Germline TP53 p.R337H and XAF1 p.E134* Variants: Prevalence in Paraguay and Comparison with Rates in Brazilian State of Paraná and Previous Findings at the Paraguayan–Brazilian Border
Abstract
1. Introduction
2. Materials and Methods
- -
- A volume of 2 µL of DNA (5 ng/µL);
- -
- A volume of 5 µL of TaqMan® Genotyping Master Mix 2X (Applied Biosystems®, Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania);
- -
- A volume of 0.125 µL of VIC®/FAM® probe (rs146752602) (Thermo Fisher Scientific, Waltham, MA, USA)
- -
- A volume of 2.875 µL of ultra-pure water.
3. Results
Results of TP53 p.R337H and XAF1 p.E134* Variants in Paraguay
- ▪
- Prevalence of TP53 p.R337H
- ▪
- Prevalence of XAF1 p.E134*
- ▪
- Prevalence of XAF1 p.E134* in Paraguay and Other Continents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Galindo, C.; Figueiredo, B.C.; Zambetti, G.P.; Ribeiro, R.C. Biology, clinical characteristics, and management of adrenocortical tumors in children. Pediatr. Blood Cancer 2005, 45, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Chang, A.; Dittmer, D.; Notterman, D.A.; Silver, A.; Thorn, K.; Welsh, D.; Wu, M. The p53 tumor suppressor gene. J. Lab. Clin. Med. 1994, 123, 817–823. [Google Scholar] [PubMed]
- Donehower, L.A.; Harvey, M.; Slagle, B.L.; McArthur, M.J.; Montgomery, C.A., Jr.; Butel, J.S.; Bradley, A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Jacks, T.; Remington, L.; Williams, B.O.; Schmitt, E.M.; Halachmi, S.; Bronson, R.T.; Weinberg, R.A. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994, 4, 1–7. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Sandrini, F.; Figueiredo, B.; Zambetti, G.P.; Michalkiewicz, E.; Lafferty, A.R.; DeLacerda, L.; Rabin, M.; Cadwell, C.; Sampaio, G.; et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl. Acad. Sci. USA 2001, 98, 9330–9335. [Google Scholar] [CrossRef]
- Latronico, A.C.; Pinto, E.M.; Domenice, S.; Fragoso, M.C.B.V.; Martin, R.M.; Zerbini, M.C.; Lucon, A.M.; Mendonca, B.B. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 2001, 86, 4970–4973. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Figueiredo, B. Childhood adrenocortical tumours. Eur. J. Cancer 2004, 40, 1117–1126. [Google Scholar] [CrossRef]
- Pianovski, M.A.; Maluf, E.M.; de Carvalho, D.S.; Ribeiro, R.C.; Rodriguez-Galindo, C.; Boffetta, P.; Zancanella, P.; Figueiredo, B.C. Mortality rate of adrenocortical tumors in children under 15 years of age in Curitiba, Brazil. Pediatr. Blood Cancer 2006, 47, 56–60. [Google Scholar] [CrossRef]
- Costa, T.E.J.; Gerber, V.K.Q.; Ibañez, H.C.; Melanda, V.S.; Parise, I.Z.S.; Watanabe, F.M.; Pianovski, M.A.D.; Fiori, C.M.C.M.; Fabro, A.L.M.R.; da Silva, D.B.; et al. Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil. Cancers 2019, 11, 1804. [Google Scholar] [CrossRef]
- Seidinger, A.L.; Mastellaro, M.J.; Paschoal Fortes, F.; Godoy Assumpção, J.; Aparecida Cardinalli, I.; Aparecida Ganazza, M.; Correa Ribeiro, R.; Brandalise, S.R.; dos Santos Aguiar, S.; Yunes, J.A. Association of the highly prevalent TP53 R337H mutation with pediatric choroid plexus carcinoma and osteosarcoma in southeast Brazil. Cancer 2011, 117, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Legal, E.F.; Ascurra, M.; Custódio, G.; Ayala, H.L.; Monteiro, M.; Vega, C.; Fernández-Nestosa, M.J.; Vega, S.; Sade, E.R.; Coelho, I.M.; et al. Prevalence of an inherited cancer predisposition syndrome associated with the germ line TP53 R337H mutation in Paraguay. Cancer Epidemiol. 