Impact of Surgical Margin Control in Index Tumors on Prognosis After Radical Prostatectomy: A Focus on Zonal Origin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Histopathological Examination
2.3. Follow-Up and Outcomes
2.4. Descriptive and Comparative Analyses
2.5. Survival and Multivariate Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RP | Radical prostatectomy |
| PSM | Positive surgical margins |
| BCR | Biochemical recurrence |
| NSM | Negative surgical margins |
| TZ | Transition zone |
| PZ | Peripheral zone |
| CZ | Central zone |
| NMSH | Nippon Medical School Hospital |
| ISUP | International Society of Urological Pathology |
| PSA | Prostate-specific antigen |
| BMI | Body mass index |
| EAU | European Association of Urology |
| Index-PSM | Index tumor surgical margin-positive |
| Index-NSM | Index tumor surgical margin-negative |
| Other-PSM | PSM in non-index tumors |
| All-NSM | NSM in all tumor foci |
| BCR-FS | BCR-free survival |
| TPV | Total prostate volume |
| pT | Pathological T |
| pN | Pathological lymph node metastasis |
| HR | Hazard ratio |
| OR | Odds ratio |
| CI | Confidence interval |
| mpMRI | Multiparametric magnetic resonance imaging |
References
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Briganti, A.; Eastham, J.A.; Epstein, J.; Graefen, M.; Montironi, R.; Touijer, K. Positive surgical margins after radical prostatectomy: A systematic review and contemporary update. Eur. Urol. 2014, 65, 303–313. [Google Scholar] [CrossRef]
- Grypari, I.M.; Zolota, V.; Tzelepi, V. Radical or Not-So-Radical Prostatectomy: Do Surgical Margins Matter? Cancers 2021, 14, 13. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, L.; Shao, Y.; An, K.; Hu, C.; Liang, X.; Wang, D. The impact of positive surgical margin parameters and pathological stage on biochemical recurrence after radical prostatectomy: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0301653. [Google Scholar] [CrossRef]
