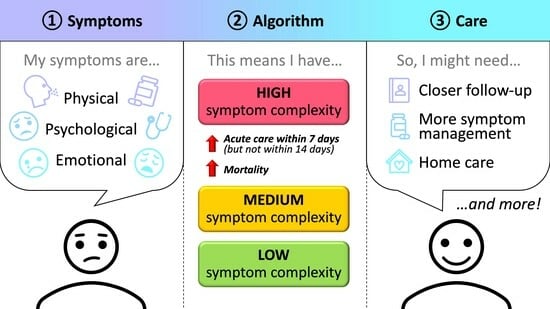
Associations Between Symptom Complexity and Acute Care Utilization Among Adult Advanced Cancer Patients Followed by a Palliative Care Service
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Context
2.2. Study Design and Population
2.3. Exposure and Outcomes
2.4. Covariates
2.5. Statistical Analysis
2.6. Ethics
3. Results
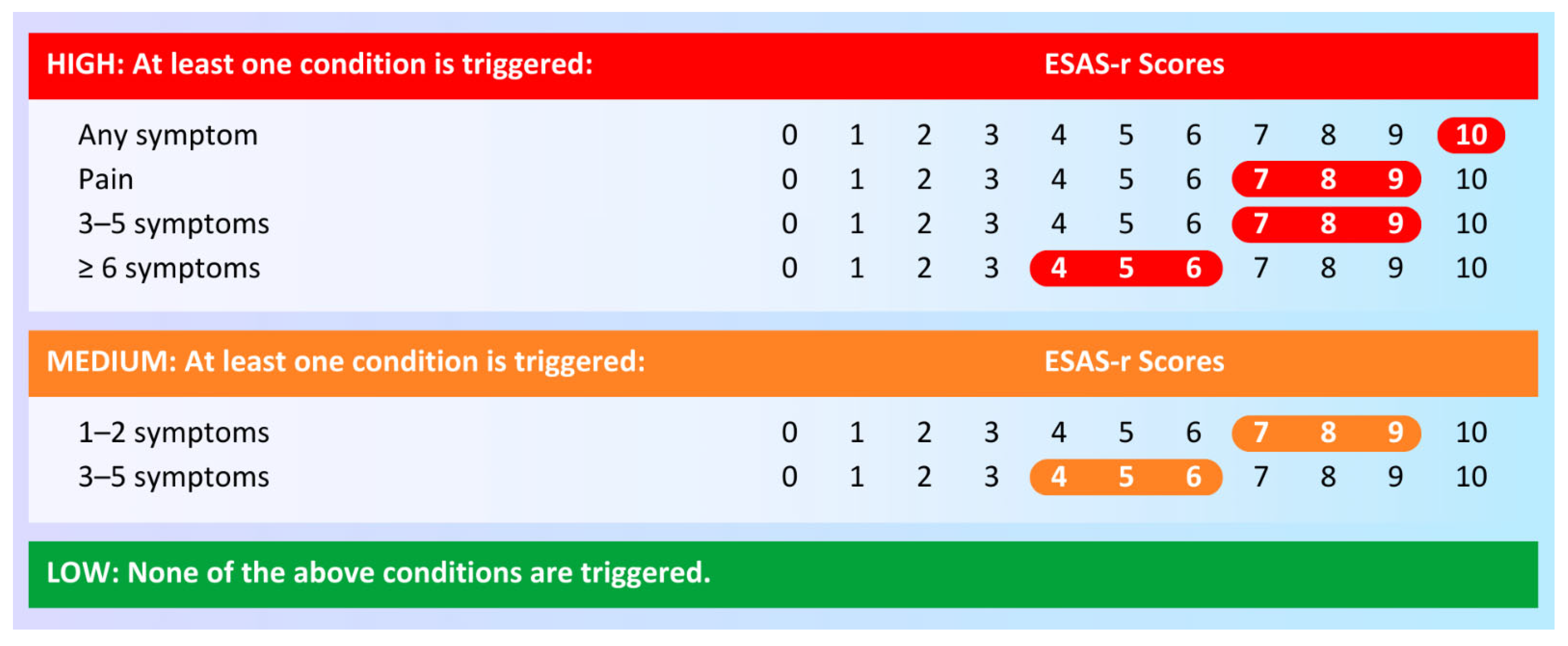
3.1. Symptom Complexity
3.2. Acute Care Utilization Within 7 Days
