Incidence and Risk Factors of Platinum-Based Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
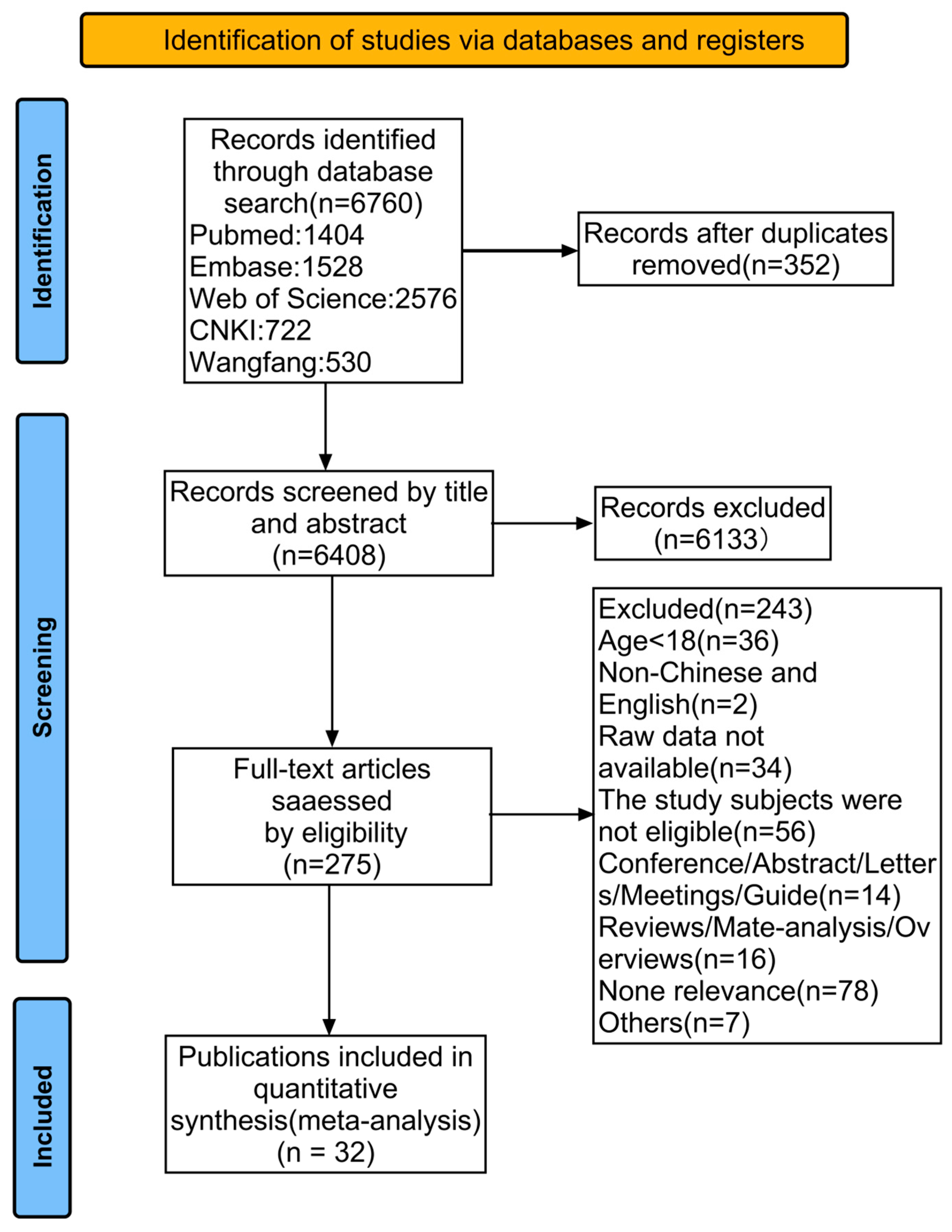
3.1. Literature Search and Study Selection
3.2. Characteristics of the Included Studies
3.3. Result of Quality Assessment
3.4. Meta-Analysis Results
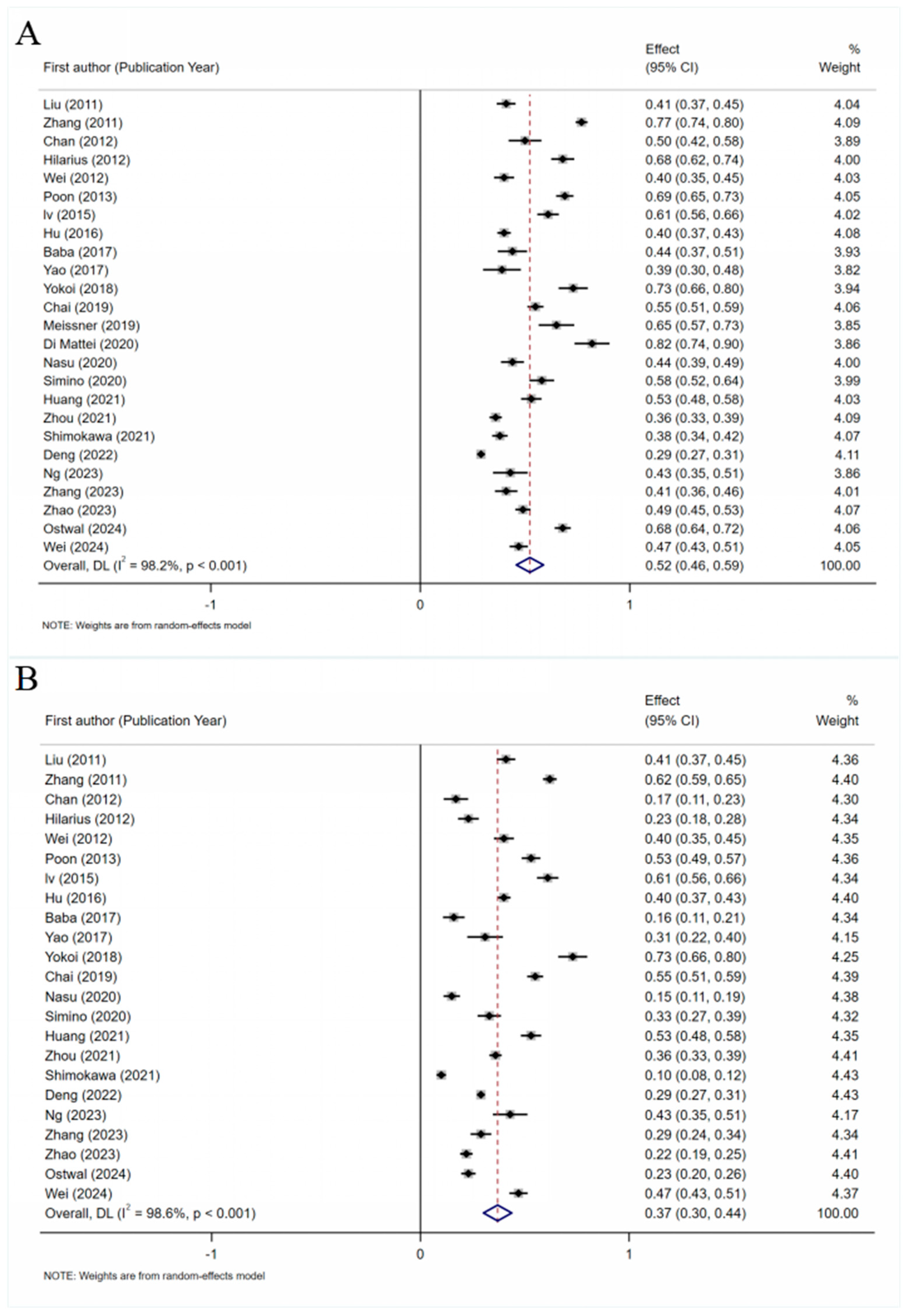
3.4.1. Total Prevalence of PINV
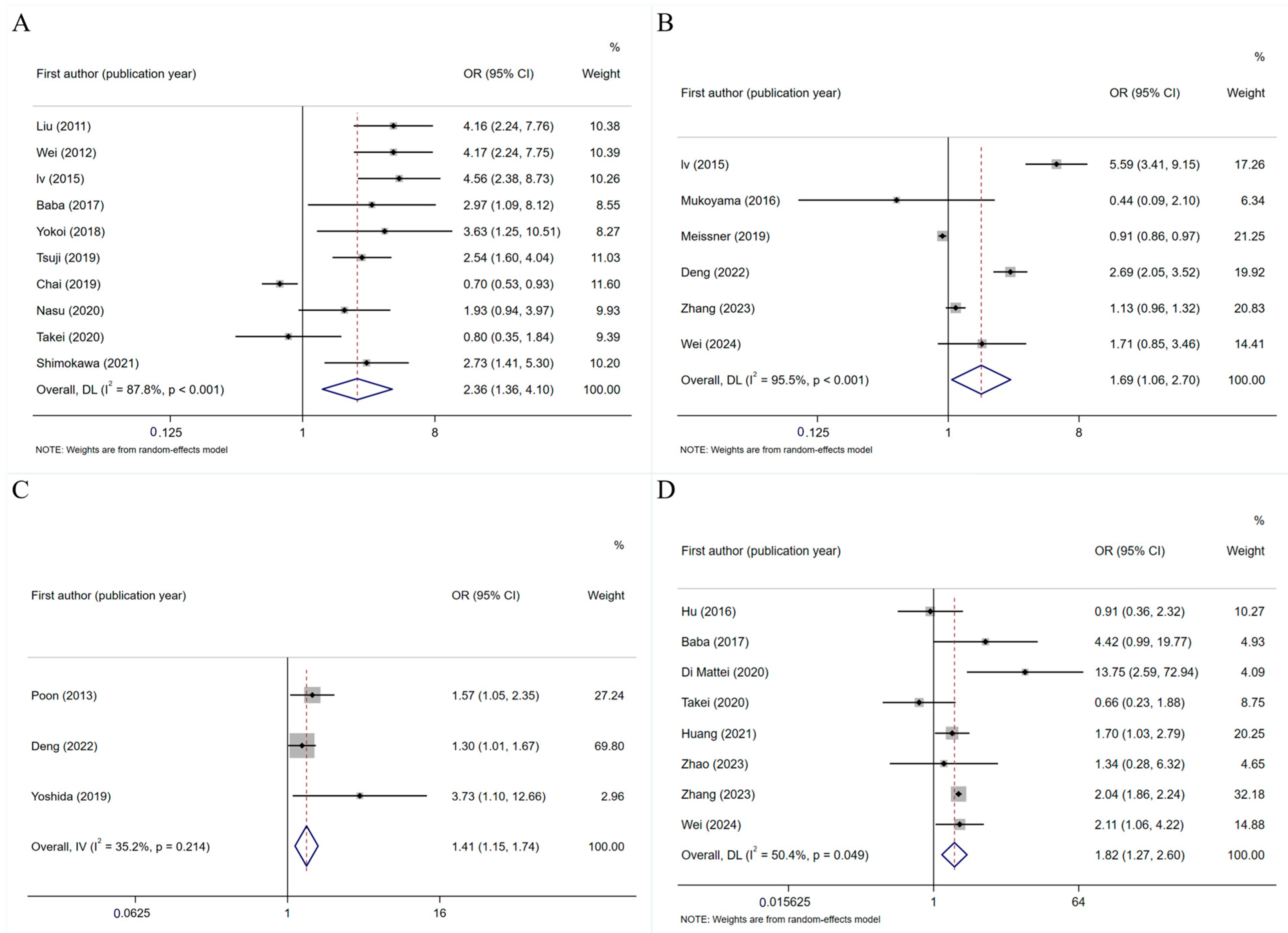
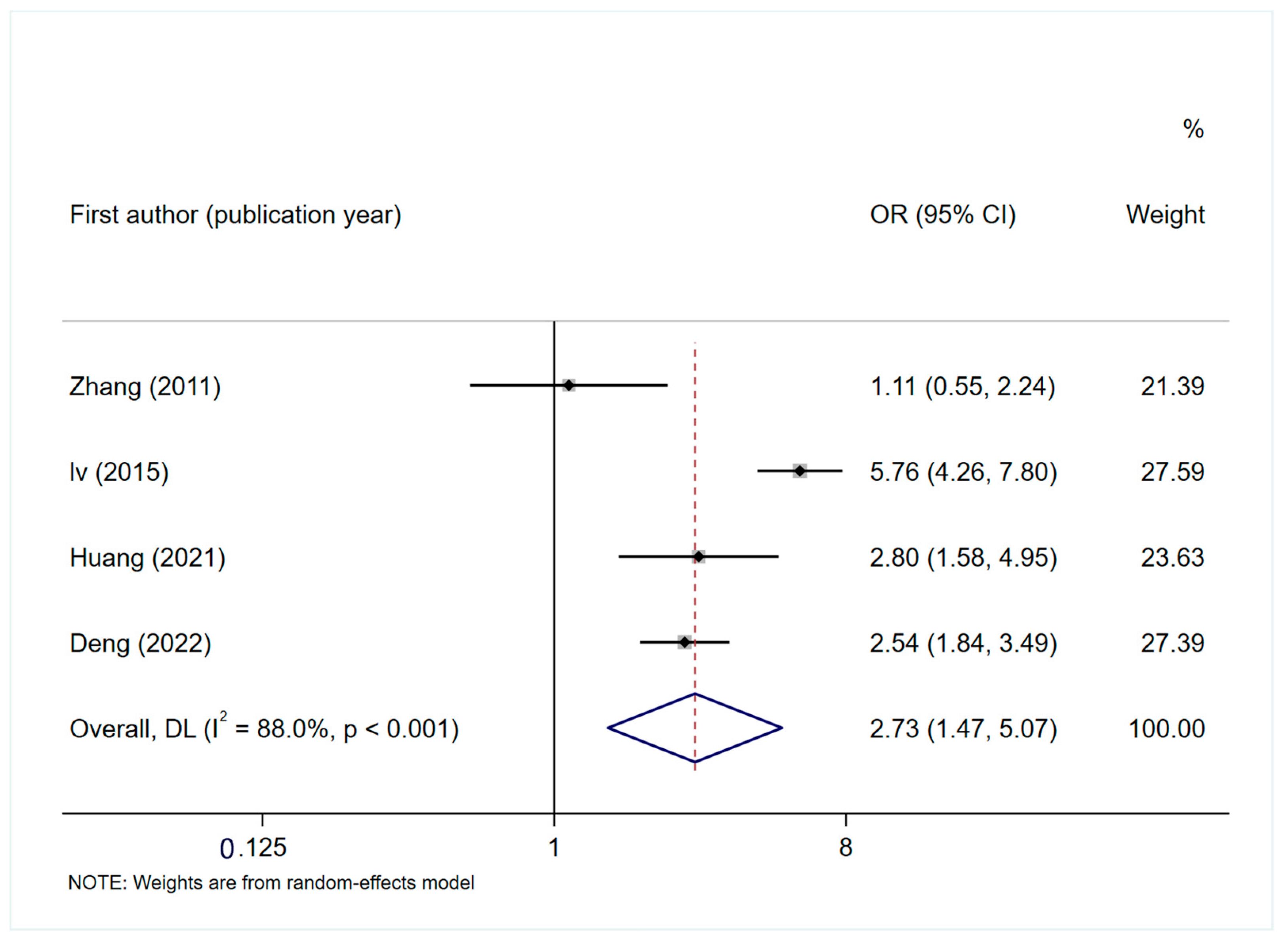
3.4.2. Factors Influencing PINV
General Factors
Disease-Related Factors
Treatment-Related Factors
3.4.3. Heterogeneity Analysis
3.4.4. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GP | Gemcitabine + Cisplatin |
| PINV | Platinum-based chemotherapy-induced nausea and vomiting |
| CINV | Chemotherapy-induced nausea and vomiting |
| CNKI | China National Knowledge Infrastructure |
| CMAJD | Chinese Medical Association Journal Database |
| VIP | China Science and Technology Journal Database |
| DP | Docetaxel + Cisplatin |
| TP | Paclitaxel + Cisplatin/carboplatin |
| NP | Vincristine + Cisplatin |
| EP | Etoposide + Cisplatin |
| XELOX | Capecitabine + Oxaliplatin |
| EN | Etoposide + Cisplatin |
| S-1 | Tegafur + Gimeracil + Oteracil |
| CDDP-P | Cisplatin + Paclitaxel |
| FOLFOX | 5-Fluorouracil/leucovorin + Oxaliplatin |
| NR | Not reported |
| MASCC | Multinational Association of Supportive Care in Cancer |
| RCT | randomized controlled trial |
References