- John, A.; Milton, T.; Gupta, A.; Nguyen, M.T.; Stretton, B.; Hewitt, J.; Virgin, J.; Kovoor, J.; Catterwell, R.; Selth, L.; et al. Impact of positive surgical margin location after radical prostatectomy: A network meta-analysis. World J. Urol. 2025, 43, 134. [Google Scholar] [CrossRef]
- Eastham, J.A.; Kuroiwa, K.; Ohori, M.; Serio, A.M.; Gorbonos, A.; Maru, N.; Vickers, A.J.; Slawin, K.M.; Wheeler, T.M.; Reuter, V.E.; et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology 2007, 70, 965–969. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, H.; He, Z.; Ma, S.; Wang, X.; Liu, R. Impact of positive surgical margin location and perineural invasion on biochemical recurrence in patients undergoing radical prostatectomy. World J. Surg. Oncol. 2020, 18, 201. [Google Scholar] [CrossRef]
- Dev, H.S.; Wiklund, P.; Patel, V.; Parashar, D.; Palmer, K.; Nyberg, T.; Skarecky, D.; Neal, D.E.; Ahlering, T.; Sooriakumaran, P. Surgical margin length and location affect recurrence rates after robotic prostatectomy. Urol. Oncol. 2015, 33, 109.e7–109.e13. [Google Scholar] [CrossRef]
- Miller, G.J.; Cygan, J.M. Morphology of prostate cancer: The effects of multifocality on histological grade, tumor volume and capsule penetration. J. Urol. 1994, 152, 1709–1713. [Google Scholar] [CrossRef]
- Wise, A.M.; Stamey, T.A.; McNeal, J.E.; Clayton, J.L. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 2002, 60, 264–269. [Google Scholar] [CrossRef]
- Arora, R.; Koch, M.O.; Eble, J.N.; Ulbright, T.M.; Li, L.; Cheng, L. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 2004, 100, 2362–2366. [Google Scholar] [CrossRef]
- Boutros, P.C.; Fraser, M.; Harding, N.J.; de Borja, R.; Trudel, D.; Lalonde, E.; Meng, A.; Hennings-Yeomans, P.H.; McPherson, A.; Sabelnykova, V.Y.; et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet. 2015, 47, 736–745. [Google Scholar] [CrossRef]