2015, 39, 166–169. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michalkiewicz, E.; Sandrini, R.; Figueiredo, B.; Miranda, E.C.; Caran, E.; Oliveira-Filho, A.G.; Marques, R.; Pianovski, M.A.; Lacerda, L.; Cristofani, L.M.; et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 2004, 22, 838–845. [Google Scholar] [CrossRef]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SV40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Linzer, D.I.H.; Levine, A.J. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979, 17, 43–52. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Greenblatt, M.S.; Bennett, W.P.; Hollstein, M.; Harris, C.C. Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994, 54, 4855–4878. [Google Scholar]
- Soussi, T.; Dehouche, K.; Béroud, C. p53 website and analysis of p53 gene mutations in human cancer: Forging a link between epidemiology and cancerogenesis. Hum. Mutat. 2000, 15, 105–113. [Google Scholar] [CrossRef]
- Srivastava, S.; Zou, Z.; Pirollo, K.; Blattner, W.; Chang, E.H. Transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature 1990, 348, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Billerbeck, A.E.C.; Villares, M.C.B.; Domenice, S.; Mendonça, B.B.; Latronico, A.C. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq. Bras. De Endocrinol. Metabol. 2004, 48, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Paskulin, D.D.; Giacomazzi, J.; Achatz, M.I.; Costa, S.; Reis, R.M.; Hainaut, P.; Dos Santos, S.E.B.; Ashton-Prolla, P. Ancestry of the Brazilian TP53 c.1010G>A (p.Arg337His, R337H) Founder Mutation: Clues from Haplotyping of Short Tandem Repeats on Chromosome 17p. PLoS ONE 2015, 10, e0143262. [Google Scholar] [CrossRef]
- Zou, B.; Chim, C.S.; Pang, R.; Zeng, H.; Dai, Y.; Zhang, R.; Lam, C.S.; Tan, V.P.; Hung, I.F.; Lan, H.Y.; et al. XIAP-associated factor 1 (XAF1), a novel target of p53, enhances p53-mediated apoptosis via post-translational modification. Mol. Carcinog. 2012, 51, 422–432. [Google Scholar] [CrossRef]
- Ng, K.C.; Campos, E.I.; Martinka, M.; Li, G. XAF1 expression is significantly reduced in human melanoma. J. Investig. Dermatol. 2004, 123, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Fridman, C.; Figueiredo, B.C.; Salvador, H.; Teixeira, M.R.; Pinto, C.; Pinheiro, M.; Kratz, C.P.; Lavarino, C.; Legal, E.A.; et al. Multiple TP53 p.R337H haplotypes and implications for tumor susceptibility. Hum. Genet. Genom. Adv. 2024, 5, 100244. [Google Scholar] [CrossRef]
- Gerber, V.K.Q.; Paraizo, M.M.; Ibañez, H.C.; Casali-da-Rocha, J.C.; Pinto, E.M.; Andrade, D.P.; Ibañez, M.V.C.; Komechen, H.; Figueiredo, M.M.O.; Custódio, G.; et al. Environmental Contaminants Modulate Breast Cancer Development and Outcome in TP53 p.R337H Carriers and Noncarriers. Cancers 2022, 14, 3014. [Google Scholar] [CrossRef]
- Custódio, G.; Komechen, H.; Figueiredo, F.R.; Fachin, N.D.; Pianovski, M.A.; Figueiredo, B.C. Molecular epidemiology of adrenocortical tumors in southern Brazil. Mol. Cell Endocrinol. 2012, 351, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística, Paraguay. Available online: https://www.ine.gov.py/ (accessed on 18 January 2025).
