A Review on the Evolving Role of Radiation Therapy in the Treatment of Locally Advanced Rectal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
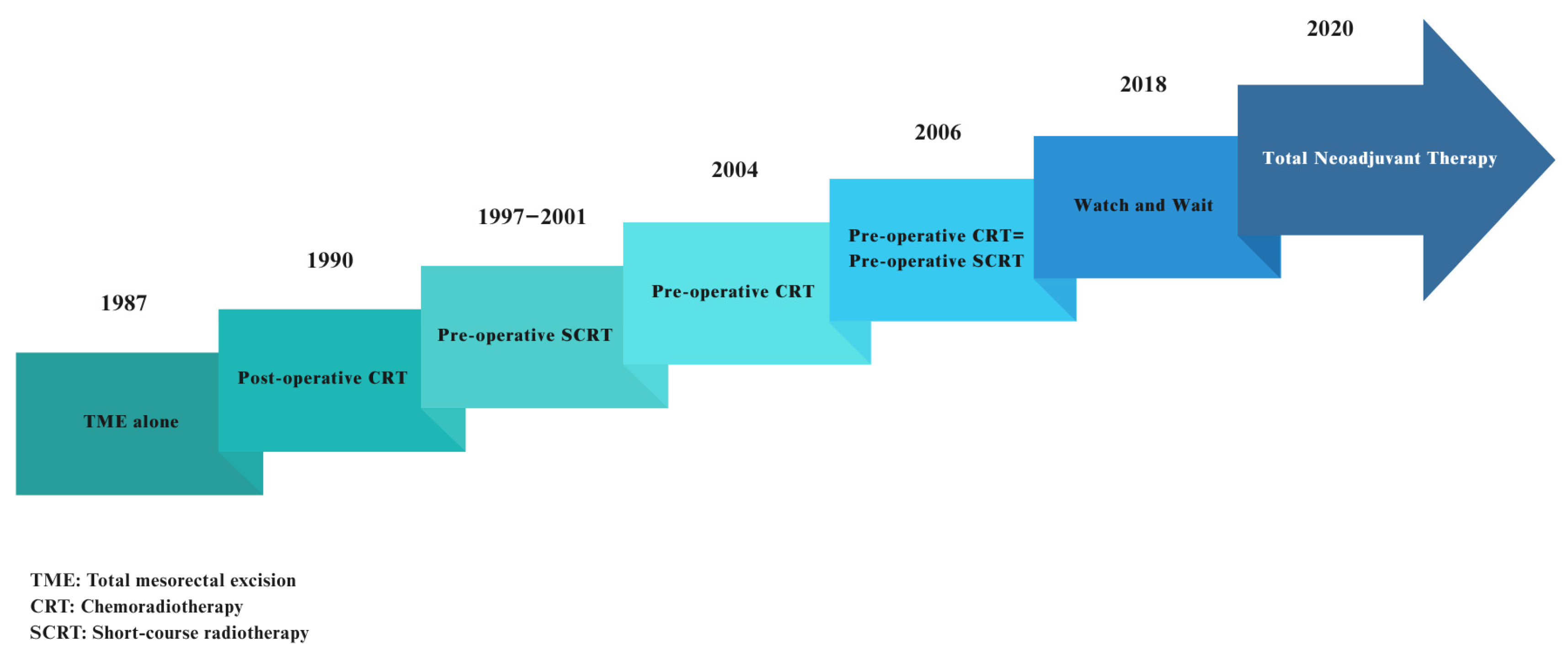
3. Radiation Treatment for LARC: From the Past to the Present
4. Radiation Therapy for LARC Today: Innovations and Updates
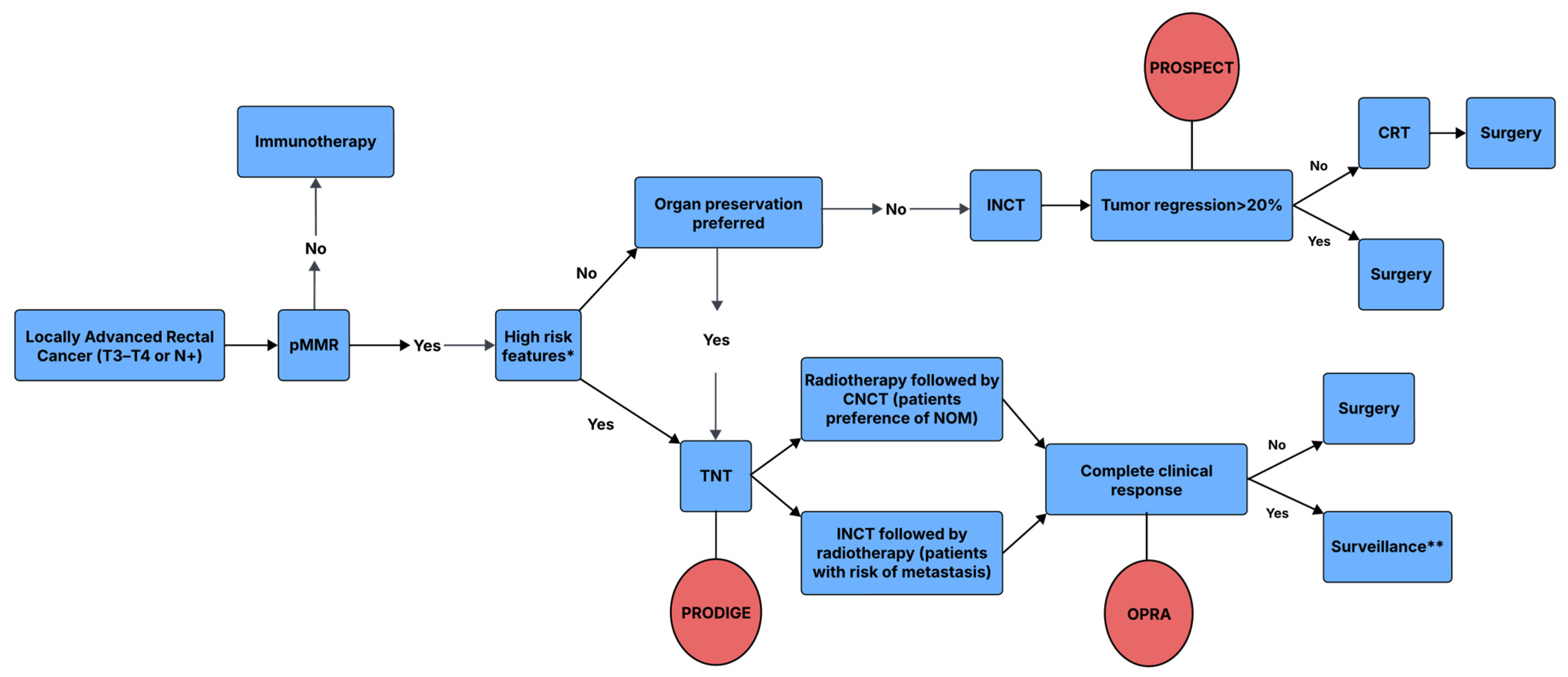
4.1. Radiotherapy as a Component of Total Neoadjuvant Therapy in LARC
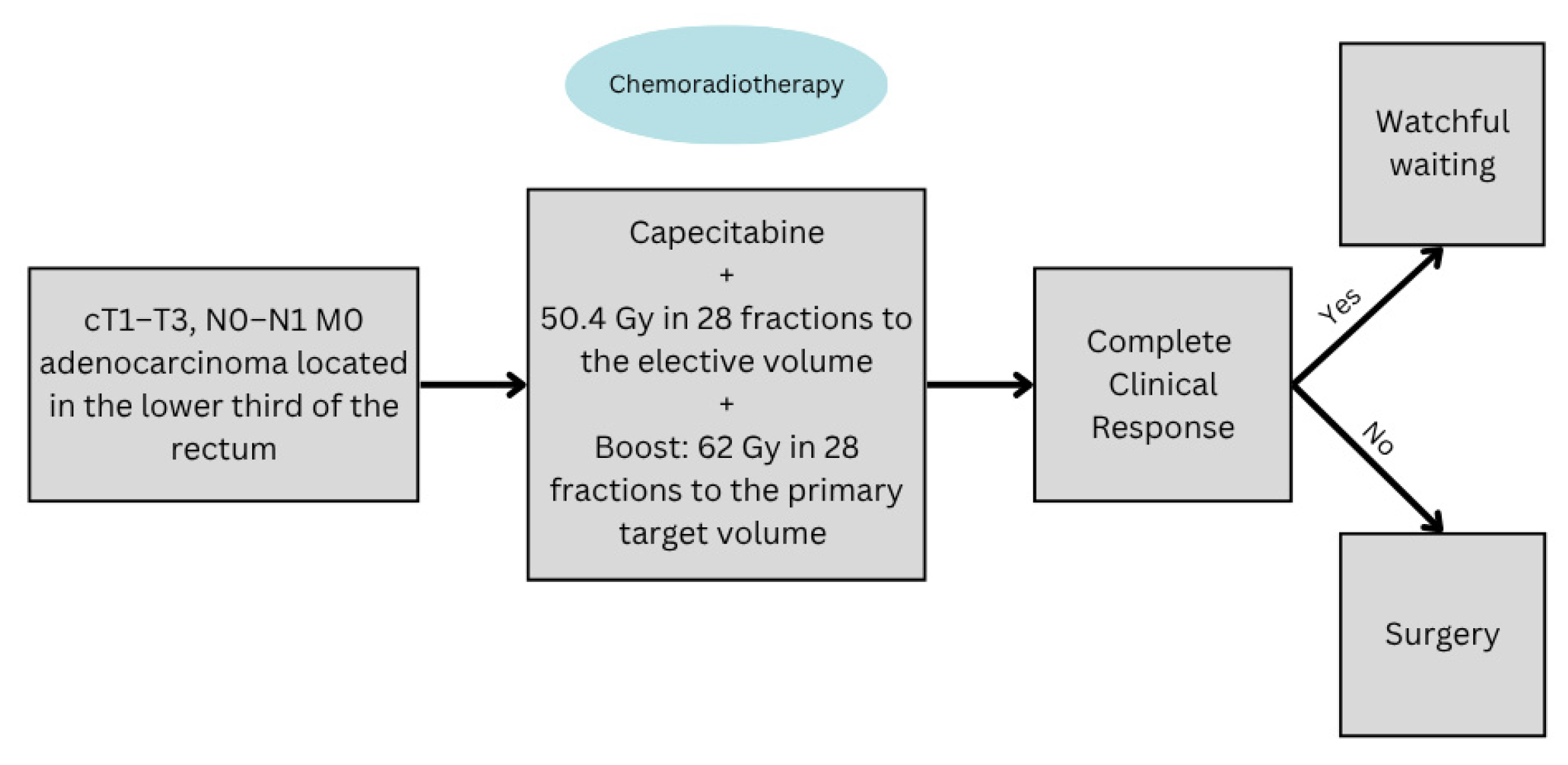
4.2. The Role of Radiotherapy in Organ Preservation for LARC
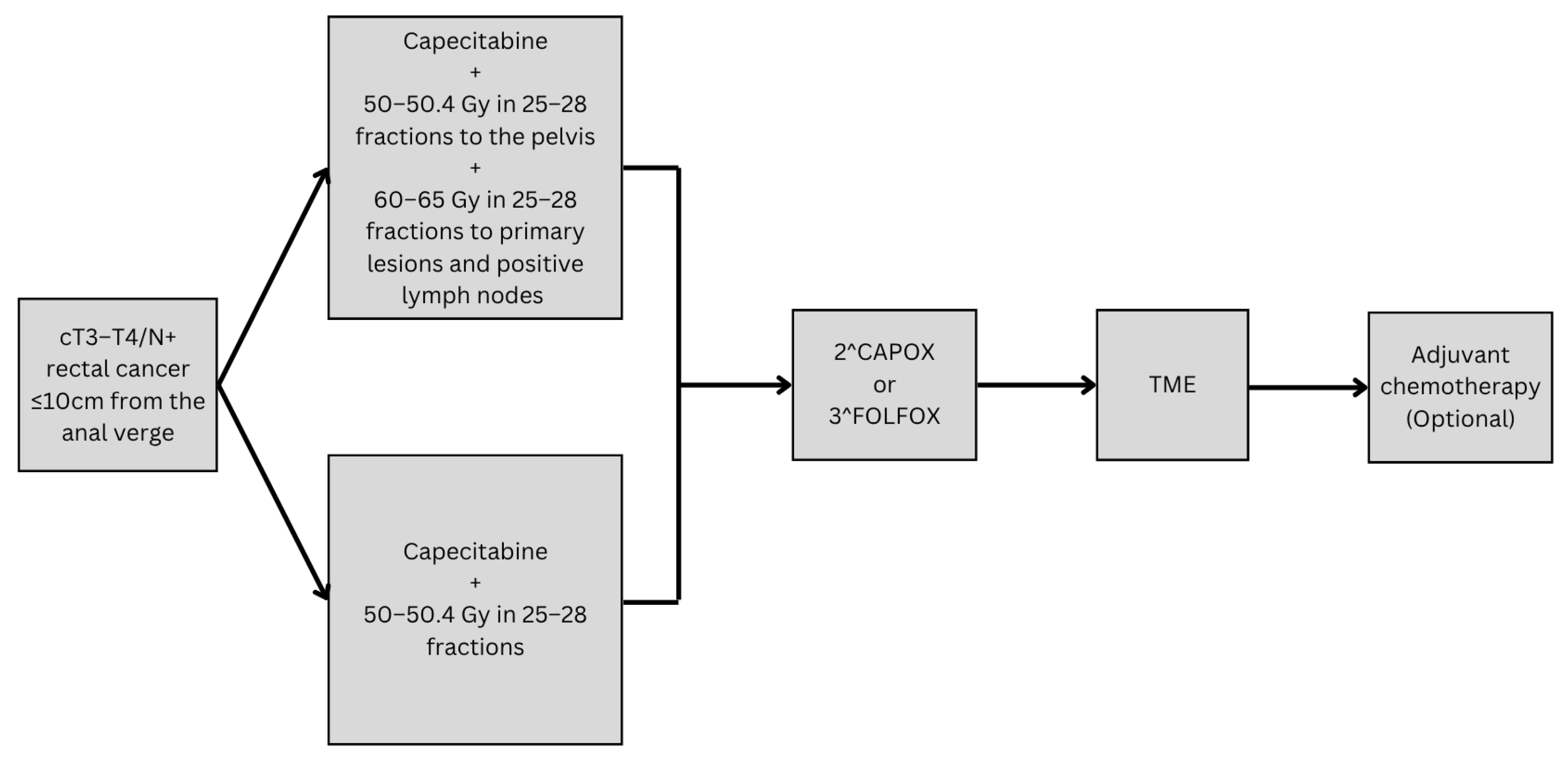
4.3. Optimizing Organ Preservation Through Radiotherapy Dose Escalation in LARC
4.4. The Selective Omission of Radiotherapy in LARC
4.5. Combining Radiotherapy with Immunotherapy
4.6. The Role of Adjuvant Radiotherapy in LARC
4.7. The Role of Intraoperative Radiotherapy (IORT) in LARC
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer?gad_source=1&gclid=CjwKCAjwxLKxBhA7EiwAXO0R0FH2aaMwGT9xFaeRB5d3wdNStTPuBpeJmaTLRV5kkKTDNvRh-E7ZvBoC2KUQAvD_BwE (accessed on 10 October 2024).
- Fazeli, M.S.; Keramati, M.R. Rectal cancer: A review. Med. J. Islam. Repub. Iran 2015, 29, 83–104. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 12 March 2025).
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Liao, L.E.; Xiao, B.Y.; Zhang, X.; Wu, A.W.; Cheng, Y.; Tang, J.H.; Jiang, W.; Kong, L.H.; Han, K.; et al. Long-Term Outcomes of dMMR/MSI-H Rectal Cancer Treated with Anti-PD-1-Based Immunotherapy as Cu-rative-Intent Treatment. J. Natl. Compr. Canc. Netw. 2024, 22, e237096. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, R.-D.; Fokas, E.; Benhaim, L.; Price, T.; Arnold, D.; Beets-Tan, R.; Guren, M.; Hospers, G.; Lonardi, S.; Nagtegaal, I.; et al. Localised rectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer, Version 2; National Comprehensive Cancer Network (NCCN): Jenkintown, PA, USA, 2025. [Google Scholar]
- Fisher, B.; Wolmark, N.; Rockette, H.; Redmond, C.; Deutsch, M.; Wickerham, D.L.; Fisher, E.R.; Caplan, R.; Jones, J.; Lerner, H.; et al. Postoperative Adjuvant Chemotherapy or Radiation Therapy for Rectal Cancer: Results From NSABP Protocol R-011. JNCI J. Natl. Cancer Inst. 1988, 80, 21–29. [Google Scholar] [CrossRef]
- Thomas, P.R.; Lindblad, A.S. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: A review of the gastrointestinal tumor study group experience. Radiother. Oncol. 1988, 13, 245–252. [Google Scholar] [CrossRef]
- Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 1996, 348, 1605–1610. [Google Scholar] [CrossRef]
- Heald, R.; Ryall, R. RECURRENCE AND SURVIVAL AFTER TOTAL MESORECTAL EXCISION FOR RECTAL CANCER. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Krook, J.E.; Moertel, C.G.; Gunderson, L.L.; Wieand, H.S.; Collins, R.T.; Beart, R.W.; Kubista, T.P.; Poon, M.A.; Meyers, W.C.; Mailliard, J.A.; et al. Effective Surgical Adjuvant Therapy for High-Risk Rectal Carcinoma. N. Engl. J. Med. 1991, 324, 709–715. [Google Scholar] [CrossRef]
- Trial, S.R.C.; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Påhlman, L.; Rutqvist, L.E.; Wilking, N. Improved Survival with Preoperative Radiotherapy in Resectable Rectal Cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; Van Krieken, J.H.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.-J.; Bardet, E.; Beny, A.; Ollier, J.-C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef]
- Bregni, G.; Telli, T.A.; Camera, S.; Deleporte, A.; Moretti, L.; Bali, A.; Liberale, G.; Holbrechts, S.; Hendlisz, A.; Sclafani, F. Adjuvant chemotherapy for rectal cancer: Current evidence and recommendations for clinical practice. Cancer Treat. Rev. 2020, 83, 101948. [Google Scholar] [CrossRef]