- Herrstedt, J.; Clark-Snow, R.; Ruhlmann, C.H.; Molassiotis, A.; Olver, I.; Rapoport, B.L.; Aapro, M.; Dennis, K.; Hesketh, P.J.; Navari, R.M.; et al. 2023 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. ESMO Open 2024, 9, 102195. [Google Scholar] [CrossRef]
- Marx, W.M.; Teleni, L.; McCarthy, A.L.; Vitetta, L.; McKavanagh, D.; Thomson, D.; Isenring, E. Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: A systematic literature review. Nutr. Rev. 2013, 71, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Apps, M.G.; Choi, E.; Wheate, N.J. The state-of-play and future of platinum drugs. Endocr. Relat. Cancer 2015, 22, R219–R233. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Omlin, A.; Higano, C.; Sweeney, C.; Martinez Chanza, N.; Mehra, N.; Kuppen, M.C.P.; Beltran, H.; Conteduca, V.; Vargas Pivato de Almeida, D.; et al. Activity of Platinum-Based Chemotherapy in Patients with Advanced Prostate Cancer with and without DNA Repair Gene Aberrations. JAMA Netw. Open 2020, 3, e2021692. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- McKeage, M.J. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995, 13, 228–244. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Zhang, M.; Yao, J.; Leng, S.; Li, X.; Lin, L.; Chen, J.; Zhang, S.; Qin, X.; et al. Randomized, Phase III Trial of Mixed Formulation of Fosrolapitant and Palonosetron (HR20013) in Preventing Cisplatin-Based Highly Emetogenic Chemotherapy-Induced Nausea and Vomiting: PROFIT. J. Clin. Oncol. 2025, 43, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Yan, Y.; Wang, Y.; Li, R.; Liu, W.; Qiu, L.; Sun, L.; Yang, Y. Research trends on chemotherapy induced nausea and vomiting: A bibliometric analysis. Front. Pharmacol. 2024, 15, 1369442. [Google Scholar] [CrossRef]