- Benitez, O.; Busson, M.; Charron, D.; Loiseau, P. HLA polymorphism in a guarani-indian population from Paraguay and its usefulness for the hispano-indian admixture study in Paraguay. Int. J. Immunogenet. 2011, 38, 7–11. [Google Scholar] [CrossRef]
- Comunidades Brasileiras No Exterior. Available online: https://www.gov.br/mre/pt-br/assuntos/portal-consular/BrasileirosnoExterior2023.pdf (accessed on 18 January 2025).
- ABraOM: Brazilian Genomic Variants. Available online: https://abraom.ib.usp.br/index.php (accessed on 2 February 2022).
- NCBI—National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 2 February 2022).
- Custodio, G.; Taques, G.R.; Figueiredo, B.C.; Gugelmin, E.S.; Oliveira Figueiredo, M.M.; Watanabe, F.; Pontarolo, R.; Lalli, E.; Bleggi Torres, L.F. Increased incidence of choroid plexus carcinoma due to the germline TP53 R337H mutation in southern Brazil. PLoS ONE 2011, 6, e18015. [Google Scholar] [CrossRef]
- Custódio, G.; Parise, G.A.; Filho, N.K.; Komechen, H.; Sabbaga, C.C.; Rosati, R.; Grisa, L.; Parise, I.Z.; Pianovski, M.A.; Fiori, C.M.; et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 2013, 31, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Li, F.P.; Fraumeni, J.F., Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann. Intern. Med. 1969, 71, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, J.R.; Pinto, E.M.; Rehg, J.E.; Clay, M.R.; Wang, J.; Neale, G.; Heath, R.J.; Lozano, G.; Lalli, E.; Figueiredo, B.C.; et al. The Common Germline TP53-R337H Mutation Is Hypomorphic and Confers Incomplete Penetrance and Late Tumor Onset in a Mouse Model. Cancer Res. 2021, 81, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Ribeiro, R.C.; Figueiredo, B.C.; Zambetti, G.P. TP53-Associated Pediatric Malignancies. Genes. Cancer 2011, 2, 485–490. [Google Scholar] [CrossRef]
- DiGiammarino, E.L.; Lee, A.S.; Cadwell, C.; Zhang, W.; Bothner, B.; Ribeiro, R.C.; Zambetti, G.; Kriwacki, R.W. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat. Struct. Biol. 2002, 9, 12–16. [Google Scholar] [CrossRef]
