Survival Outcomes in Patients with Squamous Cell Carcinoma of the Urinary Bladder: A Propensity Score-Matched Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Histopathological Evaluation
2.4. Data Collection
2.5. Survival Definitons
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rumgay, H.; Li, M.; Yu, H.; Pan, H.; Ni, J. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J. Glob. Health 2023, 13, 04109. [Google Scholar] [CrossRef] [PubMed]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef]
- Amin, H.A.A.; Kobaisi, M.H.; Samir, R.M. Schistosomiasis and Bladder Cancer in Egypt: Truths and Myths. Open Access Maced. J. Med. Sci. 2019, 7, 4023–4029. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L.; Chen, Y.; Hou, J. The prognostic value of histological subtype in patients with metastatic bladder cancer. Oncotarget 2017, 8, 28408–28417. [Google Scholar] [CrossRef]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor, J.A., 3rd; et al. What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur. Urol. Focus 2020, 6, 653–663. [Google Scholar] [CrossRef]
- Martin, J.W.; Jefferson, F.A.; Huang, M.; Sung, J.M.; Chang, J.; Piranviseh, K.; Ziogas, A.; Anton-Culver, H.; Youssef, R.F. A California Cancer Registry Analysis of Urothelial and Non-urothelial Bladder Cancer Subtypes: Epidemiology, Treatment, and Survival. Clin. Genitourin. Cancer 2020, 18, e330–e336. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.; Martin, T.; Stolzenbach, F.; Rosiello, G.; Collà Ruvolo, C.; Nocera, L.; Tian, Z.; Becker, A.; Kluth, L.; Roos, F.C.; et al. Bladder Cancer: A Comparison Between Non-urothelial Variant Histology and Urothelial Carcinoma Across All Stages and Treatment Modalities. Clin. Genitourin. Cancer 2021, 19, 60–68.e1. [Google Scholar] [CrossRef]
- Bell, S.D.; Quinn, A.E.; Bajo, A.; Mayberry, T.G.; Cowan, B.C.; Marrah, A.J.; Wakefield, M.R.; Fang, Y. Squamous Cell Bladder Cancer: A Rare Histological Variant with a Demand for Modern Cancer Therapeutics. Cancers 2025, 17, 169. [Google Scholar] [CrossRef]