- Cooper, C.S.; Eeles, R.; Wedge, D.C.; Van Loo, P.; Gundem, G.; Alexandrov, L.B.; Kremeyer, B.; Butler, A.; Lynch, A.G.; Camacho, N.; et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet. 2015, 47, 367–372. [Google Scholar] [CrossRef]
- Ahmed, H.U. The index lesion and the origin of prostate cancer. N. Engl. J. Med. 2009, 361, 1704–1706. [Google Scholar] [CrossRef]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 2009, 15, 559–565. [Google Scholar] [CrossRef]
- Bott, S.R.; Ahmed, H.U.; Hindley, R.G.; Abdul-Rahman, A.; Freeman, A.; Emberton, M. The index lesion and focal therapy: An analysis of the pathological characteristics of prostate cancer. BJU Int. 2010, 106, 1607–1611. [Google Scholar] [CrossRef]
- Barry Delongchamps, N.; Schull, A.; Anract, J.; Abecassis, J.P.; Zerbib, M.; Sibony, M.; Jilet, L.; Abdoul, H.; Goffin, V.; Peyromaure, M. Feasibility and safety of targeted focal microwave ablation of the index tumor in patients with low to intermediate risk prostate cancer: Results of the FOSTINE trial. PLoS ONE 2021, 16, e0252040. [Google Scholar] [CrossRef]
- Darr, C.; Finis, F.; Wiesenfarth, M.; Giganti, F.; Tschirdewahn, S.; Krafft, U.; Kesch, C.; Bonekamp, D.; Forsting, M.; Wetter, A.; et al. Three-dimensional Magnetic Resonance Imaging-based Printed Models of Prostate Anatomy and Targeted Biopsy-proven Index Tumor to Facilitate Patient-tailored Radical Prostatectomy-A Feasibility Study. Eur. Urol. Oncol. 2022, 5, 357–361. [Google Scholar] [CrossRef]
- McNeal, J.E. Regional morphology and pathology of the prostate. Am. J. Clin. Pathol. 1968, 49, 347–357. [Google Scholar] [CrossRef]
- Jin, S.; Wu, L.; Liang, Z.; Yan, W. The prognostic value of zonal origin in clinically localized prostate cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1248222. [Google Scholar] [CrossRef]