3.3. Acute Care Utilization Within 14 Days
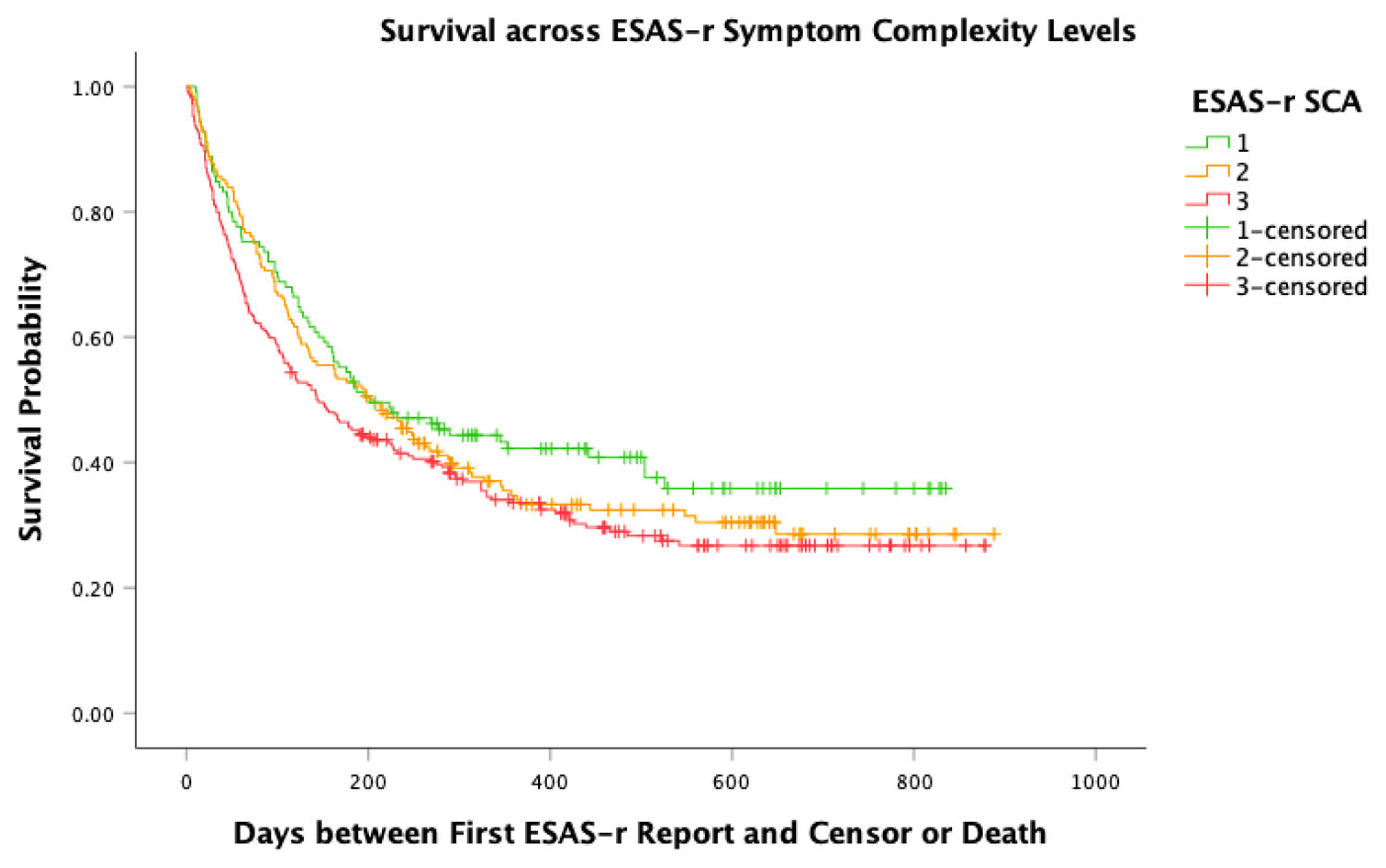
3.4. Survival over 6 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Care Ontario. Ontario Cancer Statistics 2022: Estimated Future Cancer Prevalence 2022. Available online: https://web.archive.org/web/20240417221548/https://www.cancercareontario.ca/en/data-research/view-data/statistical-reports/ontario-cancer-statistics-2022/key-findings-2022. (accessed on 23 July 2024).