- Gouda, I.; Mokhtar, N.; Bilal, D.; El-Bolkainy, T.; El-Bolkainy, N.M. Bilharziasis and bladder cancer: A time trend analysis of 9843 patients. J. Egypt Natl. Cancer Inst. 2007, 19, 158–162. [Google Scholar]
- Gellert, L.L.; Warrick, J.; Al-Ahmadie, H.A. Urothelial carcinoma with squamous differentiation—The pathologists’ perspective. Urol. Oncol. 2015, 33, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Rostom, M.; Alam, R.; Florissi, I.; Biles, M.; Rodriguez, K.; Hahn, N.M.; Johnson, B.A., 3rd; Matoso, A.; Smith, A.; et al. Clinicopathologic and Survival After Cystectomy Outcomes in Squamous Cell Carcinoma of the Bladder. Clin. Genitourin. Cancer 2023, 21, 631–638.e1. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, W.; Spiess, P.E.; Siefker-Radtke, A.; Swanson, D.; Grossman, H.B.; Kamat, A.M.; Munsell, M.F.; Guo, C.C.; Czerniak, B.A.; Dinney, C.P. Outcome and patterns of recurrence of nonbilharzial pure squamous cell carcinoma of the bladder: A contemporary review of The University of Texas M D Anderson Cancer Center experience. Cancer 2007, 110, 764–769. [Google Scholar] [CrossRef] [PubMed]
- El-Sebaie, M.; Zaghloul, M.S.; Howard, G.; Mokhtar, A. Squamous cell carcinoma of the bilharzial and non-bilharzial urinary bladder: A review of etiological features, natural history, and management. Int. J. Clin. Oncol. 2005, 10, 20–25. [Google Scholar] [CrossRef]
- Matulay, J.T.; Woldu, S.L.; Lim, A.; Narayan, V.M.; Li, G.; Kamat, A.M.; Anderson, C.B. The impact of squamous histology on survival in patients with muscle-invasive bladder cancer. Urol. Oncol. 2019, 37, 353.e17–353.e24. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Michalski, J.M.; Contreras, J.A.; Brenneman, R.; Christodouleas, J.P.; Abraham, C.D.; Kim, E.H.; Arora, V.K.; Bullock, A.D.; Carmona, R.; et al. A propensity analysis comparing definitive chemo-radiotherapy for muscle-invasive squamous cell carcinoma of the bladder vs. urothelial carcinoma of the bladder using the National Cancer Database. Clin. Transl. Radiat. Oncol. 2018, 15, 38–41. [Google Scholar] [CrossRef]
