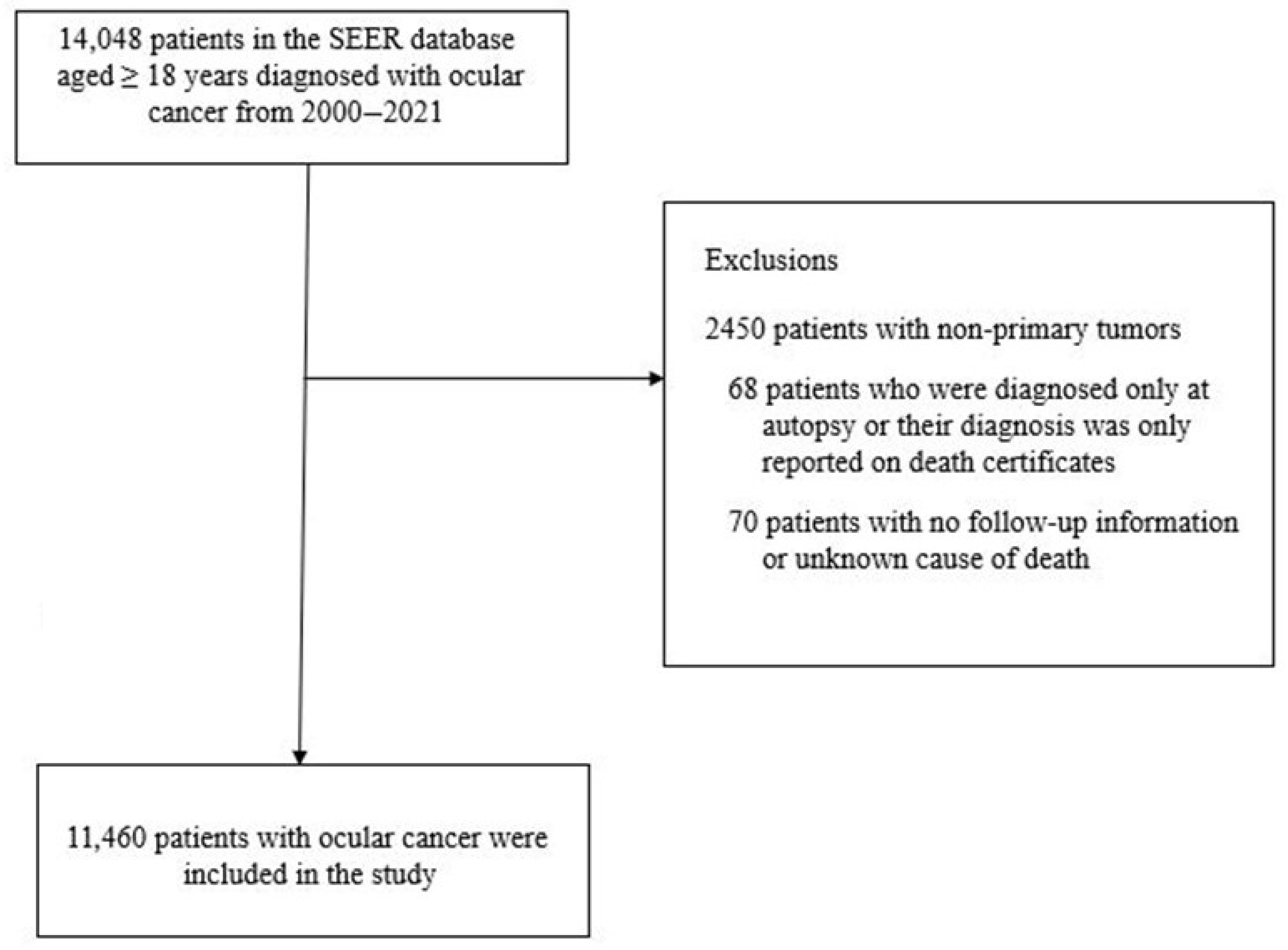
2.1. Study Population
2.2. Definition of Study Variables
2.3. Statistical Analysis
2.4. Verification Analysis
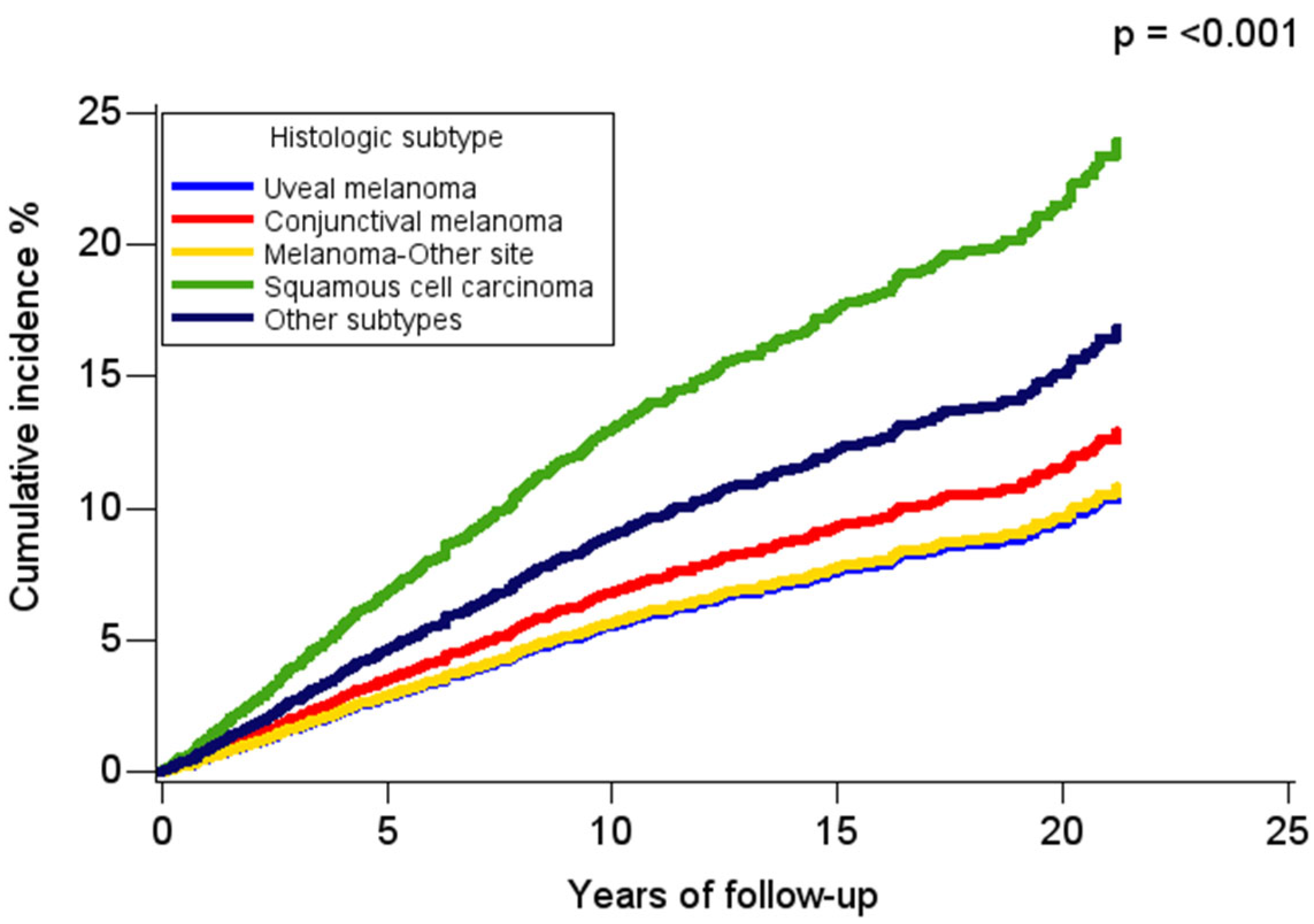
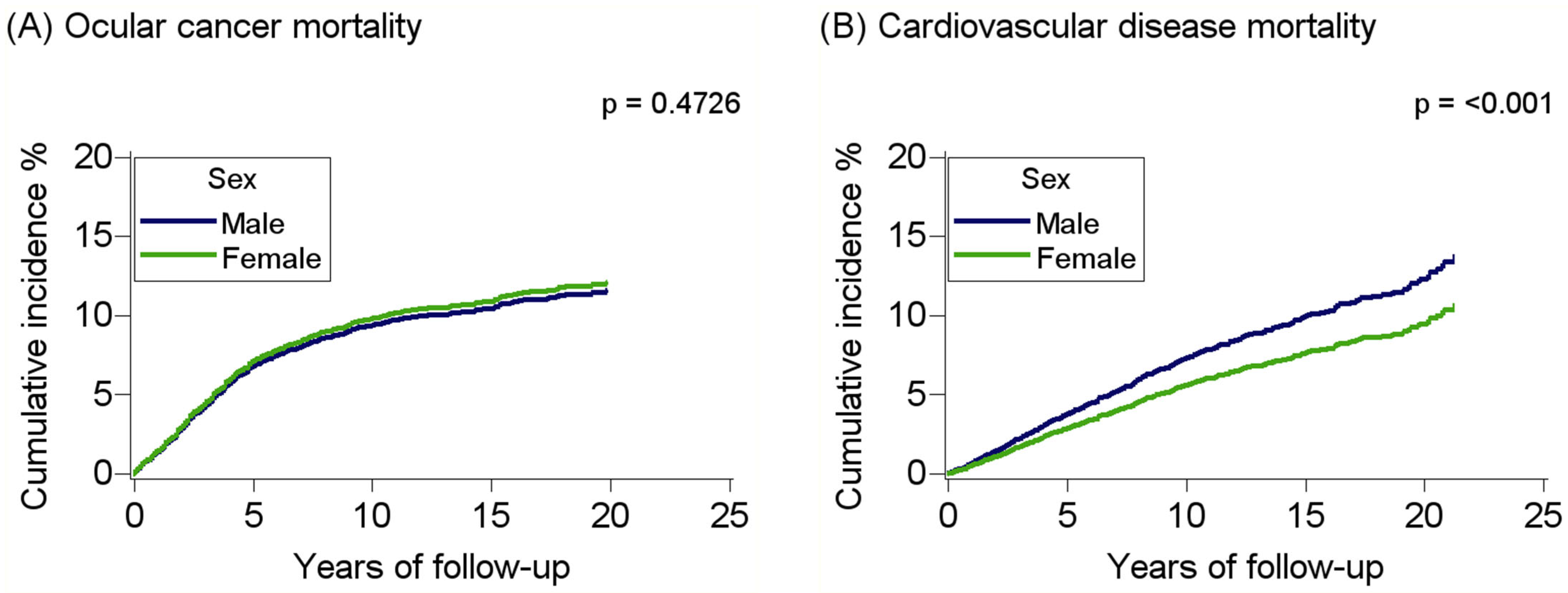
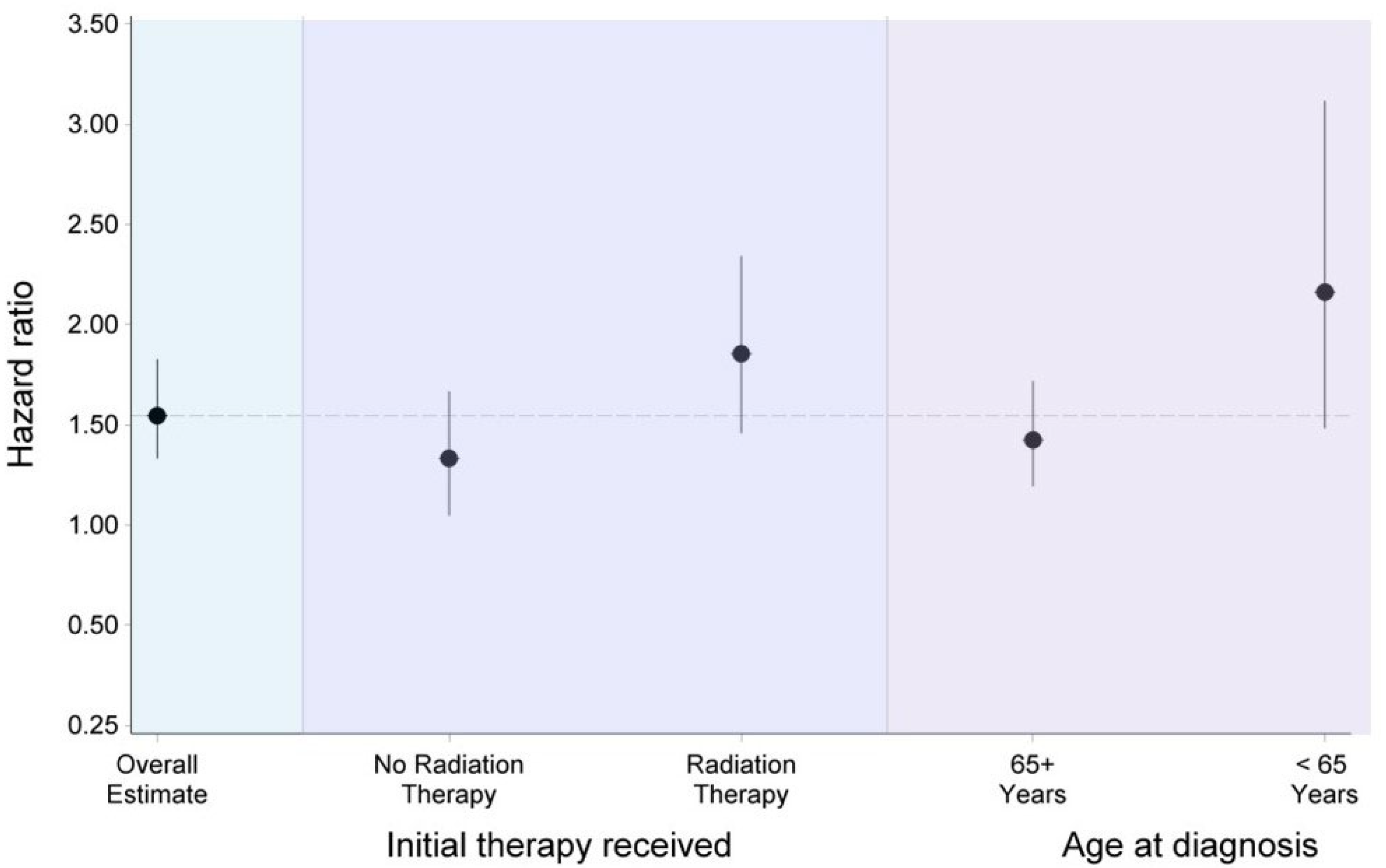
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Absolute standardized difference |
| CI | Confidence intervals |
| CVD | Cardiovascular disease |
| HR | Hazard ratio |
| ICD | International classification of diseases |
| OC | Ocular cancer |
| SEER | Surveillance, epidemiology, and end results |
References
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Disease burden, risk factors, and temporal trends of eye cancer: A global analysis of cancer registries. Clin. Exp. Ophthalmol. 2024, 52, 440–451. [Google Scholar] [CrossRef]
- Maheshwari, A.; Finger, P.T. Cancers of the eye. Cancer Metastasis Rev. 2018, 37, 677–690. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Surveillance Research Program; National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 6 January 2025).
- The Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 2001, 119, 670–676. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program (1975–2021). Available online: https://seer.cancer.gov/ (accessed on 6 January 2025).