- World Health Assembly. Strengthening of Palliative Care as a Component of Comprehensive Care Throughout the Life Course; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Shepperd, S.; Gonçalves-Bradley, D.C.; Straus, S.E.; Wee, B. Hospital at home: Home-based end-of-life care. Cochrane Database Syst. Rev. 2021, 3, CD009231. [Google Scholar] [CrossRef]
- World Health Organization. Palliative Care. 2024. Available online: https://www.who.int/health-topics/palliative-care (accessed on 23 July 2024).
- World Health Organization. Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 July 2024).
- Canadian Cancer Society. Advanced Cancer. 2024. Available online: https://cancer.ca/en/living-with-cancer/advanced-cancer (accessed on 23 July 2024).
- Verkissen, M.N.; Hjermstad, M.J.; Van Belle, S.; Kaasa, S.; Deliens, L.; Pardon, K. Quality of life and symptom intensity over time in people with cancer receiving palliative care: Results from the international European Palliative Care Cancer Symptom study. PLoS ONE 2019, 14, e0222988. [Google Scholar] [CrossRef]
- Zimmermann, C.; Pope, A.; Hannon, B.; Krzyzanowska, M.K.; Rodin, G.; Li, M.; Howell, D.; Knox, J.J.; Leighl, N.B.; Sridhar, S.; et al. Phase II Trial of Symptom Screening With Targeted Early Palliative Care for Patients With Advanced Cancer. J. Natl. Compr. Cancer Netw. 2022, 20, 361–370.e365. [Google Scholar] [CrossRef]
- Seow, H.; Sussman, J.; Martelli-Reid, L.; Pond, G.; Bainbridge, D. Do High Symptom Scores Trigger Clinical Actions? An Audit After Implementing Electronic Symptom Screening. J. Oncol. Pract. 2012, 8, e142–e148. [Google Scholar] [CrossRef]
- Pereira, J.; Green, E.; Molloy, S.; Dudgeon, D.; Howell, D.; Krzyzanowska, M.K.; Mahase, W.; Tabing, R.; Urowitz, S.; Macdougall, L. Population-Based Standardized Symptom Screening: Cancer Care Ontario’s Edmonton Symptom Assessment System and Performance Status Initiatives. J. Oncol. Pract. 2014, 10, 212–214. [Google Scholar] [CrossRef]
- Pereira, J.L.; Chasen, M.R.; Molloy, S.; Amernic, H.; Brundage, M.D.; Green, E.; Kurkjian, S.; Krzyzanowska, M.K.; Mahase, W.; Shabestari, O.; et al. Cancer Care Professionals’ Attitudes Toward Systematic Standardized Symptom Assessment and the Edmonton Symptom Assessment System After Large-Scale Population-Based Implementation in Ontario, Canada. J. Pain Symptom Manag. 2016, 51, 662–672.e668. [Google Scholar] [CrossRef]
- Dudgeon, D.; King, S.; Howell, D.; Green, E.; Gilbert, J.; Hughes, E.; Lalonde, B.; Angus, H.; Sawka, C. Cancer Care Ontario’s experience with implementation of routine physical and psychological symptom distress screening. Psycho-Oncol. 2012, 21, 357–364. [Google Scholar] [CrossRef]
- Montgomery, N.; Howell, D.; Ismail, Z.; Bartlett, S.J.; Brundage, M.; Bryant-Lukosius, D.; Krzyzanowska, M.; Moody, L.; Snyder, C.; Barbera, L.; et al. Selecting, implementing and evaluating patient-reported outcome measures for routine clinical use in cancer: The Cancer Care Ontario approach. J. Patient-Rep. Outcomes 2020, 4, 101. [Google Scholar] [CrossRef]
- Alberta Health Services. Edmonton Symptom Assessment System—Revised (ESAS-r) Administration Manual 2019. Available online: https://www.albertahealthservices.ca/assets/info/peolc/if-peolc-ed-esasr-admin-manual.pdf (accessed on 23 July 2024).