- Balci, U.; Ozer, K.; Gorgel, S.N.; Sefik, E.; Girgin, C.; Dincel, C. Do pure squamous cell carcinomas and urothelial carcinomas have similar prognosis after radical cystectomy? World J. Urol. 2013, 31, 1177–1182. [Google Scholar] [CrossRef]
- Kastritis, E.; Dimopoulos, M.A.; Antoniou, N.; Deliveliotis, C.; Chrisofos, M.; Skolarikos, A.; Gika, D.; Bamias, A. The outcome of patients with advanced pure squamous or mixed squamous and transitional urothelial carcinomas following platinum-based chemotherapy. Anticancer Res. 2006, 26, 3865–3869. [Google Scholar]
- Scosyrev, E.; Yao, J.; Messing, E. Urothelial carcinoma versus squamous cell carcinoma of bladder: Is survival different with stage adjustment? Urology 2009, 73, 822–827. [Google Scholar] [CrossRef]
- Shah, R.B.; Montgomery, J.S.; Montie, J.E.; Kunju, L.P. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: Impact of mandatory central pathology review at a large referral hospital. Urol. Oncol. 2013, 31, 1650–1655. [Google Scholar] [CrossRef]
- Weyerer, V.; Stoehr, R.; Bertz, S.; Lange, F.; Geppert, C.I.; Wach, S.; Taubert, H.; Sikic, D.; Wullich, B.; Hartmann, A.; et al. Prognostic impact of molecular muscle-invasive bladder cancer subtyping approaches and correlations with variant histology in a population-based mono-institutional cystectomy cohort. World J. Urol. 2021, 39, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Sangoi, A.R.; Beck, A.H.; Amin, M.B.; Cheng, L.; Epstein, J.I.; Hansel, D.E.; Iczkowski, K.A.; Lopez-Beltran, A.; Oliva, E.; Paner, G.P.; et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am. J. Surg. Pathol. 