- Sainato, A.; Nunzia, V.C.L.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, Å.; Broek, C.B.M.v.D.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2014, 26, 696–701. [Google Scholar] [CrossRef]
- Breugom, A.J.; Swets, M.; Bosset, J.-F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; Broek, C.B.M.v.D.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef]
- Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer. Inter-national Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U.; Silva e Sousa, A.H., Jr.; Campos, F.G., Jr.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Proscurshim, I.; Campos, F.G.; Nadalin, W.; Kiss, D.; Gama-Rodrigues, J. Patterns of Failure and Survival for Nonoperative Treatment of Stage c0 Distal Rectal Cancer Following Neoadjuvant Chemoradiation Therapy. J. Gastrointest. Surg. 2006, 10, 1319–1329. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Gama-Rodrigues, J.; Julião, G.P.S.; Proscurshim, I.; Sabbagh, C.; Lynn, P.B.; Perez, R.O. Local Recurrence After Complete Clinical Response and Watch and Wait in Rectal Cancer After Neoadjuvant Chemoradiation: Impact of Salvage Therapy on Local Disease Control. Int. J. Radiat. Oncol. 2014, 88, 822–828. [Google Scholar] [CrossRef]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-See Policy for Clinical Complete Responders After Chemoradiation for Rectal Cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar] [CrossRef]
- Renehan, A.G.; Malcomson, L.; Emsley, R.; Gollins, S.; Maw, A.; Myint, A.S.; Rooney, P.S.; Susnerwala, S.; Blower, A.; Saunders, M.P.; et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol. 2016, 17, 174–183. [Google Scholar] [CrossRef]
- Sanford, N.N.; Dee, E.C.B.; Ahn, C.; Kazmi, S.A.; Beg, M.S.; Folkert, M.R.; Aguilera, T.A.; Polanco, P.M.; Pogacnik, J.S.; Sher, D.J. Recent Trends and Overall Survival of Young Versus Older Adults with Stage II to III Rectal Cancer Treated With and Without Surgery in the United States, 2010–2015. Am. J. Clin. Oncol. 2020, 43, 694–700. [Google Scholar] [CrossRef]
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients with a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019, 5, 185896. [Google Scholar] [CrossRef] [PubMed]
- Khrizman, P.; Niland, J.C.; ter Veer, A.; Milne, D.; Dunn, K.B.; Carson, W.E.; Engstrom, P.F.; Shibata, S.; Skibber, J.M.; Weiser, M.R.; et al. Postoperative Adjuvant Chemotherapy Use in Patients with Stage II/III Rectal Cancer Treated with Neoadjuvant Therapy: A National Comprehensive Cancer Network Analysis. J. Clin. Oncol. 2013, 31, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J. Clin. Oncol. 2010, 28, 859–865. [Google Scholar]
- Glynne-Jones, R.; Counsell, N.; Quirke, P.; Mortensen, N.; Maraveyas, A.; Meadows, H.M.; Ledermann, J.; Sebag-Montefiore, D. Chronicle: Results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann. Oncol. 2014, 25, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Brown, G.; Cunningham, D.; Tait, D.; Wotherspoon, A.; Norman, A.R.; Tebbutt, N.; Hill, M.; Ross, P.J.; Massey, A.; et al. Neoadjuvant Capecitabine and Oxaliplatin Followed by Synchronous Chemoradiation and Total Mesorectal Excision in Magnetic Resonance Imaging-Defined Poor-Risk Rectal Cancer. J. Clin. Oncol. 2006, 24, 668–674. [Google Scholar] [CrossRef]
- Cercek, A.; Roxburgh, C.S.; Strombom, P.; Smith, J.J.; Temple, L.K.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef]