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Noel, C.W.; Sutradhar, R.; Zhao, H.; Delibasic, V.; Forner, D.; Irish, J.C.; Kim, J.; Husain, Z.; Mahar, A.; Karam, I.; et al. Patient-Reported Symptom Burden as a Predictor of Emergency Department Use and Unplanned Hospitalization in Head and Neck Cancer: A Longitudinal Population-Based Study. J. Clin. Oncol. 2021, 39, 675–684. [Google Scholar] [CrossRef]
- Watson, L.; Qi, S.; Deiure, A.; Photitai, E.; Chmielewski, L.; Smith, L. Validating a Patient-Reported Outcomes–Derived Algorithm for Classifying Symptom Complexity Levels Among Patients With Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 1518–1525. [Google Scholar] [CrossRef]
- Watson, L.; Qi, S.; Link, C.; DeIure, A.; Afzal, A.; Barbera, L. Patient-Reported Symptom Complexity and Acute Care Utilization Among Patients With Cancer: A Population-Based Study Using a Novel Symptom Complexity Algorithm and Observational Data . J. Natl. Compr. Cancer Netw. 2023, 21, 173–180. [Google Scholar] [CrossRef]
- Scarborough Health Network. Central East Clinical Information System 2024. Available online: https://www.shn.ca/central-east-clinical-information-system/ (accessed on 23 July 2024).
- National Cancer Institute. NCI Comorbidity Index Overview 2024. Available online: https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html (accessed on 19 December 2024).
- Van Ingen, T.; Matheson, F.I. The 2011 and 2016 iterations of the Ontario Marginalization Index: Updates, consistency and a cross-sectional study of health outcome associations. Can. J. Public Health 2022, 113, 260–271. [Google Scholar] [CrossRef]
- Public Health Ontario. Ontario Marginalization Index (ON-Marg): Ontario Agency for Health Protection and Promotion. 2025. Available online: https://www.publichealthontario.ca/en/Data-and-Analysis/Health-Equity/Ontario-Marginalization-Index (accessed on 19 December 2024).
- Salam-White, L.; Hirdes, J.P.; Poss, J.W.; Blums, J. Predictors of emergency room visits or acute hospital admissions prior to death among hospice palliative care clients in Ontario: A retrospective cohort study. BMC Palliat. Care 2014, 13, 35. [Google Scholar] [CrossRef]
- Kirkland, S.W.; Garrido Clua, M.; Kruhlak, M.; Villa-Roel, C.; Couperthwaite, S.; Yang, E.H.; Elwi, A.; O’Neill, B.; Duggan, S.; Brisebois, A.; et al. Comparison of characteristics and management of emergency department presentations between patients with met and unmet palliative care needs. PLoS ONE 2021, 16, e0257501. [Google Scholar] [CrossRef]
- Sutradhar, R.; Barbera, L.; Seow, H.-Y. Palliative homecare is associated with reduced high- and low-acuity emergency department visits at the end of life: A population-based cohort study of cancer decedents. Palliat. Med. 2017, 31, 448–455. [Google Scholar] [CrossRef]
- Seow, H.; Barbera, L.; Pataky, R.; Lawson, B.; O’Leary, E.; Fassbender, K.; McGrail, K.; Burge, F.; Brouwers, M.; Sutradhar, R. Does Increasing Home Care Nursing Reduce Emergency Department Visits at the End of Life? A Population-Based Cohort Study of Cancer Decedents. J. Pain Symptom Manag. 2016, 51, 204–212. [Google Scholar] [CrossRef]
- Michael, N.; Beale, G.; O’Callaghan, C.; Melia, A.; DeSilva, W.; Costa, D.; Kissane, D.; Shapiro, J.; Hiscock, R. Timing of palliative care referral and aggressive cancer care toward the end-of-life in pancreatic cancer: A retrospective, single-center observational study. BMC Palliat. Care 2019, 18, 13. [Google Scholar] [CrossRef]
- Qureshi, D.; Tanuseputro, P.; Perez, R.; Pond, G.R.; Seow, H.-Y. Early initiation of palliative care is associated with reduced late-life acute-hospital use: A population-based retrospective cohort study. Palliat. Med. 2019, 33, 150–159. [Google Scholar] [CrossRef]
- Patra, L.; Ghoshal, A.; Damani, A.; Salins, N. Cancer palliative care referral: Patients’ and family caregivers’ perspectives—A systematic review. BMJ Support. Palliat. Care 2024, 14, e143–e149. [Google Scholar] [CrossRef]
- Government of Ontario. Palliative and End-of-Life Care. 2023. Available online: https://www.ontario.ca/page/palliative-and-end-life-care (accessed on 23 July 2024).
- Government of Ontario. Ontario Provincial Framework for Palliative Care. 2023. Available online: https://files.ontario.ca/moh-ontario-provincial-framework-for-palliative-care-en-2021-12-07.pdf (accessed on 23 July 2024).