2010, 34, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Wood, A.; Michael, P.D.; Shin, D.; Pramod, N.; Haywood, S.C.; Eltemamy, M.; Weight, C.; Haber, G.P.; Lee, B.; et al. Impact of pathologic re-review on grade, clinical stage, and risk stratification for patients with nonmuscle invasive bladder cancer. Urol. Oncol. 2024, 42, 372.e21–372.e27. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Boorjian, S.A.; Cheville, J.C.; Sukov, W.R.; Thapa, P.; Tarrell, R.F.; Frank, I. The impact of histological reclassification during pathology re-review--evidence of a Will Rogers effect in bladder cancer? J. Urol. 2013, 190, 1692–1696. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaborators Group. Adjuvant Chemotherapy for Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis of Individual Participant Data from Randomised Controlled Trials. Eur. Urol. 2022, 81, 50–61. [Google Scholar] [CrossRef]
- Koehne, E.L.; Bakaloudi, D.R.; Ghali, F.; Nyame, Y.; Schade, G.R.; Grivas, P.; Yezefski, T.A.; Hawley, J.E.; Yu, E.Y.; Hsieh, A.C.; et al. Adjuvant Chemotherapy and Survival After Radical Cystectomy in Histologic Subtype Bladder Cancer. Clin. Genitourin. Cancer 2024, 22, 102100. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Huang, H.; Li, H.; Wen, Z.; Liu, Y.; Yang, X.S. The role of adjuvant chemotherapy after radical surgery in patients with lymph node-positive bladder cancer or locally advanced (pT3, pT4a) bladder cancer: A meta-analysis and systematic review. Int. J. Surg. 2024, 110, 7268–7280. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.Y.; Jung, J.H.; Hah, Y.S.; Cho, K.S. Role of adjuvant cisplatin-based chemotherapy following radical cystectomy in locally advanced muscle-invasive bladder cancer: Systematic review and meta-analysis of randomized trials. Investig. Clin. Urol. 2019, 60, 64–74. [Google Scholar] [CrossRef]
- Zhao, C.; Li, K.; Zhu, M.; Wang, F.; Zhang, K.; Fan, C.; Wang, J. The impact of patient sex on characteristic-adjusted bladder cancer prognosis. J. Cancer Res. Ther. 2021, 17, 1241–1247. [Google Scholar] [CrossRef]