- Van der Valk, M.J.M.; Marijnen, C.A.M.; van Etten, B.; Dijkstra, E.A.; Hilling, D.E.; Kranenbarg, E.M.; Putter, H.; Roodvoets, A.G.H.; Bahadoer, R.R.; Fokstuen, T.; et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—Results of the international randomized RAPIDO-trial. Radiother. Oncol. 2020, 147, 75–83. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.Y.; Chen, S.L.; Li, S.; Lu, N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Kryński, J.; Michalski, W.; Olędzki, J.; Kuśnierz, J.; Zając, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- Chen, L.-N.; Jiang, J.; Jiang, L.-M.; Zhou, H.-T.; Li, N.; Lu, N.-N.; Gao, Y.-H.; Liu, S.-X.; Wang, W.-L.; Wei, L.-C.; et al. Post-hoc analysis of clinicopathological factors affecting lateral lymph node metastasis based on STELLAR study for rectal cancer. Radiother. Oncol. 2024, 200, 110512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Ma, H.; Su, H.; Xu, Z.; Gao, C.; Zhou, H.; Jin, J. Number of lymph nodes retrieved in patients with locally advanced rectal cancer after total neoadjuvant therapy: Post-hoc analysis from the STELLAR trial. BJS Open 2024, 8. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Fonseca, P.; Salazar, R.; Valenti, V.; Msaouel, P.; Carmona-Bayonas, A. Is short-course radiotherapy and total neoadjuvant therapy the new standard of care in locally advanced rectal cancer? A sensitivity analysis of the RAPIDO clinical trial. Ann. Oncol. 2022, 33, 786–793. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.P.H.; et al. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared with Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 278, 766–772. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Conroy, T.; Castan, F.; Etienne, P.-L.; Rio, E.; Mesgouez-Nebout, N.; Evesque, L.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiotherapy in patients with locally advanced rectal cancer: Long-term results of the UNICANCER-PRODIGE 23 trial. Ann. Oncol. 2024, 35, 873–881. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, P.; Xiao, Y.; Meng, W.; Tang, Y.; Zhou, J.; Ding, P.-R.; Ding, K.-F.; Wang, B.; Guo, Q.; et al. Total neoadjuvant treatment with long-course radiotherapy versus concurrent chemoradiotherapy in local advanced rectal cancer with high risk factors (TNTCRT): A multicenter, randomized, open-label, phase 3 trial. J. Clin. Oncol. 2024, 42, LBA3511. [Google Scholar] [CrossRef]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 215445. [Google Scholar] [CrossRef]
- Cerdan-Santacruz, C.; Julião, G.P.S.; Vailati, B.B.; Corbi, L.; Habr-Gama, A.; Perez, R.O. Watch and Wait Approach for Rectal Cancer. J. Clin. Med. 2023, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Smith, J.J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat. Rev. Clin. Oncol. 2021, 18, 805–816. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Smith, J.J.; Chow, O.S.; Gollub, M.J.; Nash, G.M.; Temple, L.K.; Weiser, M.R.; Guillem, J.G.; Paty, P.B.; Avila, K.; Garcia-Aguilar, J.; et al. Organ Preservation in Rectal Adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolida-tion chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015, 15, 767. [Google Scholar]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.-X.; Buckley, J.T.; Thompson, H.M.; Yuval, J.B.; Kim, J.K.; Dunne, R.F.; et al. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J. Clin. Oncol. 2024, 42, 500–506. [Google Scholar] [CrossRef]
- Beets, N.R.A.M.; Verheij, F.S.; Williams, H.; Omer, D.M.; Lin, S.T.; Qin, L.-X.; Beets, G.L.; Beets-Tan, R.G.H.; Wei, I.H.; Widmar, M.; et al. Association of Lateral Pelvic Lymph Nodes with Disease Recurrence and Organ Preservation in Patients with Distal Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. Ann. Surg. 2024, 282, 311–318. [Google Scholar] [CrossRef]
- Thompson, H.M.; Omer, D.M.; Lin, S.; Kim, J.K.; Yuval, J.B.; Verheij, F.S.; Qin, L.X.; Gollub, M.J.; Wu, A.J.; Lee, M.; et al. Organ Preservation and Survival by Clinical Response Grade in Patients with Rectal Cancer Treated with Total Neoadjuvant Therapy: A Secondary Analysis of the OPRA Randomized Clinical Trial. JAMA Netw. Open 2024, 7, 2350903. [Google Scholar] [CrossRef]