- Park, A.; Lee, J.; Mun, S.; Kim, D.J.; Cha, B.H.; Moon, K.T.; Yoo, T.K.; Kang, H.G. Identification of transcription factor YY1 as a regulator of a prostate cancer specific pathway using proteomic analysis. J. Cancer 2017, 8, 2303. [Google Scholar] [CrossRef]
- Birch, J.M.; Hartley, A.L.; Tricker, K.J.; Prosser, J.; Condie, A.; Kelsey, A.M.; Harris, M.; Jones, P.H.; Binchy, A.; Crowther, D. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res. 1994, 54, 1298–1304. [Google Scholar]
- Penkert, J.; Strüwe, F.J.; Dutzmann, C.M.; Doergeloh, B.B.; Montellier, E.; Freycon, C.; Keymling, M.; Schlemmer, H.-P.; Sänger, B.; Hoffmann, B.; et al. Genotype-phenotype associations within the Li-Fraumeni spectrum: A report from the German Registry. J. Hematol. Oncol. 2022, 15, 107. [Google Scholar] [CrossRef]
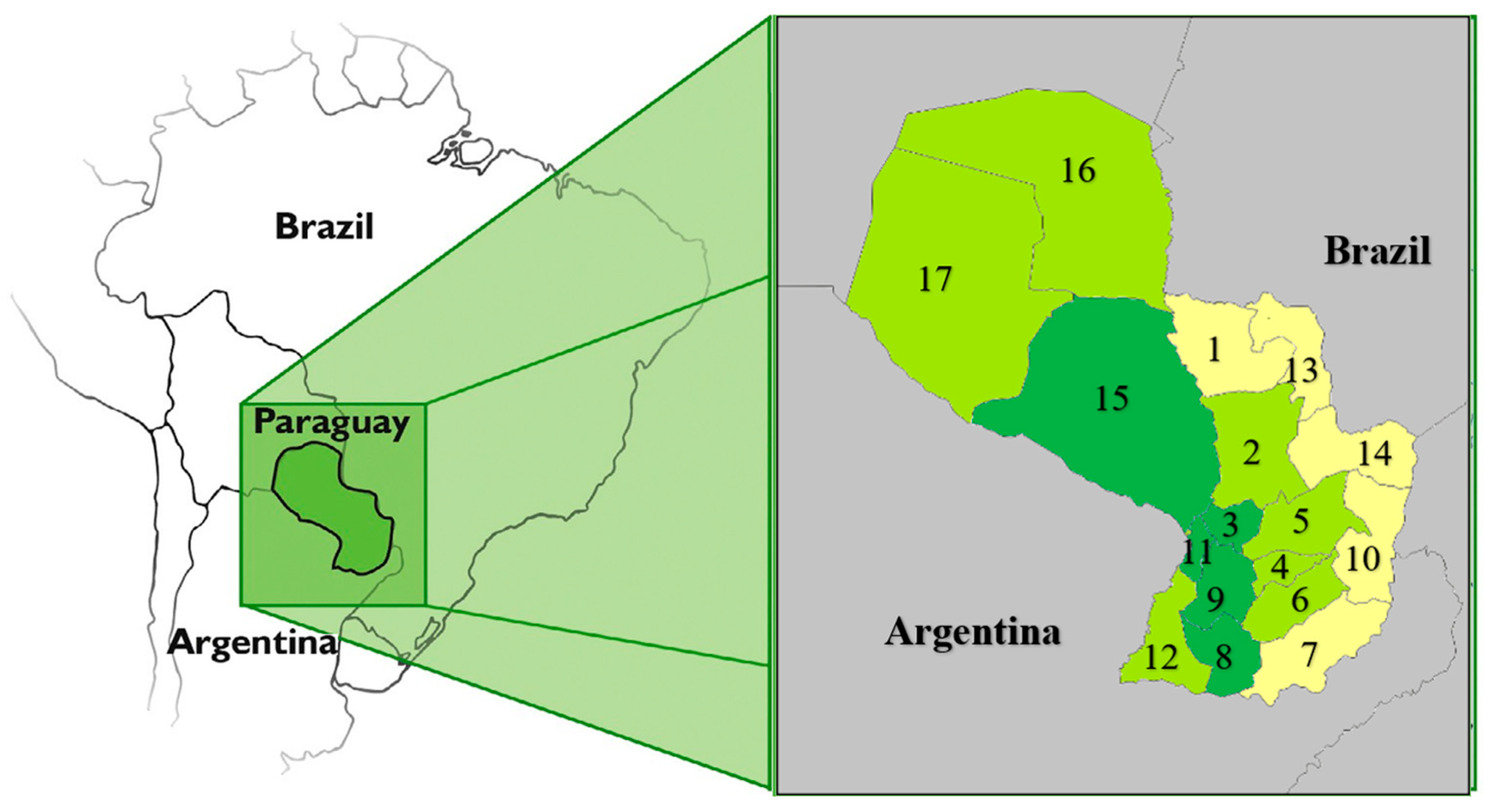
| Populational Feature | Geographical Distribution |
|---|---|
| Mixed race | Located in all departments except in central Chaco, where the majority of the population are Mennonites who have migrated mainly from Canada |
| Guarani and non-Guarani Indians | Mainly located in Departments 15, 17, 13, 14, 5, 4, and 6, in decreasing order |
| Brazilian descendants | Mainly located in Departments 10, 14, 7, 13, and 1 |
| High-density areas | Departments and codes: Central (11) + Asunción = Capital (code ASU) + Alto Paraná (10) + Caaguazú (5), and Itapúa (7), in decreasing order |
| Low-density areas | Departments and codes: San Pedro (2) + Cordillera (3) + Guairá (4) + Concepción (1) + Paraguarí (9) + Amambay (13) + Misiones (8) + Canindeyú (14) + Caazapá (6) + Presidente Hayes (15) + Ñeembucú (12) + Boquerón (17) + Alto Paraguay (16), in decreasing order |
| Department | NBs Screened for R337H Variant | NBs Screened for XAF1 p.E134* Variant | XAF1 p.E134*-Positive NB |
|---|---|---|---|
| (2) San Pedro | 3440 | 0 | |
| (3) Cordillera | 2442 | 173 | 4 (1) |
| (4) Guairá | 1833 | 0 | |
| (5) Caaguazú | 4510 | 0 | |
| (6) Caazapá | 1503 | 0 | |