- Bandieri, E.; Banchelli, F.; Borelli, E.; D’Amico, R.; Efficace, F.; Bruera, E.; Luppi, M.; Potenza, L. Edmonton symptom assessment system global distress score and overall survival among patients with advanced cancer receiving early palliative care. BMJ Support. Palliat. Care 2023, 13, e735–e736. [Google Scholar] [CrossRef]
- Koesel, N.; Tocchi, C.; Burke, L.; Yap, T.; Harrison, A. Symptom Distress: Implementation of Palliative Care Guidelines to Improve Pain, Fatigue, and Anxiety in Patients With Advanced Cancer. Clin. J. Oncol. Nurs. 2019, 23, 149–155. [Google Scholar] [CrossRef]
- Gemmell, R.; Yousaf, N.; Droney, J. “Triggers” for early palliative care referral in patients with cancer: A review of urgent unplanned admissions and outcomes. Support. Care Cancer 2020, 28, 3441–3449. [Google Scholar] [CrossRef]
- Ontario Health. Cancer System Quality Index. 2024. Available online: https://www.ontariohealth.ca/sites/ontariohealth/files/CSQI-2024-Report.pdf (accessed on 23 July 2024).
- Gilbert, J.E.; Howell, D.; King, S.; Sawka, C.; Hughes, E.; Angus, H.; Dudgeon, D. Quality improvement in cancer symptom assessment and control: The Provincial Palliative Care Integration Project (PPCIP). J Pain Symptom Manag. 2012, 43, 663–678. [Google Scholar] [CrossRef]
- Schick-Makaroff, K.; Sawatzky, R. Divergent Perspectives on the Use of the Edmonton Symptom Assessment System (Revised) in Palliative Care. J. Hosp. Palliat. Nurs. 2020, 22, 75–81. [Google Scholar] [CrossRef]
| Characteristic n = 559 | Whole Cohort n (Col %) |
|---|---|
| Total | 559 (100.0) |
| Age, mean [SD], years | 71 [13] |
| Sex | |
| Female | 284 (50.8) |
| Male | 275 (49.2) |
| Language | |
| English | 547 (97.9) |
| Other | 12 (2.1) |
| Tumour site | |
| Breast | 50 (8.9) |
| Gastrointestinal | 130 (23.3) |
| Genitourinary | 62 (11.1) |
| Gynaecological | 34 (6.1) |
| Haematological | 36 (6.4) |
| Lung | 92 (16.5) |
| Other * | 155 (27.7) |
| Used chemotherapy in 30 days prior to ESAS-r? | 166 (29.7) |
| Location of first PC visit | |
| Clinic | 526 (94.1) |
| Other ** | 33 (5.9) |
| Used home care? | 132 (23.6) |
| Latest diagnosis to first PC interaction, median [IQR], days (n = 538, 96.2%) | 74 [194] |
| Latest diagnosis to first ESAS-r report, median [IQR], days (n = 538, 96.2%) | 77 [189] |
| Location of death | |
| Hospital † | 215 (38.5) |
| Non-hospital | 171 (30.6) |
| Alive or missing ‡ | 173 (30.9) |
| Survival, median [IQR], days (n = 386, 69.1%) | 97 [160] |
| Survival | |
| Survived 0–30 days | 89 (15.9) |
| Survived 31–90 days | 100 (17.9) |
| Survived beyond 90 days | 197 (35.2) |
| Alive or missing ‡ | 173 (30.9) |
| Characteristic (n = 559) | Emergency Department Visit or Hospital Admission Within 7 Days | ||
|---|---|---|---|
| No n (Col %) | Yes n (Col %) | p Value | |
| Total (Row %) | 498 (89.1) | 61 (10.9) | |
| PC unit | - | 14 (23.0) | <0.01 |
| Other unit | - | 47 (77.0) | |
| Symptom complexity (Row %) | |||
| Low | 118 (94.4) | 7 (5.6) | 0.02 |
| Medium | 163 (90.6) | 17 (9.4) | |
| High | 217 (85.4) | 37 (14.6) | |
| Age, mean [SD], years | 71 [13] | 70 [13] | 0.64 |
| Sex | |||