- Williams, H.; Fokas, E.; Diefenhardt, M.; Lee, C.; Verheij, F.; Omer, D.; Lin, S.; Dunne, R.; Marcet, J.; Cataldo, P.; et al. Survival among patients treated with total mesorectal excision or selective watch-and-wait after total neoadjuvant therapy: A pooled analysis of the CAO/ARO/AIO-12 and OPRA randomized phase II trials. Ann. Oncol. 2025, 36, 543–547. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Shi, Q.; Dasari, A.; Garcia-Aguilar, J.; Sanoff, H.; George, T.J.; Hong, T.; Yothers, G.; Philip, P.; Nelson, G.; et al. Alliance A022104/NRG-GI010: The Janus Rectal Cancer Trial: A randomized phase II/III trial testing the efficacy of triplet versus doublet chemotherapy regarding clinical complete response and disease-free survival in patients with locally advanced rectal cancer. BMC Cancer 2024, 24, 1–17. [Google Scholar] [CrossRef]
- Rullier, E.; Vendrely, V.; Asselineau, J.; Rouanet, P.; Tuech, J.-J.; Valverde, A.; de Chaisemartin, C.; Rivoire, M.; Trilling, B.; Jafari, M.; et al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol. Hepatol. 2020, 5, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.P.; STAR-TREC Collaborative. Can we Save the rectum by watchful waiting or TransAnal surgery following (chemo)Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC)? Protocol for the international, multicentre, rolling phase II/III partially randomized patient preference trial evaluating long-course concurrent chemoradio-therapy versus short-course radiotherapy organ preservation approaches. Color. Dis. 2022, 24, 639–651. [Google Scholar]
- OncoDaily. STAR-TREC Study Shows successful Organ Preservation with Chemoradiotherapy or Short-Course Radiotherapy, Reducing the Need for Surgery in Early-Intermediate Stage Rectal Cancer Patients. Available online: https://oncodaily.com/societies/star-trec-estro-2025-press-release (accessed on 25 July 2025).
- Jensen, L.; Poulsen, L.; Risum, S.; Nielsen, J.; Mynster, T.; Ploeen, J.; Rahr, H.; Havelund, B.; Appelt, A.; Lindebjerg, J.; et al. 400MO Curative chemoradiation for low rectal cancer: Early clinical outcomes from a multicentre phase II trial. Ann. Oncol. 2020, 31, S411. [Google Scholar] [CrossRef]
- Arp, D.T.; Appelt, A.L.; Jensen, L.H.; Havelund, B.M.; Nissen, H.D.; Risumlund, S.L.; Sjölin, M.E.E.; Nielsen, M.S.; Poulsen, L.Ø. Treatment planning for patients with low rectal cancer in a multicenter prospective organ preservation study. Phys. Medica 2024, 118, 103206. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.-P.; Barbet, N.; Schiappa, R.; Magné, N.; Martel, I.; Mineur, L.; Deberne, M.; Zilli, T.; Dhadda, A.; Myint, A.S. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact x-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2–cT3 rectal adenocarcinoma (OPERA): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023, 8, 356–367. [Google Scholar] [CrossRef]
- Benezery, K.; Montagne, L.; Evesque, L.; Schiappa, R.; Hannoun-Levi, J.-M.; Francois, E.; Thamphya, B.; Gerard, J.-P. Clinical response assessment after contact X-Ray brachytherapy and chemoradiotherapy for organ preservation in rectal cancer T2-T3 M0: The time/dose factor influence. Clin. Transl. Radiat. Oncol. 2020, 24, 92–98. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Leng, B.; Zhu, K.; Jiang, S.; Feng, R.; Dou, X.; Shi, F.; Xu, L.; Yue, J. Efficacy and safety of MR-guided adaptive simultaneous integrated boost radiotherapy to primary lesions and positive lymph nodes in the neoadjuvant treatment of locally advanced rectal cancer: A randomized controlled phase III trial. Radiat. Oncol. 2024, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, P.; Shi, L.; Cui, L.; Gao, J.; Li, W.; Wei, H.; Cheng, L.; Huang, Z.; Cai, G.; et al. Neoadjuvant Modified Infusional Fluorouracil, Leucovorin, and Oxaliplatin With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Updated Results of the FOWARC Study After a Median Follow-Up of 10 Years. J. Clin. Oncol. 2025, 43, 633–640. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef]