- Aapro, M.S.; Molassiotis, A.; Olver, I. Anticipatory nausea and vomiting. Support. Care Cancer 2005, 13, 117–121. [Google Scholar] [CrossRef][Green Version]
- Hesketh, P.J.; Gralla, R.J.; du Bois, A.; Tonato, M. Methodology of antiemetic trials: Response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Support. Care Cancer 1998, 6, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Roila, F.; Donati, D.; Tamberi, S.; Margutti, G. Delayed emesis: Incidence, pattern, prognostic factors and optimal treatment. Support. Care Cancer 2002, 10, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Kiss, N.; McCarthy, A.L.; McKavanagh, D.; Isenring, L. Chemotherapy-Induced Nausea and Vomiting: A Narrative Review to Inform Dietetics Practice. J. Acad. Nutr. Diet. 2016, 116, 819–827. [Google Scholar] [CrossRef]
- Breen, D.M.; Kim, H.; Bennett, D.; Calle, R.A.; Collins, S.; Esquejo, R.M.; He, T.; Joaquim, S.; Joyce, A.; Lambert, M. GDF-15 neutralization alleviates platinum-based chemotherapy-induced emesis, anorexia, and weight loss in mice and nonhuman primates. Cell Metab. 2020, 32, 938–950.E6. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J.; Kris, M.G.; Basch, E.; Bohlke, K.; Barbour, S.Y.; Clark-Snow, R.A.; Danso, M.A.; Dennis, K.; Dupuis, L.L.; Dusetzina, S.B. Antiemetics: ASCO guideline update. J. Clin. Oncol. 2020, 38, 2782–2797. [Google Scholar] [CrossRef]