- Ogata, Y.; Akatsuka, J.; Endo, Y.; Mikami, H.; Yanagi, M.; Takeda, H.; Toyama, Y.; Yamamoto, Y.; Kimura, G.; Kondo, Y. Index tumor location affected early biochemical recurrence after radical prostatectomy in patients with negative surgical margin: A retrospective study. BMC Urol. 2024, 24, 108. [Google Scholar] [CrossRef]
- Cohen, R.J.; Shannon, B.A.; Phillips, M.; Moorin, R.E.; Wheeler, T.M.; Garrett, K.L. Central zone carcinoma of the prostate gland: A distinct tumor type with poor prognostic features. J. Urol. 2008, 179, 1762–1767, discussion 1767. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- McNeal, J.E. Normal histology of the prostate. Am. J. Surg. Pathol. 1988, 12, 619–633. [Google Scholar] [CrossRef]
- McNeal, J.E.; Redwine, E.A.; Freiha, F.S.; Stamey, T.A. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am. J. Surg. Pathol. 1988, 12, 897–906. [Google Scholar] [CrossRef]
- Anceschi, U.; Basile, S.; Prata, F.; Cellini, V.; Caviglia, A.; Flammia, R.S.; Vecchio, E.; Mazzone, E.; Tuderti, G.; Maltzman, O.; et al. Unveiling predictors of positive surgical margins in Retzius-sparing and anterograde robot-assisted radical prostatectomy: Insights from a dual-centre learning curve experience. J. Robot. Surg. 2025, 19, 429. [Google Scholar] [CrossRef]
- Sasaki, T.; Ebara, S.; Tatenuma, T.; Ikehata, Y.; Nakayama, A.; Kawase, M.; Toide, M.; Yoneda, T.; Sakaguchi, K.; Teishima, J.; et al. Prognostic differences among the positive surgical margin locations following robot-assisted radical prostatectomy in a large Japanese cohort (the MSUG94 group). Jpn. J. Clin. Oncol. 2023, 53, 443–451. [Google Scholar] [CrossRef]
- Schroeder, D.W.; Foster, B.R.; Young, D.J.; Coakley, F.V. Targeted biopsy of the prostate. Abdom. Radiol. 2025, 50, 261–271. [Google Scholar] [CrossRef]
- Donato, P.; Morton, A.; Yaxley, J.; Ranasinghe, S.; Teloken, P.E.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1843–1851. [Google Scholar] [CrossRef]