- Basch, E.; Dueck, A.C.; Mitchell, S.A.; Mamon, H.; Weiser, M.; Saltz, L.; Gollub, M.; Rogak, L.; Ginos, B.; Mazza, G.L.; et al. Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048). J. Clin. Oncol. 2023, 41, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.-J.; Wang, X.-Z.; Li, Y.-F.; Sun, Y.-M.; Yang, C.-K.; Lin, J.-Z.; Wu, Z.-G.; Zhang, R.; Wang, W.; Li, Y.; et al. Neoadjuvant Chemotherapy with CAPOX Versus Chemoradiation for Locally Advanced Rectal Cancer with Uninvolved Mesorectal Fascia (CONVERT): Initial Results of a Phase III Trial. Ann. Surg. 2022, 277, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.-R.; Wang, X.-Z.; Li, Y.-F.; Sun, Y.-M.; Yang, C.-K.; Wu, Z.-G.; Zhang, R.; Wang, W.; Zhuang, Y.-Z.; Lei, J.; et al. LBA26 Neoadjuvant chemotherapy with CAPOX versus chemoradiation for locally advanced rectal cancer with uninvolved mesorectal fascia (CONVERT): Final results of a phase III trial. Ann. Oncol. 2023, 34, S1267–S1268. [Google Scholar] [CrossRef]
- Ruppert, R.; Kube, R.; Strassburg, J.; Lewin, A.; Baral, J.; Maurer, C.A.; Sauer, J.; Junginger, T.; Hermanek, P.; Merkel, S.; et al. Avoidance of Overtreatment of Rectal Cancer by Selective Chemoradiotherapy: Results of the Optimized Surgery and MRI-Based Multimodal Therapy Trial. J. Am. Coll. Surg. 2020, 231, 413–425e2. [Google Scholar] [CrossRef]
- Group, M.S. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. Br. Med. J. 2006, 333, 779. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.D.; Simunovic, M.; Jhaveri, K.; Kirsch, R.; Brierley, J.; Drolet, S.; Brown, C.; Vos, P.M.; Xiong, W.; MacLean, T.; et al. Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients with “Good Prognosis” Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol. 2019, 5, 961–966. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.-T.; Liu, C.-C.; Wang, L.-H.; Ju, H.-X.; Huang, X.-F.; Chi, P.; Du, J.-L.; Wang, J.-P.; Xiao, Y.; et al. Primary Surgery Followed by Selective Chemoradiotherapy Versus Preoperative Chemoradiotherapy Followed by Surgery for Locally Advanced Rectal Cancer: A Randomized Clinical Trial. Int. J. Radiat. Oncol. 2024, 119, 884–895. [Google Scholar] [CrossRef]
- He, W.; Huang, J.; Liao, G.; Li, W.; Shen, J.; Fu, Y.; Tang, N.; He, F.; Zhao, Y.; Liu, Z.; et al. Short-course radiation(SCRT) followed by 6 cycles of cadonilimab plus mFOLFOX6 as neoadjuvant therapy for patients with locally advanced rectal cancer (LARC): A multicenter, single arm, phase II trial (NeoCaCRT). J. Clin. Oncol. 2025, 43. [Google Scholar] [CrossRef]
- Shamseddine, A.; Turfa, R.; Chehade, L.; Zeidan, Y.H.; El Husseini, Z.; Kreidieh, M.; Bouferraa, Y.; Elias, C.; Kattan, J.; Khalifeh, I.; et al. Short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced microsatellite stable rectal adenocarcinoma: The Averectal study. Eur. J. Cancer 2025, 222, 115428. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Wu, F.; Chi, Z.; Xiao, L.; Wang, X.; Liang, Z.; Li, X.; Yu, Q.; Lin, X.; et al. The efficacy and safety of neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer patients: A systematic review. Front. Immunol. 2024, 15, 1392499. [Google Scholar] [CrossRef]
- Thong, D.W.; Chakraborty, P.; Rajan, R.; Theophilus, M. Adjuvant Radiotherapy in Incidental Positive Nodal Disease in Rectal Cancer—A Systemic Review. J. Surg. Oncol. 2025. [Google Scholar] [CrossRef]