- Mori, K.; Yanagisawa, T.; Katayama, S.; Laukhtina, E.; Pradere, B.; Mostafaei, H.; Quhal, F.; Rajwa, P.; Moschini, M.; Soria, F.; et al. Impact of sex on outcomes after surgery for non-muscle-invasive and muscle-invasive bladder urothelial carcinoma: A systematic review and meta-analysis. World J. Urol. 2023, 41, 909–919. [Google Scholar] [CrossRef]
- Mun, D.H.; Kimura, S.; Shariat, S.F.; Abufaraj, M. The impact of gender on oncologic outcomes of bladder cancer. Curr. Opin. Urol. 2019, 29, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Sjödahl, G.; Abrahamsson, J.; Holmsten, K.; Bernardo, C.; Chebil, G.; Eriksson, P.; Johansson, I.; Kollberg, P.; Lindh, C.; Lövgren, K.; et al. Different Responses to Neoadjuvant Chemotherapy in Urothelial Carcinoma Molecular Subtypes. Eur. Urol. 2022, 81, 523–532. [Google Scholar] [CrossRef]
- Majumdar, S.; Gong, E.M.; Di Vizio, D.; Dreyfuss, J.; Degraff, D.J.; Hager, M.H.; Park, P.J.; Bellmunt, J.; Matusik, R.J.; Rosenberg, J.E.; et al. Loss of Sh3gl2/endophilin A1 is a common event in urothelial carcinoma that promotes malignant behavior. Neoplasia 2013, 15, 749–760. [Google Scholar] [CrossRef]
- Adam, R.M.; DeGraff, D.J. Molecular mechanisms of squamous differentiation in urothelial cell carcinoma: A paradigm for molecular subtyping of urothelial cell carcinoma of the bladder. Urol. Oncol. 2015, 33, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Narita, S.; Matsui, Y.; Kanda, S.; Hidaka, Y.; Abe, H.; Tsuzuki, T.; Ito, K.; Kojima, T.; Kato, M.; et al. Impact of histological variants on outcomes in patients with urothelial carcinoma treated with pembrolizumab: A propensity score matching analysis. BJU Int. 2022, 130, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Minato, A.; Furubayashi, N.; Harada, M.; Negishi, T.; Sakamoto, N.; Song, Y.; Hori, Y.; Tomoda, T.; Tamura, S.; Kuroiwa, K.; et al. Efficacy of Pembrolizumab in Patients with Variant Urothelial Carcinoma: A Multicenter Retrospective Study. Clin. Genitourin. Cancer 2022, 20, 499.e1–499.e8. [Google Scholar] [CrossRef]