- Lasorsa, F.; Biasatti, A.; Orsini, A.; Bignante, G.; Farah, G.M.; Pandolfo, S.D.; Lambertini, L.; Reddy, D.; Damiano, R.; Ditonno, P.; et al. Focal therapy for prostate cancer: Recent advances and insights. Curr. Oncol. 2024, 32, 15. [Google Scholar] [CrossRef]
- Freedland, S.J.; Samjoo, I.A.; Rosta, E.; Lansing, A.; Worthington, E.; Niyazov, A.; Nazari, J.; Arondekar, B. The impact of race on survival in metastatic prostate cancer: A systematic literature review. Prostate Cancer Prostatic Dis. 2023, 26, 461–474. [Google Scholar] [CrossRef]
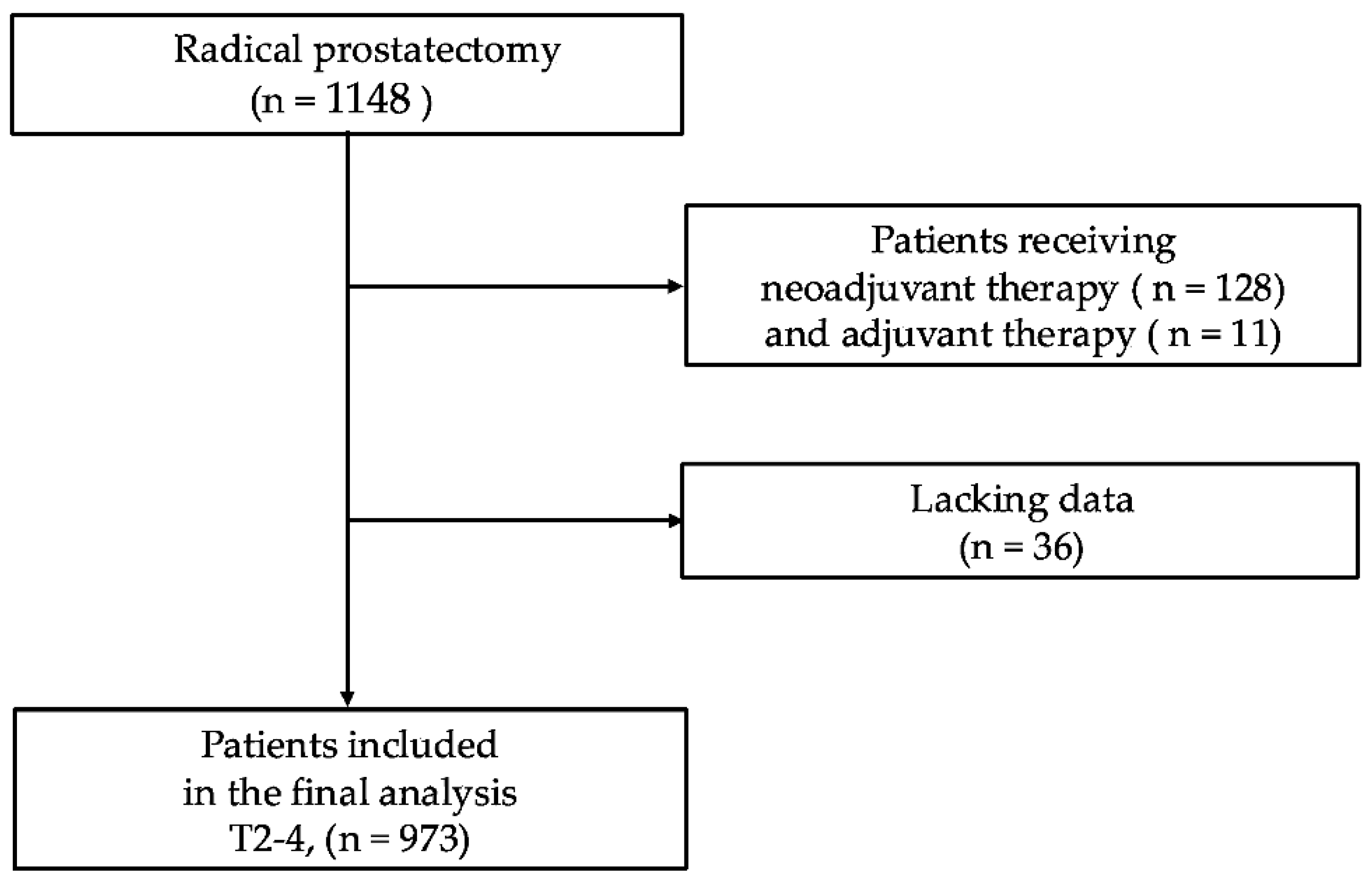
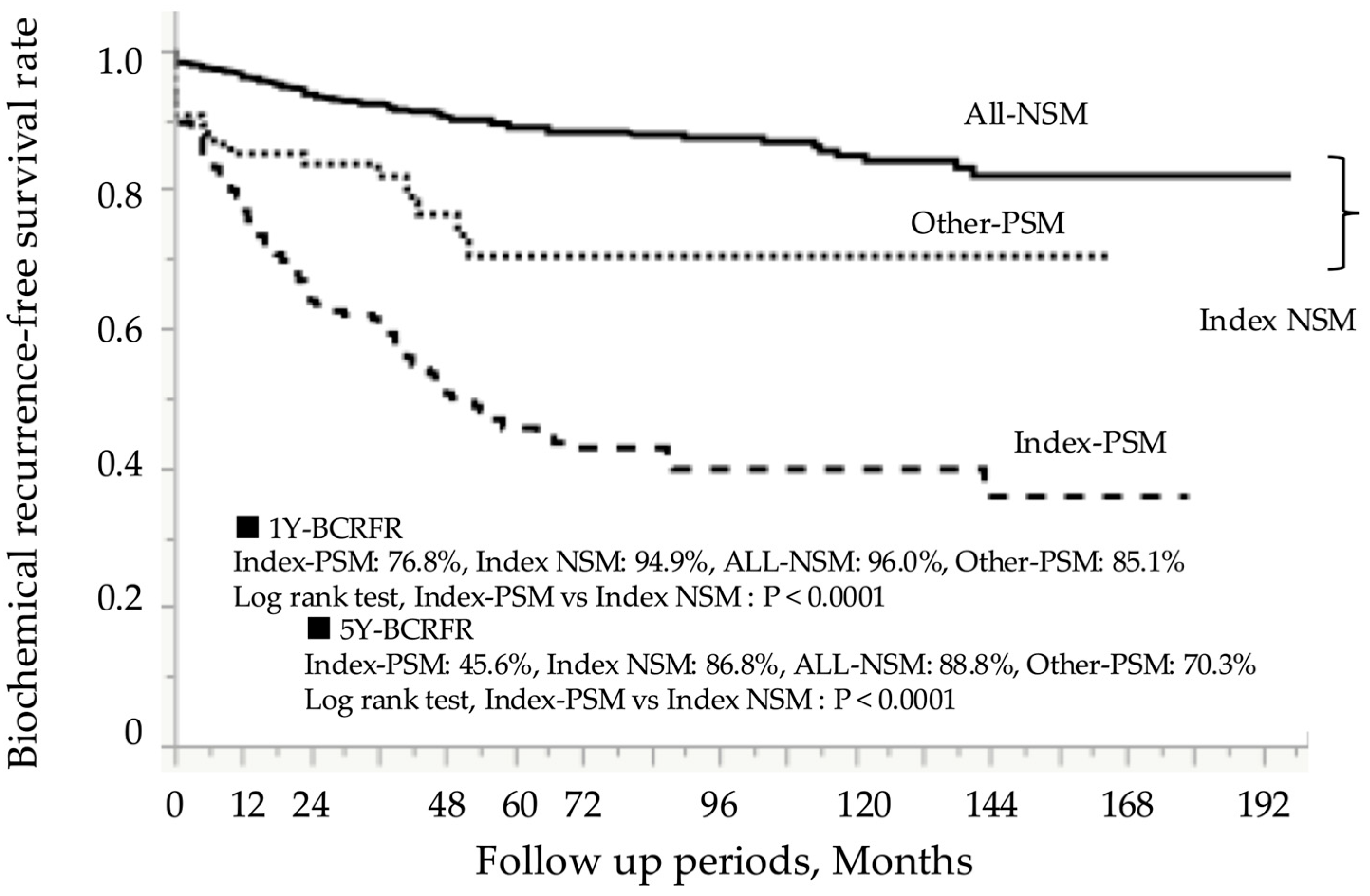
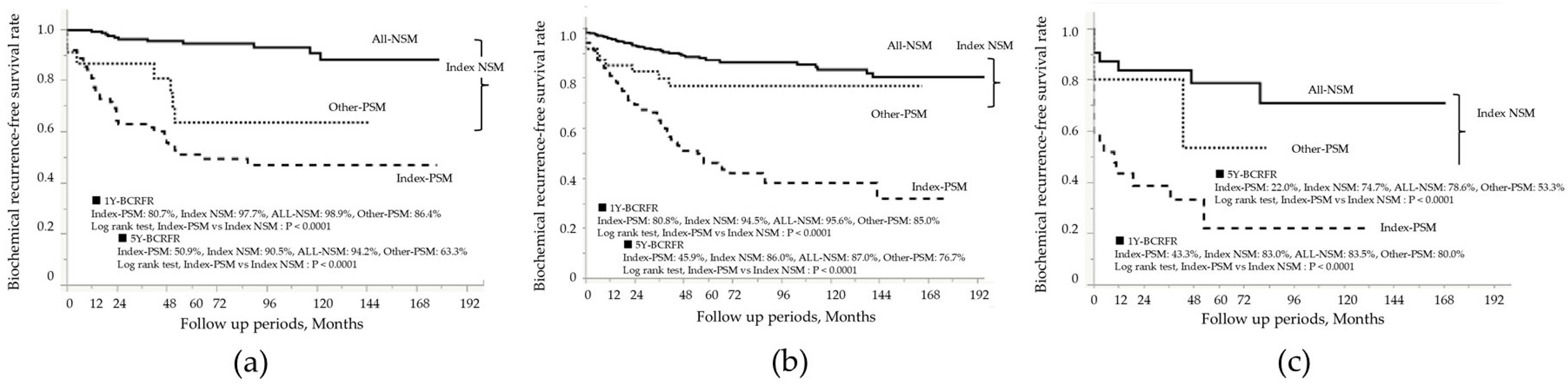
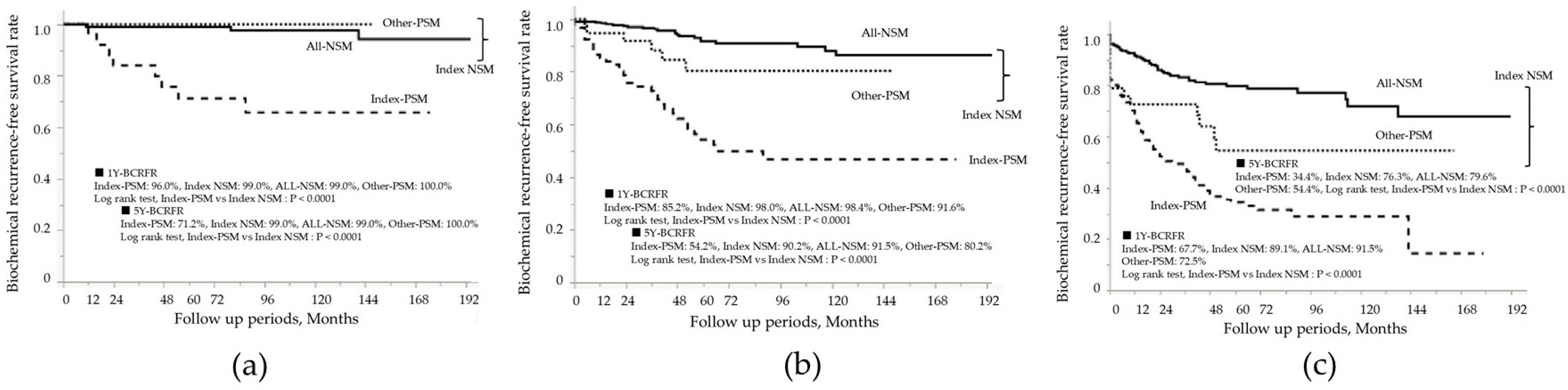
| Patient in the Entire Cohort: N = 973 | |||
|---|---|---|---|
| Age, years, average ± SD | 66.8 | ±6.0 | |
| BMI, kg/m2, average ± SD | 23.6 | ±2.9 | |
| TPV, mL, average ± SD | 30.7 | ±15.0 | |
| Preoperative PSA, (ng/mL), n, (%) | ≤10 | 592 | 60.8 |
| 10–20 | 266 | 27.3 | |
| >20 | 115 | 11.8 | |
| cT stage, n, (%) | T1 | 254 | 26.1 |
| T2 | 634 | 65.2 | |
| T3–4 | 85 | 8.7 | |
| Biopsy ISUP grade, n, (%) | 1–2 | 524 | 53.9 |
| 3 | 190 | 19.5 | |
| 4–5 | 259 | 26.6 | |
| EAU risk, n, (%) | Low | 131 | 13.5 |
| Intermediate | 440 | 45.2 | |
| High | 402 | 41.3 | |
| Prostatectomy ISUP grade, n, (%) | 1–2 | 422 | 43.4 |
| 3 | 207 | 21.3 | |
| 4–5 | 344 | 35.4 | |
| pT stage, n, (%) | T2 | 654 | 67.2 |
| T3–4 | 319 | 32.8 | |
| Nerve sparing surgery, n, (%) | Unilateral/Bilateral | 263/44 | 27.0/4.5 |
| None | 666 | 68.4 | |
| Lymph node dissection, n, (%) | Limited/Extended | 418/38 | 43.0/3.9 |
| None | 517 | 53.1 | |
| pN positive, n, (%) | 14 | 1.4 | |
| PSM, n, (%) | 331 | 34.0 | |
| Index tumor location, n, (%) | TZ | 316 | 32.5 |
| PZ | 594 | 61.0 | |
| CZ | 63 | 6.5 | |
| Index-PSM (n = 257:26.4%) | Index-NSM (n = 716:73.6%) | Index-PSM vs. Index-NSM | ||||||
|---|---|---|---|---|---|---|---|---|
| Other-PSM (n = 74:7.6%) | All-NSM (n = 642:66.0%) | |||||||
| Age, y, average ± SD | 67.4 | ±5.7 | 67.3 | ±5.4 | 66.5 | ±6.2 | 0.15 | |