- Adel, N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am. J. Manag. Care 2017, 23, S259–S265. [Google Scholar]
- Fleishman, S.B.; Mahajan, D.; Rosenwald, V.; Nugent, A.V.; Mirzoyev, T. Prevalence of Delayed Nausea and/or Vomiting in Patients Treated With Oxaliplatin-Based Regimens for Colorectal Cancer. J. Oncol. Pract. 2012, 8, 136–140. [Google Scholar] [CrossRef]
- Yokoi, M.; Tsuji, D.; Suzuki, K.; Kawasaki, Y.; Nakao, M.; Ayuhara, H.; Kogure, Y.; Shibata, K.; Hayashi, T.; Hirai, K.; et al. Genetic risk factors for chemotherapy-induced nausea and vomiting in patients with cancer receiving cisplatin-based chemotherapy. Support. Care Cancer 2018, 26, 1505–1513. [Google Scholar] [CrossRef]
- Mizukami, N.; Yamauchi, M.; Koike, K.; Watanabe, A.; Ichihara, K.; Masumori, N.; Yamakage, M. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients Receiving Highly or Moderately Emetogenic Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Study. J. Pain Symptom Manag. 2014, 47, 542–550. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; D’Cruz, A.; Fey, M.; Pollock, R.E.; Vermorken, J.; Huang, S.H. UICC Manual of Clinical Oncology; Wiley Online Library: Hoboken, NJ, USA, 2015; Volume 267. [Google Scholar]
- Butrum, R.R.; Clifford, C.K.; Lanza, E. NCI dietary guidelines: Rationale. Am. J. Clin. Nutr. 1988, 48, 888–895. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, Y.; Hu, Y.; Xing, W. The joanna briggs institute critical appraisal tools for use in systematic review: Prevalence study and analytical cross sectional study. J. Nurses Train. 