- Jindal, T.; Zhang, L.; Deshmukh, P.; Reyes, K.; Chan, E.; Kumar, V.; Zhu, X.; Maldonado, E.; Feng, S.; Johnson, M.; et al. Impact of Squamous Histology on Clinical Outcomes and Molecular Profiling in Metastatic Urothelial Carcinoma Patients Treated with Immune Checkpoint Inhibitors or Enfortumab Vedotin. Clin. Genitourin. Cancer 2023, 21, e394–e404. [Google Scholar] [CrossRef]
- Minato, A.; Furubayashi, N.; Nagata, Y.; Tomoda, T.; Masaoka, H.; Song, Y.; Hori, Y.; Kiyoshima, K.; Negishi, T.; Kuroiwa, K.; et al. Prognostic Impact of Histologic Subtype and Divergent Differentiation in Patients with Metastatic Urothelial Carcinoma Treated with Enfortumab Vedotin: A Multicenter Retrospective Study. Curr. Oncol. 2024, 31, 862–871. [Google Scholar] [CrossRef]

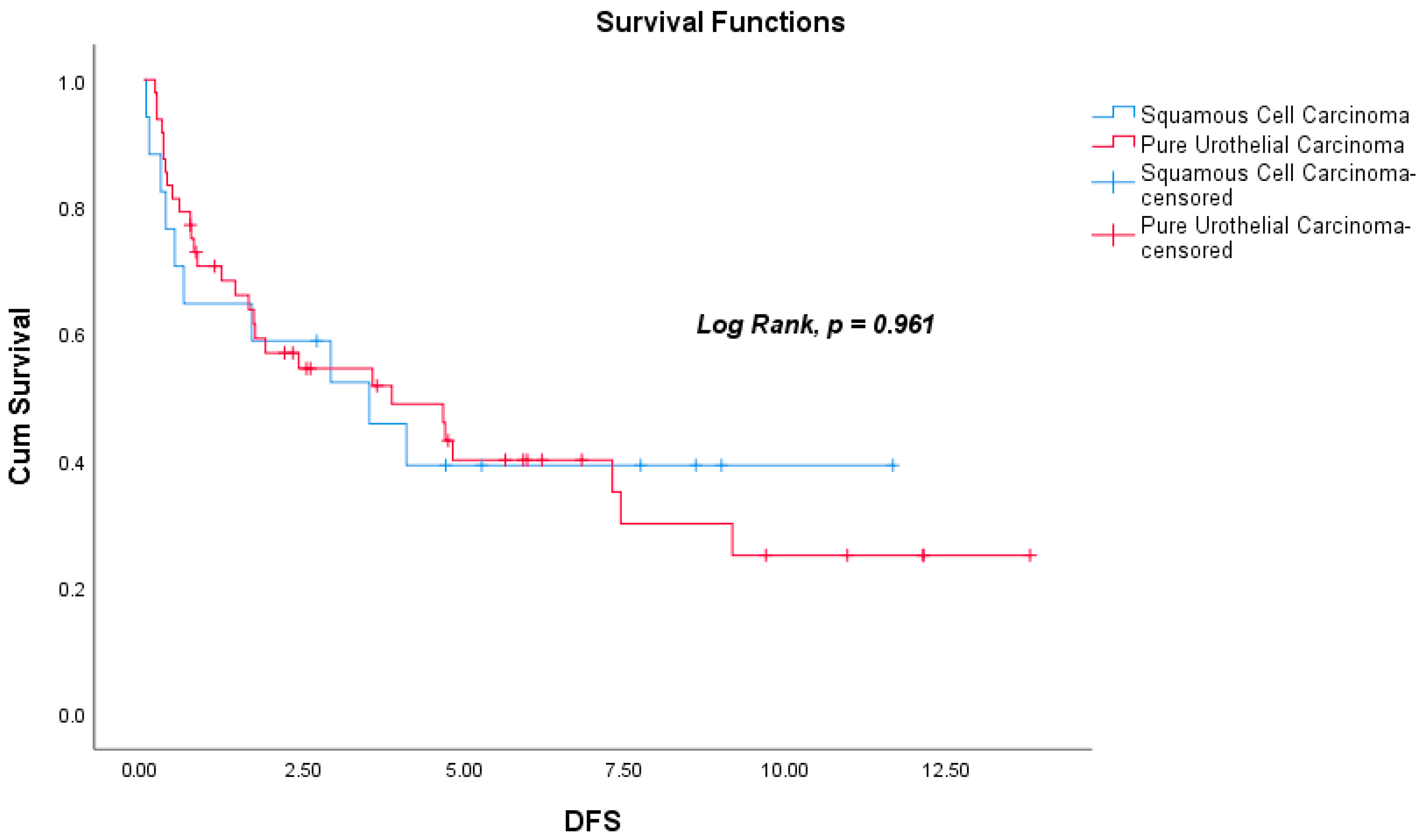
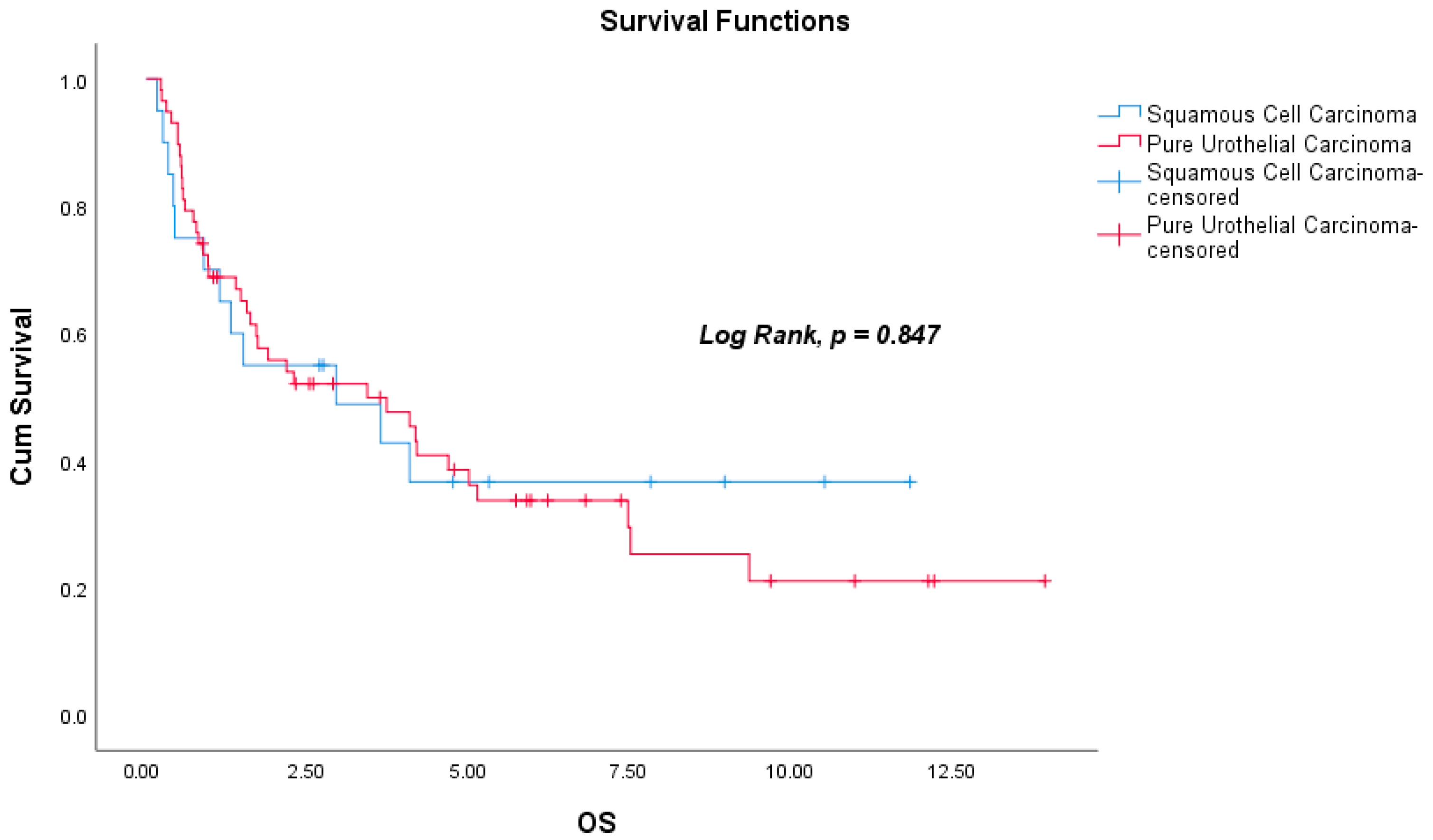
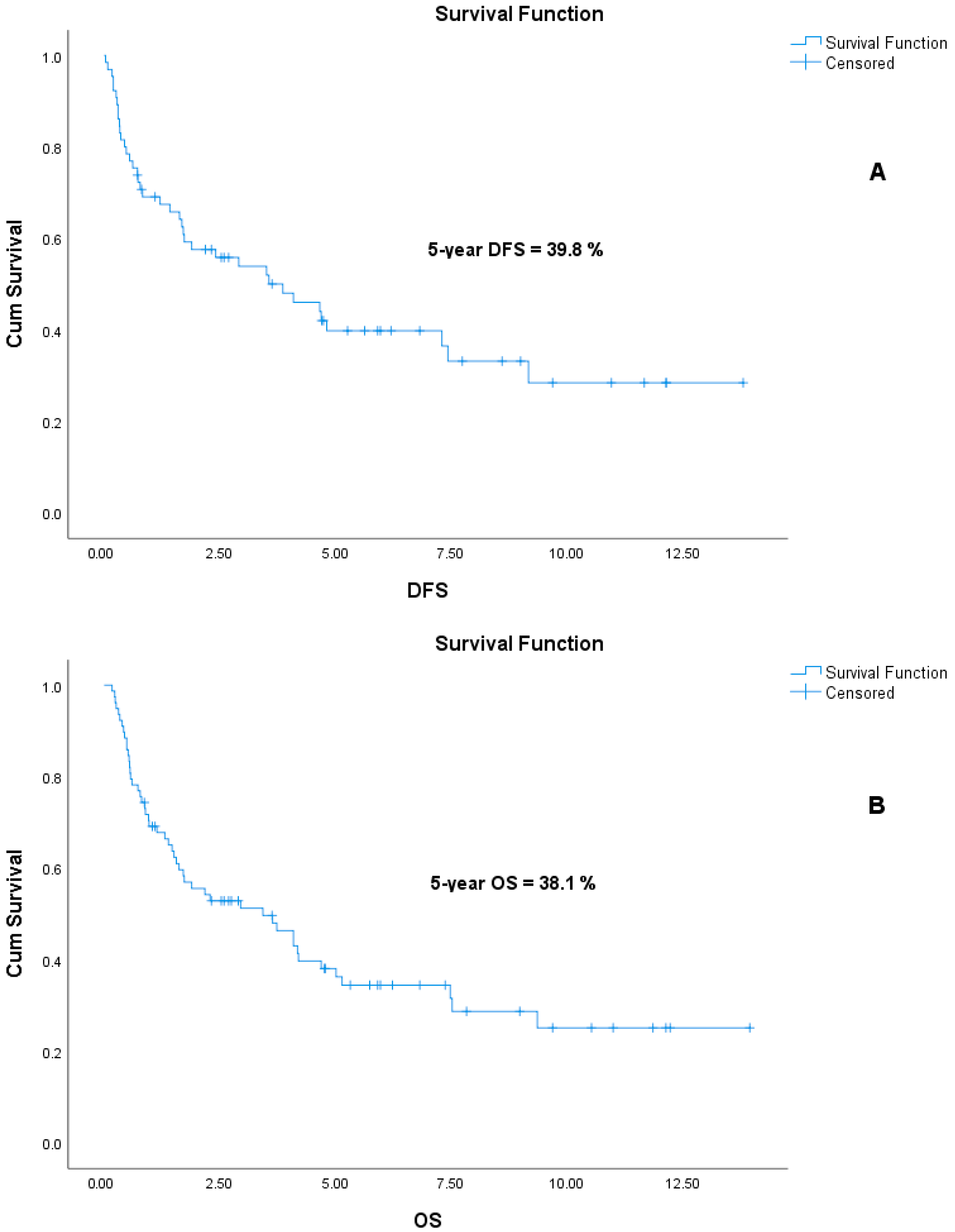
| Characteristics (N = 78) | SqCC (n = 20) | PUC (n = 58) | p |
|---|---|---|---|
| Age, Median (Min–Max) | 65.0 (53.0–84.0) | 66.5 (48.0–90.0) | 0.948 * |
| Gender | 0.853 ** | ||
| Male | 18 (90%) | 53 (91.4%) | |
| Female | 2 (10%) | 5 (8.6%) | |
| Smoking Status | 0.846 ** | ||
| Yes | 6 (30%) | 21 (36.2%) | |
| No | 1 (5%) | 5 (8.6%) | |
| Smoking (pack/year) (mean ± SD) | 42.6 ± 16.6 | 37.0 ± 22.4 | 0.613 * |
| Diagnosis T Stage | 0.080 ** | ||
| T1-T2 a | 9 (45%) | 14 (24.1%) | |
| T3-T4 b | 11 (55%) | 44 (75.9%) | |
| Diagnosis N Stage | 0.954 ** | ||
| Node negative | 14 (70%) | 41 (70.7%) | |
| Node positive | 6 (30%) | 17 (29.3%) | |
| Diagnosis M Stage | 0.818 ** | ||
| M0 | 17 (85%) | 48 (82.8%) | |
| M1 | 3 (15%) | 10 (17.2%) | |
| Diagnosis TNM Stage | 0.856 ** | ||
| Stage II | 5 (25%) | 14 (24.1%) | |
| Stage III | 12 (60%) | 34 (58.6%) | |
| Stage IV | 3 (15%) | 10 (17.2%) | |
| Neoadjuvant CT | 2 (10%) | 6 (10.3%) | 0.937 ** |