- Polamraju, P.; Haque, W.; Verma, V.; Wiederhold, L.; Hatch, S.; Butler, E.B.; Teh, B.S. Adjuvant Management of Pathologic Node–Positive Disease After Definitive Surgery for Clinical T1-2 N0 Rectal Cancer. Clin. Color. Cancer 2018, 17, e519–e530. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, X.; Ding, Y.; Shi, D.; Wu, H.; Cai, S. Is adjuvant radiotherapy warranted in resected pT1-2 node-positive rectal cancer? Radiat. Oncol. 2013, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Akeel, N.; Lan, N.; Stocchi, L.; Costedio, M.M.; Dietz, D.W.; Gorgun, E.; Kalady, M.F.; Karagkounis, G.; Kessler, H.; Remzi, F.H. Clinically Node Negative, Pathologically Node Positive Rectal Cancer Patients Who Did Not Receive Neoad-juvant Therapy. J. Gastrointest. Surg. 2017, 21, 49–55. [Google Scholar] [CrossRef]
- Huh, J.W.; Lim, S.W.; Kim, H.R.; Kim, Y.J. Effects of Postoperative Adjuvant Radiotherapy on Recurrence and Survival in Stage III Rectal Cancer. J. Gastrointest. Surg. 2011, 15, 963–970. [Google Scholar] [CrossRef]
- Kariv, Y.; Kariv, R.; Hammel, J.P.; Lavery, I.C. Postoperative Radiotherapy for Stage IIIA Rectal Cancer: Is It Justified? Dis. Colon Rectum 2008, 51, 1459–1466. [Google Scholar] [CrossRef]
- Komori, K.; Kimura, K.; Kinoshita, T.; Sano, T.; Ito, S.; Abe, T.; Senda, Y.; Misawa, K.; Ito, Y.; Uemura, N.; et al. Complications Associated with Postoperative Adjuvant Radiation Therapy for Advanced Rectal Cancer. Int. Surg. 2014, 99, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Pilar, A.; Gupta, M.; Laskar, S.G.; Laskar, S. Intraoperative radiotherapy: Review of techniques and results. ecancermedicalscience 2017, 11, 750. [Google Scholar] [CrossRef]
- Haddock, M.G. Intraoperative radiation therapy for colon and rectal cancers: A clinical review. Radiat. Oncol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Amarnath, S.R. The Role of Intraoperative Radiotherapy Treatment of Locally Advanced Rectal Cancer. Clin. Colon Rectal Surg. 2023, 37, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ge, L.; Wang, J.; Chen, Y.-Q.; Ma, S.-X.; Ma, P.-L.; Zhang, Y.-Q.; Yang, K.-H.; Cai, H. Efficacy and safety of intraoperative radiotherapy in rectal cancer: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2020, 13, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.C.; Joshi, N.; Vicini, F.; Chang, A.J.; Hong, T.S.; Showalter, T.N.; Chao, S.T.; Wolden, S.; Wu, A.J.; Martin, D.; et al. The American Brachytherapy Society consensus statement on intraoperative radiation therapy. Brachytherapy 2019, 18, 242–257. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sole, C.V.; Rutten, H.J.; Poortmans, P.; Asencio, J.M.; Serrano, J.; Aristu, J.; Roeder, F.; Dries, W.J. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in primary locally advanced rectal cancer. Clin. Transl. Radiat. Oncol. 2020, 25, 29–36. [Google Scholar] [CrossRef]

| Trial | STELLAR | RAPIDO | PRODIGE-23 | TNTCRT |
|---|---|---|---|---|
| Eligibility | cT3 or cT4 and/or N+, M0, tumor located in the distal or middle third of the rectum | cT4a,b, extramural invasion, cN2 disease, enlarged lateral lymph nodes, or involved mesorectal fascia | cT3 with a risk of local recurrence or cT4 resectable rectal cancer with no metastasis | cT4a,b that is resectable, cT3c,d with extramural venous invasion, cN2, involved mesorectal fascia, or enlarged lateral lymph nodes |
| Experimental group | SCRT → 4^CAPOX → TME → 2^CAPOX | SCRT → 6^CAPOX or 9^FOLFOX → TME | 6^FOLFIRINOX → CRT → TME → 6^FOLFOX6 or 4 capecitabine | LCRT → 6^CAPOX → TME |
| Control group | LCRT → TME → 6^CAPOX | LCRT → TME → +/− 8^CAPOX or 12^FOLFOX4 | LCRT → TME → 12^FOLFOX6 or 8^ capecitabine | LCRT → TME → adjuvant CAPOX |
| Primary outcome | Disease-free survival | Disease-related treatment failure | Disease-free survival | Disease-free survival |
| Median follow-up | 2.9 years | 5.6 years | 6.9 years | 3.7 years |