2018, 33, 219–221. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhou, S.; Peterson, C.B.; Wang, Y.; Fang, X.; Wang, M.L.; Shen, C. Meta-Analysis of Censored Adverse Events. N. Engl. J. Stat. Data Sci. 2024, 2, 380–392. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, Z.; Peterson, C.B.; Bao, L.; Zhou, S. Shiny-MAGEC: A Bayesian R Shiny Application for Meta-analysis of Censored Adverse Events. arXiv 2024, arXiv:2503.05982. [Google Scholar]
- Min, L.; Wen, C.; Deai, L. Logistic regression analysis of risk factors associated with nausea and vomiting. Qilu Med. J. 2011, 26, 209–210+213. [Google Scholar]
- Zhang, Y.; Kang, S.; Shen, Q.; Chen, X. Analysis of the Impact of Nausea and Vomiting on Patients with Malignant Tumors Undergoing Chemotherapy. Fujian J. Med. 2011, 33, 157–158. [Google Scholar] [CrossRef]
- Chan, A.; Tan, S.H.; Low, X.H.; Yap, K.Y. Antiemetic effectiveness and nausea and vomiting incidence during capecitabine and oxaliplatin chemotherapy. Nurs. Res. 2012, 61, 405–412. [Google Scholar] [CrossRef]
- Hilarius, D.L.; Kloeg, P.H.; van der Wall, E.; van den Heuvel, J.J.; Gundy, C.M.; Aaronson, N.K. Chemotherapy-induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support. Care Cancer 2012, 20, 107–117. [Google Scholar] [CrossRef]
- Huajun, W. Logistic control study on factors related to nausea and vomiting in patients with lung cancer after chemotherapy. Contemp. Med. 2012, 18, 105–106.64. [Google Scholar]