| Female | 254 (51.0) | 30 (49.2) | 0.79 |
| Male | 244 (49.0) | 31 (50.8) | |
| Language | |||
| English | 486 (97.6) | 61 (100.0) | 0.22 |
| Other | 12 (2.4) | 0 (0.0) | |
| Tumour site | |||
| Breast | 46–50 | 1–5 | 0.75 |
| Gastrointestinal | 115 (23.1) | 15 (24.6) | |
| Genitourinary | 56 (11.2) | 6 (9.8) | |
| Gynaecological | 26–30 | 1–5 | |
| Haematological | 31–35 | 1–5 | |
| Lung | 78 (15.7) | 14 (23.0) | |
| Other * | 139 (27.9) | 16 (26.2) | |
| Used chemotherapy in 30 days prior to ESAS-r? | 155 (31.1) | 11 (18.0) | 0.04 |
| Location of first PC visit | |||
| Clinic | 468 (94.0) | 58 (95.1) | 0.73 |
| Other ** | 26–30 | 1–5 | |
| Used home care? | 117 (23.5) | 15 (24.6) | 0.85 |
| Latest diagnosis to first PC interaction, median [IQR], days | 81 [196] (n = 479) | 21 [80] (n = 59) | <0.01 |
| Latest diagnosis to first ESAS-r report, median [IQR], days | 85 [192] (n = 479) | 20 [101] (n = 59) | <0.01 |
| Location of death | |||
| Hospital | 172 (34.5) | 43 (70.5) | <0.01 |
| Non-hospital | 164 (32.9) | 7 (11.5) | |
| Alive or missing ‡ | 162 (32.5) | 11 (18.0) | |
| Survival, median [IQR], days | 101 [173] (n = 336, 67.5%) | 25 [96] (n = 50, 82.0%) | <0.01 |
| Survival † | |||
| Survived 0–30 days | 60 (12.0) | 29 (47.5) | <0.01 |
| Survived 31–90 days | 93 (18.7) | 7 (11.5) | |
| Survived beyond 90 days | 183 (36.7) | 14 (23.0) | |
| Alive or missing ‡ | 162 (32.5) | 11 (18.0) | |
| Characteristic (n = 559) | Emergency Department Visit or Hospital Admission Within 7 Days | |
|---|---|---|
| aOR (95% CI) | p Value | |
| Symptom complexity | ||
| Low | Ref | |
| Medium | 1.56 (0.60–4.05) | 0.37 |
| High | 2.83 (1.18–6.77) | 0.02 |
| Sex | ||
| Female | Ref | |
| Male | 1.18 (0.62–2.23) | 0.62 |
| Tumour site | ||
| Breast | Ref | |
| Gastrointestinal | 1.83 (0.45–7.43) | 0.40 |
| Genitourinary | 1.51 (0.31–7.33) | 0.61 |
| Gynaecological | 2.63 (0.51–13.45) | 0.25 |
| Haematological | 1.32 (0.22–7.77) | 0.76 |
| Lung | 2.69 (0.66–10.98) | 0.17 |
| Other *** | 1.78 (0.45–7.07) | 0.42 |
| Chemotherapy in 30 days prior to ESAS-r | ||
| No | Ref | |
| Yes | 0.48 (0.23–0.98) | 0.04 |
| Involvement of home care | ||
| No | Ref | |
| Yes | 0.89 (0.46–1.77) | 0.76 |
| Days from latest diagnosis to first PC interaction, median [IQR], days | 1.00 (1.00–1.00) | 0.05 |
| Characteristic (n = 559) | Emergency Department Visit or Hospital Admission Within 14 Days | ||
|---|---|---|---|
| No n (Col %) | Yes n (Col %) | p Value | |
| Total (Row %) | 451 (80.7) | 108 (19.3) | |
| PC unit | - | 25 (23.1) | < 0.01 |
| Other unit | - | 83 (76.9) | |
| Symptom complexity (Row %) | |||
| Low | 107 (85.6) | 18 (14.4) | 0.02 |
| Medium | 152 (84.4) | 28 (15.6) | |
| High | 192 (75.6) | 62 (24.4) | |
| Age, mean [SD], years | 71 [13] | 70 [12] | 0.33 |
| Sex | |||
| Female | 237 (52.5) | 47 (43.5) | 0.09 |
| Male | 214 (47.5) | 61 (56.5) | |
| Language | |||
| English | 440 (97.6) | 107 (99.1) | 0.33 |
| Other | 11–15 | 1–5 | |
| Tumour site | |||
| Breast | 46–50 | 1–5 | 0.33 |
| Gastrointestinal | 108 (23.9) | 22 (20.4) | |
| Genitourinary | 47 (10.4) | 15 (13.9) | |
| Gynaecological | 25 (5.5) | 9 (8.3) | |