| (8) Misiones | 1024 | 73 | 0 |
| (9) Paraguarí | 2163 | 153 | 0 |
| (11) Central and Asuncion | 20,727 | 1478 | 4 (2) |
| (12) Ñeembucú | 755 | 0 | |
| (15) Presidente Hayes | 963 | 69 | 4 (3) |
| (16) Alto Paraguay | 146 | 0 | |
| (17) Boquerón | 494 | 0 | |
| Total | 40,000 | 2000 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcon-de Legal, E.; Ascurra, M.; Vega-Paredes, R.; Sade, E.; Monteiro, M.; Paraízo, M.; Colman, M.; Florentin, A.G.; Ojeda, C.; Legal-Ayala, H.; et al. Germline TP53 p.R337H and XAF1 p.E134* Variants: Prevalence in Paraguay and Comparison with Rates in Brazilian State of Paraná and Previous Findings at the Paraguayan–Brazilian Border. Curr. Oncol. 2025, 32, 333. https://doi.org/10.3390/curroncol32060333
Falcon-de Legal E, Ascurra M, Vega-Paredes R, Sade E, Monteiro M, Paraízo M, Colman M, Florentin AG, Ojeda C, Legal-Ayala H, et al. Germline TP53 p.R337H and XAF1 p.E134* Variants: Prevalence in Paraguay and Comparison with Rates in Brazilian State of Paraná and Previous Findings at the Paraguayan–Brazilian Border. Current Oncology. 2025; 32(6):333. https://doi.org/10.3390/curroncol32060333
Chicago/Turabian StyleFalcon-de Legal, Edith, Marta Ascurra, Rosa Vega-Paredes, Elis Sade, Magna Monteiro, Mariana Paraízo, Magali Colman, Angeles Gutierrez Florentin, Cesar Ojeda, Horacio Legal-Ayala, and et al. 2025. "Germline TP53 p.R337H and XAF1 p.E134* Variants: Prevalence in Paraguay and Comparison with Rates in Brazilian State of Paraná and Previous Findings at the Paraguayan–Brazilian Border" Current Oncology 32, no. 6: 333. https://doi.org/10.3390/curroncol32060333
APA StyleFalcon-de Legal, E., Ascurra, M., Vega-Paredes, R., Sade, E., Monteiro, M., Paraízo, M., Colman, M., Florentin, A. G., Ojeda, C., Legal-Ayala, H., & Ries, A. (2025). Germline TP53 p.R337H and XAF1 p.E134* Variants: Prevalence in Paraguay and Comparison with Rates in Brazilian State of Paraná and Previous Findings at the Paraguayan–Brazilian Border. Current Oncology, 32(6), 333. https://doi.org/10.3390/curroncol32060333