- Zloto, O.; Pe’er, J.; Frenkel, S. Gender Differences in Clinical Presentation and Prognosis of Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 652–656. [Google Scholar] [CrossRef]
- Park, S.J.; Oh, C.-M.; Yeon, B.; Cho, H.; Park, K.H. Sex Disparity in Survival of Patients with Uveal Melanoma: Better Survival Rates in Women Than in Men in South Korea. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1909–1915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romanowska-Dixon, B.; Dębicka-Kumela, M.; Śmigielski, J.; Nowak, M.S. Sex Differences in the Treatment of Uveal Melanoma in a Group of 1336 Patients. J. Pers. Med. 2023, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.J.; Malik, K.; Considine, S.P.; Acaba-Berrocal, L.A.; Selzer, E.B.; Newman, J.H.; Shields, J.A.; Shields, C.L. Uveal Metastasis Based on Patient Sex in 2214 Tumors of 1111 Patients. A Comparison of Female Versus Male Clinical Features and Outcomes. Asia-Pac. J. Ophthalmol. 2019, 8, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, B.; Ruder, E.; Lin, S.W.; Abnet, C.; Hollenbeck, A.; Mbulaiteye, S. Incidence of squamous-cell carcinoma of the conjunctiva and other eye cancers in the NIH-AARP Diet and Health Study. Ecancermedicalscience 2012, 6, 254. [Google Scholar] [CrossRef]
- Yu, G.-P.; Hu, D.-N.; McCormick, S.; Finger, P.T. Conjunctival melanoma: Is it increasing in the United States? Am. J. Ophthalmol. 2003, 135, 800–806. [Google Scholar] [CrossRef]
- Stålhammar, G. Sex-based differences in early and late uveal melanoma-related mortality. Cancer Med. 2023, 12, 6700–6710. [Google Scholar] [CrossRef]
- Stålhammar, G.; See, T.R.; Filì, M.; Seregard, S. No Gender Differences in Long-Term Survival after Brachytherapy of 1,541 Patients with Uveal Melanoma. Ocul. Oncol. Pathol. 2019, 5, 432–439. [Google Scholar] [CrossRef]
- Ituarte, B.E.; Pitchyaiah, P.; Taylor, M.A.; Thomas, S.; Sharma, D.; Voss, V.B. Male sex as an independent predictor of poor disease-specific survival in conjunctival melanoma. Int. J. Dermatol. 2024, 63, e450–e451. [Google Scholar] [CrossRef]
- He, L.F.; Mou, P.; Wei, R.L. Epidemiology and survival outcomes of patients with orbital region non-cutaneous squamous cell carcinoma: A population-based analysis. Front. Oncol. 2023, 13, 1152337. [Google Scholar] [CrossRef]
- Guan, T.; Jiang, Y.; Tu, P.; Ye, B.; Zeng, L.; Luo, Z.; Chi, K.; Liang, H.; Yang, Y.; Huang, J.; et al. Risk classification for non-cancer death in middle-aged cancer patients. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Ng, H.S.; Meng, R.; Marin, T.S.; Damarell, R.A.; Buckley, E.; Selvanayagam, J.B.; Koczwara, B. Cardiovascular mortality in people with cancer compared to the general population: A systematic review and meta-analysis. Cancer Med. 2024, 13, e70057. [Google Scholar] [CrossRef]
- Appiah, D.; Chaudhury, H.; Chaudhury, T.; Iweh, M.; Shabaneh, O.; De La Cruz, N. The Risk of Cardiovascular Disease Risk Among Adults with Vision Impairment from Low-, Middle- and High-Income Countries. Ophthalmic Epidemiol. 2024, 32, 163–170. [Google Scholar] [CrossRef]
- De La Cruz, N.; Shabaneh, O.; Appiah, D. The Association of Ideal Cardiovascular Health and Ocular Diseases Among US Adults. Am. J. Med. 2021, 134, 252–259.e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, Y.; Li, Y.; Liu, Y.; Chen, N.; Cui, J. Association Between Cardiovascular Health and Retinopathy in US Adults: From NHANES 2005–2008. Am. J. Ophthalmol. 2024, 266, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tan, Z.; Sawut, A.; Li, L.; Chen, C. Association between Life’s Essential 8 and cataract among US adults. Sci. Rep. 2024, 14, 13101. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Law, D.T.W.; Kan, A.H.S.; Cruz, F.F.P.; Jhanji, V.; Yuen, H.K.L.; Ni, M.Y. The Epidemiology of Eye Cancer, Eyelid Cancer, and Ophthalmic Lymphoma in a Chinese Population in Hong Kong: A Population-Based Registry Study 2005–2018. Investig. Ophthalmol. Vis. Sci. 2025, 66, 15. [Google Scholar] [CrossRef]