| Outcomes at 3 years (experimental vs. control) | ||||
| DFS % | 64.5 vs. 62.3 * | 76 vs. 69 * | 77 vs. 67.9 * | |
| DrTF % | 23.7 vs. 30.4 * | |||
| OS % | 86.5 vs. 75.1 * | 89.1 vs. 88.8 | 91 vs. 88 | 90.3 vs. 87.9 |
| LRF % | 8.4 vs. 11 | 8.3 vs. 6 | 4 vs. 6 | |
| MFS % | 77.1 vs. 75.3 | 20 vs. 26.8 *a | 79 vs. 72 * | 83 vs. 74.2 * |
| pCR % | 21.8 vs. 12.3 * | 28 vs. 14 * | 28 vs. 12 * | 27.5 vs. 9.9 * |
| Outcomes at 5 years (experimental vs. control) | ||||
| DrTF % | 27.8 vs. 34 * | |||
| OS % | 81.7 vs. 80.2 | |||
| LRF % | 11.7 vs. 8.1 (10.2 vs. 6.1 *†) | |||
| DM % | 23 vs. 30.4 * | |||
| Outcomes at 7 years (Experimental vs. Control) | ||||
| DFS % | 67.6 vs. 62.5 * | |||
| OS % | 81.9 vs. 76.1 * | |||
| LRF % | 5.3 vs. 8.1 | |||
| MFS % | 79.2 vs. 72.3 * | |||
| Trial | CAO/ARO/AIO-12 |
|---|---|
| Eligibility | cT3–T4 and/or N+ in the middle or lower third of the rectum, where cT3 in the middle third should be >cT3b |
| Group A (induction chemotherapy) | 3^FOLFOX → CRT → TME |
| Group B (consolidation chemotherapy) | CRT → 3^FOLFOX → TME |
| Primary outcome | Pathologic complete response |
| Median follow-up | 3.6 years |
| Outcomes at 3 years (induction vs. consolidation) | |
| pCR % | 17 vs. 25 * |
| DFS % | 73 vs. 73 (p-value = 0.82) |
| OS % | 92 vs. 92 (p-value = 0.81) |
| LRR % | 6 vs. 5 (p-value = 0.67) |
| DM % | 18 vs. 16 (p-value = 0.52) |
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| CEA | q3 months | q3 months | q3 months | q6 months | q6 months |
| DRE, endoscopy, and pelvic MRI | q3–4 months | q3–4 months | q6 months | q6 months | q6 months |
| Chest and/or abdominal CT | q6–12 months | q1 year | q1 year | q1 year | q1 year |
| Complete Response | Near-Complete Response | Incomplete Response | |
|---|---|---|---|
| Endoscopy | Flat, white scar Telangiectasia No ulcer No nodularity | Irregular mucosa Small mucosal nodules or minor mucosal abnormality Superficial ulceration Mild persisting erythema of the scar | Visible tumor |
| Digital Rectal Exam | Normal | Smooth induration or minor mucosal abnormalities | Palpable tumor nodules |
| MRI-T2W | Only a dark T2 signal, no intermediate T2 signal AND No visible lymph nodes | Mostly a dark T2 signal, some remaining intermediate signal AND/OR Partial regression of the lymph nodes | More intermediate than a dark T2 signal, no T2 scar AND/OR No regression of the lymph nodes |
| MRI-DW | No visible tumor on B800–B1000 signal AND/OR Lack of a signal or a low signal in ADC map A uniform, linear signal in the wall above the tumor is okay | Significant regression of the signal on B800–B1000 AND/OR Minimal or low residual signal in ADC map | Insignificant regression of signal on B800–B1000 AND/OR Obvious low signal in ADC map |
| Trial | OPRA |
|---|---|
| Eligibility | cT3–T4 and/or cN+ |
| Group A (induction chemotherapy) | 8^FOLFOX or 5^CAPEOX → CRT → response-guided TME or WW |
| Group B (consolidation chemotherapy) | CRT → 8^FOLFOX or 5^CAPEOX → response-guided TME or WW |
| Primary outcome | Disease-free survival |
| Median follow-up | 5.1 years |
| Outcomes at 3 years (induction vs. consolidation) | |
| DFS % | 76 vs. 76 |
| TME-free survival % | 47 vs. 60 * |
| LRFS % | 94 vs. 94 |
| DMFS % | 84 vs. 82 |
| Outcomes at 5 years (induction vs. consolidation) | |
| DFS % | 71 vs. 69 |
| TME-free survival % | 39 vs. 54 * |
| OS % | 88 vs. 85 |
| LRFS % | 94 vs. 90 |
| DMFS % | 80 vs. 78 |
| CCR | NCR | ICR | ||
|---|---|---|---|---|
| Outcomes at 3 Years | ||||
| OP % | 77 | 40 | - | p-value < 0.01 |
| DFS % | 88 | 69 | 56 | p-value < 0.001 |
| LE Group | TME Group | |
|---|---|---|
| Outcomes at 5 Years | ||
| LR % | 7 | 7 * |
| DM % | 18 | 19 * |
| OS % | 84 | 82 * |
| DFS % | 70 | 72 * |