- Poon, K.S.; Un, M.K.; Low, X.H.; Cheung, Y.T.; Yap, K.Y.; Chan, A. Impact of cancer-related fatigue on chemotherapy-induced nausea and vomiting in Asian cancer patients. Pharmacoepidemiol. Drug Saf. 2013, 22, 1345–1351. [Google Scholar] [CrossRef]
- Jiao, L.H.; Beiwei, Y.; Xing, F. Risk factors of chemotherapy-induced nausea and vomiting in cancer patients. PLA Nurs. J. 2015, 32, 10–13. [Google Scholar]
- Hu, Z.; Liang, W.; Yang, Y.; Keefe, D.; Ma, Y.; Zhao, Y.; Xue, C.; Huang, Y.; Zhao, H.; Chen, L.; et al. Personalized Estimate of Chemotherapy-Induced Nausea and Vomiting: Development and External Validation of a Nomogram in Cancer Patients Receiving Highly/Moderately Emetogenic Chemotherapy. Medicine 2016, 95, e2476. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, N.; Yoshimi, A.; Goto, A.; Kotani, H.; Ishikawa, K.; Miyazaki, N.; Miyazaki, M.; Yamada, K.; Kikkawa, F.; Hasegawa, Y.; et al. An Analysis of Behavioral and Genetic Risk Factors for Chemotherapy-Induced Nausea and Vomiting in Japanese Subjects. Biol. Pharm. Bull. 2016, 39, 1852–1858. [Google Scholar] [CrossRef][Green Version]
- Baba, Y.; Baba, H.; Yamamoto, S.; Shimada, H.; Shibata, T.; Miyazaki, T.; Yoshikawa, T.; Nakajima, Y.; Tsuji, Y.; Shimokawa, M.; et al. Chemotherapy-induced nausea and vomiting is less controlled at delayed phase in patients with esophageal cancer: A prospective registration study by the CINV Study Group of Japan. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, C.; He, Y.; Pan, Y. Relationship between Helicobacter pylori infection and vomiting induced by gastrointestinal cancer chemotherapy. Intern. Med. J. 2017, 47, 792–797. [Google Scholar] [CrossRef]