- Friedman, S.; Negoita, S. History of the Surveillance, Epidemiology, and End Results (SEER) Program. J. Natl. Cancer Inst. Monogr. 2024, 2024, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Mahendraraj, K.; Shrestha, S.; Lau, C.S.M.; Chamberlain, R.S. Ocular melanoma-when you have seen one, you have not seen them all: A clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973–2012). Clin. Ophthalmol. 2017, 11, 153–160. [Google Scholar] [CrossRef]
- Appiah, D.; Mai, M.; Parmar, K. A Prospective Population-Based Study of Cardiovascular Disease Mortality following Treatment for Breast Cancer among Men in the United States, 2000–2019. Curr. Oncol. 2022, 30, 284–297. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Makram, O.M.; Okwuosa, T.; Addison, D.; Cortes, J.; Dent, S.; Bevel, M.; Ganatra, S.; Al-Kindi, S.; Hedrick, C.C.; Weintraub, N.L.; et al. Cardiovascular Diseases Increase Cancer Mortality in Adults: NHANES-Continuous Study. J. Am. Heart Assoc. 2024, 13, e035500. [Google Scholar] [CrossRef]
- Lima-Fontes, M.; Barata, P.; Falcão, M.; Carneiro, Â. Ocular findings in metabolic syndrome: A review. Porto Biomed. J. 2020, 5, e104. [Google Scholar] [CrossRef]
- Wong, T.Y.; Duncan, B.B.; Golden, S.H.; Klein, R.; Couper, D.J.; Klein, B.E.; Hubbard, L.D.; Sharrett, A.R.; Schmidt, M.I. Associations between the metabolic syndrome and retinal microvascular signs: The Atherosclerosis Risk In Communities study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2949–2954. [Google Scholar] [CrossRef]
- Dreyfuss, J.L.; Giordano, R.J.; Regatieri, C.V. Ocular Angiogenesis. J. Ophthalmol. 2015, 2015, 892043. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Obesity and familial obesity and risk of cancer. Eur. J. Cancer Prev. 2011, 20, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef]
- Sabazade, S.; Opalko, A.; Herrspiegel, C.; Gill, V.T.; Plastino, F.; André, H.; Stålhammar, G. Obesity paradox in uveal melanoma: High body mass index is associated with low metastatic risk. Br. J. Ophthalmol. 2024, 108, 578–587. [Google Scholar] [CrossRef]
- Gu, L.; Ma, G.; Li, C.; Lin, J.; Zhao, G. New insights into the prognosis of intraocular malignancy: Interventions for association mechanisms between cancer and diabetes. Front. Oncol. 2022, 12, 958170. [Google Scholar] [CrossRef]
- Tura, A.; Thieme, C.; Brosig, A.; Merz, H.; Ranjbar, M.; Vardanyan, S.; Zuo, H.; Maassen, T.; Kakkassery, V.; Grisanti, S. Lower Levels of Adiponectin and Its Receptor Adipor1 in the Uveal Melanomas with Monosomy-3. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef]
- Liau, S.; Wang, J.Z.; Zagarella, E.; Paulus, P.; Dang, N.; Rawling, T.; Murray, M.; Zhou, F. An update on inflammation in uveal melanoma. Biochimie 2023, 212, 114–122. [Google Scholar] [CrossRef]
- Madani, A.; Omar, N.E.; Mustafa, G.; Petkar, M.; Mohamed, S.; Al Kuwari, M.; Karim, S.A.; Mohsen, R. Cardiac Metastases from Choroidal Melanoma. Clin. Case Rep. 2022, 10, e6080. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, T.; Kivelä, T. Cardiac metastasis from uveal melanoma. Arch. Ophthalmol. 2001, 119, 139–140. [Google Scholar]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Chiu, C.-Y.; Johnson, D.L.; Miller, P.W.; Glazer, E.S.; Wu, Z.; Wilson, M.W. The Sex Differences in Uveal Melanoma: Potential Roles of EIF1AX, Immune Response and Redox Regulation. Curr. Oncol. 2021, 28, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Kapil, V.; Velmurugan, S.; Khambata, R.S.; Siddique, U.; Khan, S.; Van Eijl, S.; Gee, L.C.; Bansal, J.; Pitrola, K.; et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Investig. 2017, 127, 169–182. [Google Scholar] [CrossRef]
- Ferraro, A.; Laborante, M.; Cutrupi, F.; Salerno, A.; Coassin, M.; Di Zazzo, A. Immunological Impact of Sex Hormones at Ocular Surface: A Narrative Review. Semin. Ophthalmol. 2025, 1–10. [Google Scholar] [CrossRef]
- Miller, M.; Schoenfield, L.; Abdel-Rahman, M.; Cebulla, C.M. Is Uveal Melanoma a Hormonally Sensitive Cancer? A Review of the Impact of Sex Hormones and Pregnancy on Uveal Melanoma. Ocul. Oncol. Pathol. 2021, 7, 239–250. [Google Scholar] [CrossRef]
- Scarabin, P.Y. Endogenous sex hormones and cardiovascular disease in postmenopausal women: New but conflicting data. Ann. Transl. Med. 2018, 6, 448. [Google Scholar] [CrossRef]