| Primary Surgery Status | 0.976 ** | ||
| Yes | 19 (95%) | 55 (94.8%) | |
| No | 1 (5%) | 3 (5.2%) | |
| Type of Surgery | 0.021 ** | ||
| RC | 16 (80%) | 54 (93.1%) | |
| PC | 3 (15%) | 1 (1.7%) | |
| Adjuvant CT | 5 (25%) | 14 (24.1%) | 0.985 ** |
| Follow-up (year) (mean ± SD) | 3.59 ± 3.60 | 3.51 ± 3.46 | 0.877 ** |
| Factor | DFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | %95 CI | p | Multivariate | %95 CI | p | |||
| HR | Lower | Upper | HR | Lower | Upper | |||
| Age a | 1.292 | 0.641 | 2.607 | 0.474 | ||||
| Gender b | 0.227 | 0.084 | 0.614 | 0.003 | 0.663 | 0.231 | 1.902 | 0.445 * |
| Smoking status c | 0.785 | 0.219 | 2.820 | 0.711 | ||||
| Smoking (pack/year) | 1.006 | 0.975 | 1.038 | 0.713 | ||||
| Pathological subtype d | 1.018 | 0.495 | 2.093 | 0.961 | ||||
| Diagnosis T stage e | 2.059 | 0.975 | 4.351 | 0.058 | 4.026 | 1.719 | 9.428 | 0.001 * |
| Diagnosis nodal status f | 2.039 | 1.028 | 4.046 | 0.042 | 3.128 | 1.462 | 6.691 | 0.003 * |
| Diagnosis TNM stage g | ||||||||
| Stage III | 2.325 | 1.023 | 5.281 | 0.044 | ||||
| Neoadjuvant CT h | 2.423 | 1.063 | 5.524 | 0.035 | ||||
| Neoadjuvant CT Cisplatin status i | 0.712 | 0.156 | 3.241 | 0.660 | ||||
| Neoadjuvant CT Number of cycles | 1.521 | 0.535 | 4.320 | 0.431 | ||||
| Surgery type j | 3.805 | 1.314 | 11.021 | 0.014 | ||||
| Adjuvant CT k | 0.249 | 0.105 | 0.590 | 0.002 | 0.120 | 0.045 | 0.317 | 0.000 * |
| Adjuvant CT cisplatin status l | 0.379 | 0.089 | 1.606 | 0.188 | ||||
| Adjuvant CT number of cycles | 1.022 | 0.590 | 1.771 | 0.938 | ||||
| Factor | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | %95 CI | p | Multivariate | %95 CI | p | |||
| HR | Lower | Upper | HR | Lower | Upper | |||
| Age a | 1.268 | 0.699 | 2.302 | 0.434 | ||||
| Gender b | 0.283 | 0.116 | 0.692 | 0.006 | 0.389 | 0.154 | 0.986 | 0.047 * |
| Smoking status c | 0.853 | 0.246 | 2.958 | 0.802 | ||||
| Smoking (pack/year) | 0.992 | 0.968 | 1.016 | 0.509 | ||||