| TPV, mL, average ± SD | 30.5 | ±16.5 | 30.8 | ±14.5 | 30.7 | ±14.5 | 0.66 | |
| BMI, kg/m2, average ± SD | 23.7 | ±3.0 | 24.3 | ±2.9 | 23.5 | ±2.8 | 0.90 | |
| Preoperative PSA (ng/mL), n, (%) | ≤10 | 117 | 45.5 | 42 | 56.8 | 433 | 67.4 | <0.0001 |
| 10–20 | 73 | 28.4 | 24 | 32.4 | 169 | 26.3 | ||
| >20 | 67 | 26.1 | 8 | 10.8 | 40 | 6.2 | ||
| Prostatectomy ISUP grade, n, (%) | 1–2 | 73 | 28.4 | 27 | 36.5 | 322 | 50.2 | <0.0001 |
| 3 | 56 | 21.8 | 22 | 29.7 | 129 | 20.1 | ||
| 4–5 | 128 | 49.8 | 25 | 33.8 | 191 | 29.8 | ||
| pT stage, n, (%) | T2 | 111 | 43.2 | 38 | 51.4 | 505 | 78.7 | <0.0001 |
| T3–4 | 146 | 56.8 | 36 | 48.6 | 137 | 21.3 | ||
| Index tumor location, n, (%) | TZ | 96 | 37.4 | 22 | 29.7 | 198 | 30.8 | 0.003 |
| PZ | 134 | 52.1 | 47 | 63.5 | 413 | 64.3 | ||
| CZ | 27 | 10.5 | 5 | 6.8 | 31 | 4.8 | ||
| Nerve sparing surgery, n, (%) | 68 | 26.5 | 18 | 24.3 | 221 | 34.4 | 0.04 | |
| Lymph node dissection, n, (%) | 155 | 60.3 | 42 | 56.8 | 259 | 40.3 | <0.0001 | |
| pN, n, (%) | 9 | 3.5 | 3 | 4.1 | 2 | 0.3 | 0.003 | |
| TZ (316: 32.5%) | PZ (594: 61.0%) | CZ (63: 6.5%) | TZ vs. PZ | TZ vs. CZ | PZ vs. CZ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years, average ± SD | 66.7 | ±5.7 | 66.9 | ±6.2 | 66.4 | ±5.8 | 0.34 | 0.70 | 0.40 | |
| BMI, kg/m2, average ± SD | 23.6 | ±2.9 | 23.6 | ±2.8 | 24.2 | ±3.2 | 0.97 | 0.17 | 0.14 | |
| Preoperative PSA (ng/mL), n, (%) | ≤10 | 166 | 52.5 | 401 | 67.5 | 25 | 39.7 | <0.0001 | 0.03 | <0.0001 |
| 10–20 | 86 | 27.2 | 152 | 25.6 | 28 | 44.4 | ||||
| >20 | 64 | 20.3 | 41 | 6.9 | 10 | 15.9 | ||||
| EAU risk classification, n, (%) | Low | 33 | 10.4 | 94 | 15.8 | 4 | 6.3 | 0.06 | 0.01 | <0.0001 |
| Intermediate | 148 | 46.8 | 276 | 46.5 | 16 | 25.4 | ||||
| High | 135 | 42.7 | 224 | 37.7 | 43 | 68.3 | ||||
| Prostatectomy ISUP grade, n, (%) | 1–2 | 156 | 49.4 | 254 | 42.8 | 12 | 19.0 | 0.16 | <0.0001 | <0.001 |