- Henderson, M.; Tuteja, S.Y.; Lockington, D. Literature Review of Sex Hormones and Cataract Development, with Modern Implications. Clin. Exp. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Dedania, V.S.; Zacks, D.N.; Pan, W.; VanderBeek, B.L. Testosterone supplementation and retinal vascular disease. Retina 2018, 38, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Byun, S.J.; Woo, S.J.; Park, K.H.; Park, S.J. A 12-year nationwide cohort study on the association between central retinal artery occlusion and cancer. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1893–1900. [Google Scholar] [CrossRef]
- Narowska, G.; Gandhi, S.; Tzeng, A.; Hamad, E.A. Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance. J. Cardiovasc. Dev. Dis. 2023, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, L.; Ye, Z.; Zhou, Y.; Wang, F.; Zhang, Y. Radiotherapy has a survival advantage over surgery in patients with choroidal melanoma: A retrospective cohort study of 6,871 patients. Front. Surg. 2025, 12, 1577775. [Google Scholar] [CrossRef] [PubMed]
- Santaballa Bertrán, A.; Marcos Rodríguez, J.A.; Cardeña-Gutiérrez, A.; Martinez-Callejo, V.; Higuera, O.; Bernardez, B.; Moreno-Martínez, M.E.; Majem, M. Sex-related differences in the efficacy and toxicity of cancer treatments. Clin. Transl. Oncol. 2025. [Google Scholar] [CrossRef]
- Little, M.P.; Azizova, T.V.; Hamada, N. Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: A review of the epidemiology. Int. J. Radiat. Biol. 2021, 97, 782–803. [Google Scholar] [CrossRef]
- Zemba, M.; Dumitrescu, O.M.; Gheorghe, A.G.; Radu, M.; Ionescu, M.A.; Vatafu, A.; Dinu, V. Ocular Complications of Radiotherapy in Uveal Melanoma. Cancers 2023, 15, 333. [Google Scholar] [CrossRef]
- Nemet, A.Y.; Vinker, S.; Levartovsky, S.; Kaiserman, I. Is cataract associated with cardiovascular morbidity? Eye 2010, 24, 1352–1358. [Google Scholar] [CrossRef]
- Geiger, M.D.; Lynch, A.M.; Palestine, A.G.; Grove, N.C.; Christopher, K.L.; Davidson, R.S.; Taravella, M.J.; Mandava, N.; Patnaik, J.L. Are there sex-based disparities in cataract surgery? Int. J. Ophthalmol. 2024, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Martel, A.; Matet, A.; Loria, O.; Kodjikian, L.; Nguyen, A.M.; Rosier, L.; Herault, J.; Nahon-Estève, S.; Mathis, T. Non-Cancer Effects following Ionizing Irradiation Involving the Eye and Orbit. Cancers 2022, 14, 1194. [Google Scholar] [CrossRef]
- Durkin, S.R.; Roos, D.; Higgs, B.; Casson, R.J.; Selva, D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol. Scand. 2007, 85, 240–250. [Google Scholar] [CrossRef]
- Nuzzi, R.; Trossarello, M.; Bartoncini, S.; Marolo, P.; Franco, P.; Mantovani, C.; Ricardi, U. Ocular Complications After Radiation Therapy: An Observational Study. Clin. Ophthalmol. 2020, 14, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Boldt, H.C.; Melia, B.M.; Liu, J.C.; Reynolds, S.M. I-125 brachytherapy for choroidal melanoma photographic and angiographic abnormalities: The Collaborative Ocular Melanoma Study: COMS Report No. 30. Ophthalmology 2009, 116, 106–115.e1. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xing, Y.; Cormier, J.N.; Chang, G.J. The validity of cause of death coding within the Surveillance, Epidemiology, and End Results (SEER) Registry. J. Clin. Oncol. 2009, 27, 6544. [Google Scholar] [CrossRef]
| Sex | p Value | ||
|---|---|---|---|
| Characteristics | Male (N = 6307) | Female (N = 5153) | |
| Age, years, % | 0.012 | ||
| 18–44 | 794 (12.6) | 705 (13.7) | |
| 45–64 | 2755 (43.7) | 2114 (41.0) | |
| ≥65 | 2758 (43.7) | 2334 (45.3) | |
| Year of diagnosis, % | 0.610 | ||
| 2000–2004 | 1271 (20.2) | 1020 (19.8) | |
| 2005–2009 | 1298 (20.6) | 1103 (21.4) | |
| 2010–2014 | 1549 (24.6) | 1227 (23.8) | |
| 2015–2021 | 2189 (34.7) | 1803 (35.0) | |
| Race and ethnicity, % | 0.406 | ||
| Non-Hispanic White | 5383 (85.3) | 4417 (85.7) | |
| Non-Hispanic Black | 107 (1.7) | 93 (1.8) | |
| Non-Hispanic Asian or Pacific Islander | 163 (2.6) | 117 (2.3) | |
| Hispanic | 518 (8.2) | 436 (8.5) | |
| Other 1 | 136 (2.2) | 90 (1.7) | |
| Region, % | 0.049 | ||
| Midwest | 382 (6.1) | 322 (6.2) | |
| Northeast | 902 (14.3) | 829 (16.1) | |
| South | 1437 (22.8) | 1166 (22.6) | |
| West | 3586 (56.9) | 2836 (55.0) | |
| Marital status, married, % | 4025 (63.8) | 2602 (50.5) | <0.001 |
| Median household income, % | 0.507 | ||