| Haematological | 29 (6.4) | 7 (6.5) | |
| Lung | 73 (16.2) | 19 (17.6) | |
| Other * | 123 (27.3) | 32 (29.6) | |
| Used radiotherapy in 30 days prior to ESAS-r? | 58 (12.9) | 23 (21.3) | 0.03 |
| Location of first PC visit | |||
| Clinic | 424 (94.0) | 102 (94.4) | 0.86 |
| Other ** | 27 (6.0) | 6 (5.6) | |
| Used home care? | 105 (23.3) | 27 (25.0) | 0.71 |
| Days from diagnosis to first PC interaction, median [IQR], days | 86 [197] (n = 432) | 35 [99] (n = 106) | <0.01 |
| Days from diagnosis to first ESAS-r report, median [IQR], days | 88 [202] (n = 432) | 34 [105] (n = 106) | <0.01 |
| Location of death | |||
| Hospital | 149 (33.0) | 66 (61.1) | <0.01 |
| Non-hospital | 146 (32.4) | 25 (23.1) | |
| Alive or missing ‡ | 156 (34.6) | 17 (15.7) | |
| Survival, median [IQR], days | 107 [177] (n = 295) | 36 [100] (n = 91) | <0.01 |
| Survival † | |||
| 0–30 | 46 (10.2) | 43 (39.8) | <0.01 |
| 31–90 | 83 (18.4) | 17 (15.7) | |
| 91+ | 166 (36.8) | 31 (28.7) | |
| Alive or missing ‡ | 156 (34.6) | 17 (15.7) | |
| Characteristic (n = 559) | Emergency Department Visit or Hospital Admission Within 14 Days | |
|---|---|---|
| aOR (95% CI) | p Value | |
| Symptom complexity | ||
| Low | Ref | |
| Medium | 0.99 (0.50–1.95) | 0.98 |
| High | 1.78 (0.97–3.28) | 0.07 |
| Sex | ||
| Female | Ref | |
| Male | 1.54 (0.92–2.58) | 0.10 |
| Tumour site | ||
| Breast | Ref | |
| Gastrointestinal | 1.59 (0.48–5.30) | 0.45 |
| Genitourinary | 2.49 (0.67–8.88) | 0.17 |
| Gynaecological | 4.55 (1.21–17.04) | 0.03 |
| Haematological | 2.29 (0.56–9.32) | 0.25 |
| Lung | 2.11 (0.62–7.14) | 0.23 |
| Other * | 2.57 (0.80–8.24) | 0.11 |
| Radiotherapy in 30 days prior to ESAS-r | ||
| No | Ref | |
| Yes | 1.70 (0.95–3.04) | 0.07 |
| Involvement of home care | ||
| No | Ref | |
| Yes | 1.02 (0.60–1.72) | 0.96 |
| Days from latest diagnosis to first PC interaction, median [IQR], days | 1.00 (1.00–1.00) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranajaya, P.; Ho, V.; Jiang, M.; Tran, V.; Sinnarajah, A. Associations Between Symptom Complexity and Acute Care Utilization Among Adult Advanced Cancer Patients Followed by a Palliative Care Service. Curr. Oncol. 2025, 32, 388. https://doi.org/10.3390/curroncol32070388
Pranajaya P, Ho V, Jiang M, Tran V, Sinnarajah A. Associations Between Symptom Complexity and Acute Care Utilization Among Adult Advanced Cancer Patients Followed by a Palliative Care Service. Current Oncology. 2025; 32(7):388. https://doi.org/10.3390/curroncol32070388
Chicago/Turabian StylePranajaya, Philip, Vincent Ho, Mengzhu Jiang, Vance Tran, and Aynharan Sinnarajah. 2025. "Associations Between Symptom Complexity and Acute Care Utilization Among Adult Advanced Cancer Patients Followed by a Palliative Care Service" Current Oncology 32, no. 7: 388. https://doi.org/10.3390/curroncol32070388
APA StylePranajaya, P., Ho, V., Jiang, M., Tran, V., & Sinnarajah, A. (2025). Associations Between Symptom Complexity and Acute Care Utilization Among Adult Advanced Cancer Patients Followed by a Palliative Care Service. Current Oncology, 32(7), 388. https://doi.org/10.3390/curroncol32070388