| 3 | 64 | 20.3 | 131 | 22.1 | 12 | 19.0 | ||||
| 4–5 | 96 | 30.1 | 209 | 35.2 | 39 | 61.9 | ||||
| pT stage, n, (%) | T2 | 221 | 69.9 | 412 | 69.4 | 21 | 33.3 | 0.88 | <0.0001 | <0.001 |
| T3–4 | 95 | 30.1 | 182 | 30.6 | 42 | 66.7 | ||||
| Nerve sparing surgery, n, (%) | 97 | 30.7 | 192 | 32.3 | 18 | 28.6 | 0.65 | 0.88 | 0.57 | |
| Lymph node dissection, n, (%) | 142 | 44.9 | 272 | 45.8 | 42 | 66.7 | 0.83 | 0.002 | 0.002 | |
| pN positive, n, (%) | 3 | 0.9 | 5 | 0.8 | 6 | 9.5 | 1.0 | 0.0009 | 0.0002 | |
| Index-PSM, n, (%) | 96 | 30.4 | 134 | 22.6 | 27 | 42.9 | 0.01 | 0.06 | 0.001 | |
| Variable | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age, years | ≤67 | - | - | ||
| >67 | 0.9 (0.7–1.1) | 0.25 | |||
| Preoperative PSA, ng/mL | ≤10 | - | - | - | - |
| 10–20 | 3.1 (2.2–4.2) | <0.0001 | 2.1 (1.5–3.0) | <0.0001 | |
| >20 | 5.5 (3.9–7.8) | <0.0001 | 2.0 (1.3–3.0) | 0.001 | |
| EAU risk | Low | - | - | ||
| Intermediate | 2.2 (1.2–4.4) | 0.01 | |||
| High | 6.5 (3.5–12.1) | <0.0001 | |||
| Prostatectomy ISUP grade | 1–2 | - | - | - | - |
| 3 | 3.4 (2.3–5.0) | <0.0001 | 1.9 (1.3–2.9) | 0.003 | |
| 4–5 | 8.6 (5.9–13) | <0.0001 | 3.4 (2.2–5.5) | <0.0001 | |
| pT stage | T2 | - | - | - | - |
| T3-4 | 4.7 (3.5–6.2) | <0.0001 | 1.9 (1.4–2.5) | 0.0001 | |
| Index tumor location | TZ | - | - | - | - |
| PZ | - | - | 1.5 (1.1–2.0) | 0.01 | |
| CZ | 2.9 (1.8–4.6) | <0.0001 | 1.8 (1.1–2.9) | 0.02 | |
| Index-PSM | Negative | - | - | - | - |
| Positive | 5.3 (4.0–6.9) | <0.0001 | 3.4 (2.5–4.5) | <0.0001 | |
| Variable | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age, years | ≤67 | - | - | ||
| >67 | 0.9 (0.6–1.4) | 0.59 | |||
| Preoperative PSA, ng/mL | ≤10 | - | - | - | - |
| 10–20 | 4.3 (2.5–7.3) | <0.0001 | 2.6 (1.4–4.8) | 0.002 | |
| >20 | 9.5 (5.3–17.1) | <0.0001 | 3.5 (1.7–7.5) | 0.0008 | |
| EAU risk | Low | - | - | ||
| Intermediate | - | - | |||
| High | 14.1 (3.4–58.2) | 0.0003 | |||
| Prostatectomy ISUP grade | 1–2 | - | - | - | - |