| <$75,000 | 3470 (55.0) | 2867 (55.6) | |
| ≥$75,000 | 2837 (45.0) | 2286 (44.4) | |
| Location, rural, % | 899 (14.3) | 706 (13.7) | 0.399 |
| Primary site of tumor, % | <0.001 | ||
| Choroid | 3811 (60.4) | 3431 (66.6) | |
| Ciliary body | 513 (8.1) | 531 (10.3) | |
| Conjunctiva | 1019 (16.2) | 520 (10.1) | |
| Orbit | 305 (4.8) | 190 (3.7) | |
| Other sites/unspecified | 659 (10.4) | 481 (9.3) | |
| Histology, % | <0.001 | ||
| Uveal melanoma | 4308 (68.3) | 3949 (76.6) | |
| Conjunctival melanoma | 286 (4.5) | 263 (5.1) | |
| Melanoma, other sites | 313 (5.0) | 280 (5.4) | |
| Conjunctival squamous cell carcinoma | 683 (10.8) | 222 (4.3) | |
| Other histologic subtypes | 717 (11.4) | 439 (8.5) | |
| Tumor stage, % | 0.686 | ||
| Localized | 4101 (65.0) | 3373 (65.5) | |
| Regional | 442 (7.0) | 332 (6.4) | |
| Distant | 177 (2.8) | 147 (2.9) | |
| Unknown/unstaged | 1586 (25.1) | 1301 (25.2) | |
| Laterality, % | 0.243 1 | ||
| Unilateral | 6252 (99.1) | 5097 (98.9) | |
| Bilateral | 55 (0.9) | 56 (1.1) | |
| Diagnostic confirmation, % | <0.001 | ||
| Clinical diagnosis | 335 (5.3) | 364 (7.1) | |
| Direct visualization | 736 (11.7) | 706 (13.7) | |
| Other methods | 417 (6.6) | 391 (7.6) | |
| Positive histology | 3839 (60.9) | 2730 (53.0) | |
| Radiography | 886 (14.0) | 854 (16.6) | |
| Unknown | 94 (1.5) | 108 (2.1) | |
| Time from diagnosis to treatment, % | <0.001 | ||
| <1 month | 3280 (52.0) | 2478 (48.1) | |
| 1–2 months | 1327 (21.0) | 1276 (24.8) | |
| ≥3 months | 250 (4.0) | 216 (4.2) | |
| Unknown | 1450 (23.0) | 1183 (23.0) | |
| Initial treatment, % 2 | |||
| Surgery | 2873 (46.0) | 1987 (38.8) | <0.001 |
| Chemotherapy | 329 (5.2) | 208 (4.0) | 0.003 |
| Radiation | 3403 (54.2) | 3162 (61.7) | <0.001 |
| Age-Adjusted | Multivariable Adjusted 2 | Propensity Score Matched | ||
|---|---|---|---|---|
| Mortality outcomes | N | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| All-cause | 4561 | 1.14 (1.07–1.21) | 1.20 (1.13–1.27) | 1.17 (1.09–1.25) |
| Ocular cancer | 946 | 0.99 (0.87–1.12) | 1.02 (0.89–1.16) | 1.01 (0.87–1.16) |
| Cardiovascular disease | 694 | 1.41 (1.21–1.64) | 1.54 (1.31–1.81) | 1.52 (1.27–1.82) |
| Sex | ASD | p Value | ||
|---|---|---|---|---|
| Characteristics | Male (N = 4490) | Female (N = 4490) | ||
| Age, years, % | 0.732 | |||
| 18–44 | 670 (14.9) | 678 (15.1) | ||
| 45–64 | 1985 (44.2) | 1948 (43.4) | 0.009 | |
| ≥65 | 1835 (40.9) | 1864 (41.5) | 0.015 | |
| Year of diagnosis, % | 0.741 | |||
| 2000–2004 | 888 (19.8) | 851 (19.0) | ||
| 2005–2009 | 941 (21.0) | 967 (21.5) | 0.006 | |
| 2010–2014 | 1083 (24.1) | 1077 (24.0) | 0.002 | |
| 2015–2021 | 1578 (35.1) | 1595 (35.5) | 0.011 | |
| Race and ethnicity, % | 0.013 | 0.557 | ||
| White | 3838 (85.5) | 3812 (84.9) | ||
| Non-white | 652 (14.5) | 678 (15.1) | ||
| Region, % | 0.301 | |||
| Midwest | 258 (5.7) | 273 (6.1) | 0.003 | |
| Northeast | 689 (15.3) | 747 (16.6) | 0.023 | |
| South | 998 (22.2) | 991 (22.1) | 0.002 | |
| West | 2545 (56.7) | 2479 (55.2) | ||
| Marital status, married, % | 2625 (58.5) | 2591 (57.7) | 0.016 | 0.467 |
| Median household income, % | 0.013 | 0.687 | ||
| <USD 75,000 | 2462 (54.8) | 2481 (55.3) | ||
| ≥USD 75,000 | 2028 (45.2) | 2009 (44.7) | ||
| Location, rural, % | 623 (13.9) | 605 (13.5) | 0.583 | |
| Histology, % | 0.894 | |||
| Uveal melanoma | 3425 (76.3) | 3428 (76.3) | 0.003 | |
| Conjunctival melanoma | 226 (5.0) | 222 (4.9) | 0.003 | |
| Melanoma, other sites | 229 (5.1) | 233 (5.2) | 0.020 | |
| Conjunctival squamous cell carcinoma | 386 (8.6) | 367 (8.2) | 0.018 | |
| Other histologic subtypes | 224 (5.0) | 240 (5.3) | ||
| Tumor stage, % | 0.870 | |||
| Localized | 2983 (66.4) | 2982 (66.4) | 0.003 | |
| Regional | 276 (6.1) | 278 (6.2) | 0.002 | |
| Distant | 108 (2.4) | 120 (2.7) | 0.003 | |
| Unknown/unstaged | 1123 (25.0) | 1110 (24.7) | ||
| Laterality, % | 0.000 | 0.529 | ||
| Unilateral | 4447 (99.0) | 4441 (98.9) | ||
| Bilateral | 43 (1.0) | 49 (1.1) | ||
| Diagnostic confirmation, % | 0.335 | |||
| Clinical diagnosis | 308 (6.9) | 350 (7.8) | 0.028 | |
| Direct visualization | 617 (13.7) | 627 (14.0) | 0.000 | |
| Other methods | 338 (7.5) | 332 (7.4) | 0.003 | |
| Positive histology | 2402 (53.5) | 2316 (51.6) | 0.019 | |