| Pathological subtype d | 1.066 | 0.557 | 2.042 | 0.847 | ||||
| Diagnosis T stage e | 2.162 | 1.078 | 4.333 | 0.030 | 1.574 | 0.761 | 3.256 | 0.221 * |
| Diagnosis nodal status f | 2.533 | 1.425 | 4.504 | 0.002 | 1.652 | 0.870 | 3.137 | 0.125 * |
| Diagnosis metastasis status g | 4.797 | 2.415 | 9.527 | 0.000 | 3.533 | 1.699 | 7.344 | 0.001 * |
| Diagnosis TNM stage h | 0.000 | |||||||
| Stage III | 2.109 | 0.925 | 4.811 | 0.076 | ||||
| Stage IV | 8.466 | 3.227 | 22.214 | 0.000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coskun, A.; Sahin, A.B.; Kabul, S.; Celik, M.A.; Sali, M.; Ozcelik, E.E.; Deligonul, A.; Cubukcu, E.; Kurt, M.; Savci, G.; et al. Survival Outcomes in Patients with Squamous Cell Carcinoma of the Urinary Bladder: A Propensity Score-Matched Analysis. Curr. Oncol. 2025, 32, 394. https://doi.org/10.3390/curroncol32070394
Coskun A, Sahin AB, Kabul S, Celik MA, Sali M, Ozcelik EE, Deligonul A, Cubukcu E, Kurt M, Savci G, et al. Survival Outcomes in Patients with Squamous Cell Carcinoma of the Urinary Bladder: A Propensity Score-Matched Analysis. Current Oncology. 2025; 32(7):394. https://doi.org/10.3390/curroncol32070394
Chicago/Turabian StyleCoskun, Alper, Ahmet Bilgehan Sahin, Selva Kabul, Muhammed Abdurrahman Celik, Mursel Sali, Ender Eren Ozcelik, Adem Deligonul, Erdem Cubukcu, Meral Kurt, Gursel Savci, and et al. 2025. "Survival Outcomes in Patients with Squamous Cell Carcinoma of the Urinary Bladder: A Propensity Score-Matched Analysis" Current Oncology 32, no. 7: 394. https://doi.org/10.3390/curroncol32070394
APA StyleCoskun, A., Sahin, A. B., Kabul, S., Celik, M. A., Sali, M., Ozcelik, E. E., Deligonul, A., Cubukcu, E., Kurt, M., Savci, G., Evrensel, T., & Yavascaoğlu, I. (2025). Survival Outcomes in Patients with Squamous Cell Carcinoma of the Urinary Bladder: A Propensity Score-Matched Analysis. Current Oncology, 32(7), 394. https://doi.org/10.3390/curroncol32070394