| Cancer-specific mortality % | 7 | 10 * |
| OPERA Trial | Group A (External Beam Radiotherapy Group) | Group B (Contact X-Ray Brachytherapy Group) |
|---|---|---|
| CRT → EBRT Boost → Response-Guided TME or LE | (CRT → CXB Boost → Response-Guided TME or LE) † | |
| Outcomes at 3 years | ||
| Overall OP % | 59 | 81 * |
| Tumors < 3 cm OP % | 63 | 97 * |
| Tumors ≥ 3 cm OP % | 55 | 68 |
| Early toxicity grade 2–3% | 36 | 44 |
| Late toxicity grade 1–2% | 11.6 | 62.7 * |
| Trial | FOWARC |
|---|---|
| Eligibility | Stage II/III |
| Group 1 | 5^fluorouracil → radiotherapy → TME → 7^fluorouracil |
| Group 2 | 5^mFOLFOX6 → radiotherapy → TME → 6–8^mFOLFOX6 |
| Group 3 | 4–6^mFOLFOX6 → TME → 6–8^mFOLFOX6 |
| Primary outcome | Disease-free survival |
| Median follow-up | 10 years |
| Outcomes at 3 years (group 1 vs. 2 vs. 3) | |
| DFS % | 72.9 vs. 77.2 vs. 73.5 * |
| LR % | 8.0 vs. 7.0 vs. 8.3 * |
| OS % | 91.3 vs. 89.1 vs. 90.7 * |
| Outcomes at 5 years (group 1 vs. 2 vs. 3) | |
| DFS % | 52.5 vs. 62.6 vs. 60.5 * |
| LR % | 10.8 vs. 8.0 vs. 9.6 * |
| OS % | 65.9 vs. 72.3 vs. 73.4 * |
| PROSPECT | CONVERT | OCUM | PSSR | |
|---|---|---|---|---|
| Eligibility | cT2N+, cT3N−/+ rectal cancer, ≤4 enlarged lymph nodes, no threatened CRM | cT3–T4a and/or N+ rectal cancer with no MRF involvement | cT2–T4, any cN, cM0, rectal cancer undergoing elective surgery with curative intent (R0, R1) | cT3–T4 and/or N+, tumor 6 to 12 cm from the anal verge, and MRI-proven MRF > 1 mm |
| Experimental group | 6^FOLFOX → response-guided CRT → TME → +/− 6^FOLFOX or CAPOX | 4^CAPOX → TME → 4^CAPOX | (Primary TME) a | (Primary TME) c |
| Control group | CRT → TME → +/− 8^FOLFOX or CAPOX | CRT → TME → 6^CAPOX | (CRT → TME) b | CRT → TME → 5^capecitabine |
| Primary outcome | Disease-free survival | Locoregional recurrence free survival | Local recurrence | Disease-free survival |
| Median follow-up | 4.8 years | 4 years | 5 years | 2.9 years |
| Outcomes at 3 years (experimental vs. control) | ||||
| LRFS % | 97.4 vs. 96.3 | |||
| OS % | 94.1 vs. 95 | |||
| DFS % | 89.2 vs. 87.9 | 81.82 vs. 85.37 (20 vs. 11.11 *) ‡ | ||
| LR % | 2.2 vs. 4.3 * (3.6 vs. 4.2) † | 4.29 vs. 0 * | ||
| DM % | 12.5 vs. 23.7 * | 12.14 vs. 10.37 | ||
| pCRM negative % | 97.9 vs. 91.5 * | |||
| pCR | 11.0 vs 13.8 | |||
| Outcomes at 5 years (experimental vs. control) | ||||
| DFS % | 80.8 vs. 78.6 | |||
| OS % | 89.5 vs. 90.2 | |||
| LRFS % | 98.2 vs. 98.4 | |||
| pCR % | 21.9 vs. 24.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dandash, Z.; Mobayed, T.; Temraz, S.; Shamseddine, A.; Doughan, S.; Deeba, S.; Ayoub, Z.; Eid, T.; Youssef, B.; Hilal, L. A Review on the Evolving Role of Radiation Therapy in the Treatment of Locally Advanced Rectal Cancer. Curr. Oncol. 2025, 32, 443. https://doi.org/10.3390/curroncol32080443
Dandash Z, Mobayed T, Temraz S, Shamseddine A, Doughan S, Deeba S, Ayoub Z, Eid T, Youssef B, Hilal L. A Review on the Evolving Role of Radiation Therapy in the Treatment of Locally Advanced Rectal Cancer. Current Oncology. 2025; 32(8):443. https://doi.org/10.3390/curroncol32080443
Chicago/Turabian StyleDandash, Zeinab, Tala Mobayed, Sally Temraz, Ali Shamseddine, Samer Doughan, Samer Deeba, Zeina Ayoub, Toufic Eid, Bassem Youssef, and Lara Hilal. 2025. "A Review on the Evolving Role of Radiation Therapy in the Treatment of Locally Advanced Rectal Cancer" Current Oncology 32, no. 8: 443. https://doi.org/10.3390/curroncol32080443
APA StyleDandash, Z., Mobayed, T., Temraz, S., Shamseddine, A., Doughan, S., Deeba, S., Ayoub, Z., Eid, T., Youssef, B., & Hilal, L. (2025). A Review on the Evolving Role of Radiation Therapy in the Treatment of Locally Advanced Rectal Cancer. Current Oncology, 32(8), 443. https://doi.org/10.3390/curroncol32080443