| Radiography | 742 (16.5) | 770 (17.1) | 0.001 | |
| Unknown | 83 (1.8) | 95 (2.1) | ||
| Time from diagnosis to treatment, % | 0.455 | |||
| <1 month | 2203 (49.1) | 2155 (48.0) | 0.015 | |
| 1–2 months | 1084 (24.1) | 1140 (25.4) | 0.039 | |
| ≥3 months | 177 (3.9) | 190 (4.2) | 0.006 | |
| Unknown | 1026 (22.9) | 1005 (22.4) | ||
| Initial treatment, % 1 | ||||
| Surgery | 1718 (38.6) | 1690 (37.9) | 0.004 | 0.467 |
| Chemotherapy | 180 (4.0) | 187 (4.2) | 0.011 | 0.709 |
| Radiation | 2780 (61.9) | 2815 (62.7) | 0.020 | 0.446 |
| Sex | p Value | ||
|---|---|---|---|
| Characteristics | Male (N = 1501) | Female (N = 1331) | |
| Age, years, % | 0.092 | ||
| 18–44 | 223 (14.9) | 206 (15.5) | |
| 45–64 | 634 (42.2) | 509 (38.2) | |
| ≥65 | 644 (42.9) | 616 (46.3) | |
| Year of diagnosis, % | 0.340 | ||
| 1995–1999 | 217 (14.5) | 197 (14.8) | |
| 2000–2004 | 272 (18.1) | 226 (17.0) | |
| 2005–2009 | 286 (19.1) | 257 (19.3) | |
| 2010–2014 | 269 (17.9) | 275 (20.7) | |
| 2015–2019 | 457 (30.4) | 376 (28.2) | |
| Race and ethnicity, % | 0.096 | ||
| Non-Hispanic White | 1279 (85.2) | 1111 (83.5) | |
| Non-Hispanic Black | 25 (1.7) | 32 (2.4) | |
| Hispanic | 180 (12.0) | 160 (12.0) | |
| Other 1 | 17 (1.1) | 28 (2.1) | |
| Health insurance | 0.675 | ||
| Not insured | 49 (3.3) | 45 (3.4) | |
| Medicaid | 19 (1.3) | 11 (0.8) | |
| Medicare | 277 (18.5) | 273 (20.5) | |
| Private | 387 (25.8) | 332 (24.9) | |
| Other | 223 (14.9) | 195 (14.7) | |
| Unknown | 546 (36.4) | 475 (35.7) | |
| Census tract poverty indicator, % | 0.166 | ||
| <10% | 687 (45.8%) | 619 (46.5%) | |
| 10–20% | 462 (30.8%) | 436 (32.8%) | |
| >20% | 301 (20.1%) | 246 (18.5%) | |
| Unknown | 51 (3.4%) | 30 (2.3%) | |
| Location, rural, % | 274 (18.3) | 242 (18.2) | 0.960 |
| Primary site of tumor, % | 0.004 | ||
| Choroid | 829 (55.2) | 776 (58.3) | |
| Ciliary body | 170 (11.3) | 172 (12.9) | |
| Conjunctiva | 175 (11.7) | 101 (7.6) | |
| Other sites/unspecified | 327 (20.4) | 282 (21.2) | |
| Histology, % | 0.004 | ||
| Uveal melanoma | 991 (66.0) | 942 (70.8) | |
| Conjunctival melanoma | 57 (3.8) | 60 (4.5) | |
| Other histologic subtypes | 453 (30.2) | 329 (24.7) | |
| Tumor stage, % | 0.046 | ||
| Localized | 883 (58.8) | 747 (56.1) | |
| Regional | 121 (8.1) | 126 (9.5) | |
| Distant | 78 (5.2) | 49 (3.7) | |
| Unknown/unstaged | 419 (27.9) | 409 (30.7) | |
| Laterality, % | 0.822 | ||
| Unilateral | 1447 (96.4) | 1281 (96.2) | |
| Bilateral | 54 (3.6) | 50 (3.8) | |
| Initial treatment, % 2 | |||
| Surgery | 670 (44.6) | 500 (37.6) | <0.001 |
| Radiation | 380 (25.3) | 380 (28.5) | 0.127 |
| Chemotherapy | 65 (4.3) | 55 (4.1) | 0.550 |
| Age-Adjusted | Multivariable Adjusted 2 | ||
|---|---|---|---|
| Mortality outcomes | N | HR (95%CI) | HR (95%CI) |
| All-cause | 1302 | 1.20 (1.07–1.33) | 1.14 (1.02–1.28) |
| Ocular cancer | 712 | 1.03 (0.89–1.19) | 1.03 (0.88–1.19) |
| Cardiovascular disease | 242 | 1.45 (1.13–1.88) | 1.34 (1.03–1.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, D.; Almosa, A.; Heath, E.; De La Cruz, N.; Shabaneh, O. A Population-Based Study of Sex Differences in Cardiovascular Disease Mortality Among Adults with Ocular Cancer in the United States, 2000–2021. Curr. Oncol. 2025, 32, 447. https://doi.org/10.3390/curroncol32080447
Appiah D, Almosa A, Heath E, De La Cruz N, Shabaneh O. A Population-Based Study of Sex Differences in Cardiovascular Disease Mortality Among Adults with Ocular Cancer in the United States, 2000–2021. Current Oncology. 2025; 32(8):447. https://doi.org/10.3390/curroncol32080447
Chicago/Turabian StyleAppiah, Duke, Abdulkader Almosa, Eli Heath, Noah De La Cruz, and Obadeh Shabaneh. 2025. "A Population-Based Study of Sex Differences in Cardiovascular Disease Mortality Among Adults with Ocular Cancer in the United States, 2000–2021" Current Oncology 32, no. 8: 447. https://doi.org/10.3390/curroncol32080447
APA StyleAppiah, D., Almosa, A., Heath, E., De La Cruz, N., & Shabaneh, O. (2025). A Population-Based Study of Sex Differences in Cardiovascular Disease Mortality Among Adults with Ocular Cancer in the United States, 2000–2021. Current Oncology, 32(8), 447. https://doi.org/10.3390/curroncol32080447