| 3 | 5.1 (2.3–11.2) | <0.0001 | 2.7 (1.1–6.3) | 0.03 | |
| 4–5 | 20.3 (9.4–43.6) | <0.0001 | 6.8 (2.8–17.0) | <0.0001 | |
| pT stage | T2 | - | - | - | - |
| T3–4 | 6.2 (3.9–9.9) | <0.0001 | 1.8 (1.1–3.2) | 0.03 | |
| Index tumor location | TZ | - | - | - | - |
| PZ | - | - | - | - | |
| CZ | 6.4 (3.2–12.5) | <0.0001 | 3.7 (1.6–8.5) | 0.002 | |
| Index-PSM | Negative | - | - | - | - |
| Positive | 5.5 (3.5–8.6) | <0.0001 | 3.1 (1.8–5.1) | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akatsuka, J.; Ogata, Y.; Obayashi, K.; Takadate, M.; Ikuma, S.; Hasegawa, H.; Mikami, H.; Takeda, H.; Endo, Y.; Takahashi, T.; et al. Impact of Surgical Margin Control in Index Tumors on Prognosis After Radical Prostatectomy: A Focus on Zonal Origin. Curr. Oncol. 2025, 32, 445. https://doi.org/10.3390/curroncol32080445
Akatsuka J, Ogata Y, Obayashi K, Takadate M, Ikuma S, Hasegawa H, Mikami H, Takeda H, Endo Y, Takahashi T, et al. Impact of Surgical Margin Control in Index Tumors on Prognosis After Radical Prostatectomy: A Focus on Zonal Origin. Current Oncology. 2025; 32(8):445. https://doi.org/10.3390/curroncol32080445
Chicago/Turabian StyleAkatsuka, Jun, Yoshihiko Ogata, Kotaro Obayashi, Mami Takadate, Shunsuke Ikuma, Hiroya Hasegawa, Hikaru Mikami, Hayato Takeda, Yuki Endo, Takayuki Takahashi, and et al. 2025. "Impact of Surgical Margin Control in Index Tumors on Prognosis After Radical Prostatectomy: A Focus on Zonal Origin" Current Oncology 32, no. 8: 445. https://doi.org/10.3390/curroncol32080445
APA StyleAkatsuka, J., Ogata, Y., Obayashi, K., Takadate, M., Ikuma, S., Hasegawa, H., Mikami, H., Takeda, H., Endo, Y., Takahashi, T., Ono, K., Toyama, Y., Yamamoto, Y., Kimura, G., & Kondo, Y. (2025). Impact of Surgical Margin Control in Index Tumors on Prognosis After Radical Prostatectomy: A Focus on Zonal Origin. Current Oncology, 32(8), 445. https://doi.org/10.3390/curroncol32080445