- Limin, C.; Junting, J.; Jing, Y.; Zhiying, H. Analysis of high risk factors for nausea and vomiting induced by chemotherapy. Chinese Medicine and Clinical Practice. 2019, 19, 2770–2772. [Google Scholar]
- Meissner, K.; Talsky, N.; Olliges, E.; Jacob, C.; Stötzer, O.J.; Salat, C.; Braun, M.; Flondor, R. Individual Factors Contributing to Nausea in First-Time Chemotherapy Patients: A Prospective Cohort Study. Front. Pharmacol. 2019, 10, 410. [Google Scholar] [CrossRef]
- Tsuji, D.; Suzuki, K.; Kawasaki, Y.; Goto, K.; Matsui, R.; Seki, N.; Hashimoto, H.; Hama, T.; Yamanaka, T.; Yamamoto, N.; et al. Risk factors associated with chemotherapy-induced nausea and vomiting in the triplet antiemetic regimen including palonosetron or granisetron for cisplatin-based chemotherapy: Analysis of a randomized, double-blind controlled trial. Support. Care Cancer 2019, 27, 1139–1147. [Google Scholar] [CrossRef]
- Yoshida, N.; Taguchi, T.; Nakanishi, M.; Inoue, K.; Okayama, T.; Ishikawa, T.; Otsuji, E.; Takayama, K.; Kuroboshi, H.; Kanazawa, M.; et al. Efficacy of the combination use of aprepitant and palonosetron for improving nausea in various moderately emetogenic chemotherapy regimens. BMC Pharmacol. Toxicol. 2019, 20, 6. [Google Scholar] [CrossRef]
- Di Mattei, V.E.; Carnelli, L.; Taranto, P.; Mazzetti, M.; Perego, G.; Rottoli, S.; Rancoita, P.M.V.; Bergamini, A.; Petrone, M.; Rabaiotti, E.; et al. Chemotherapy-induced nausea in a sample of gynaecological cancer patients: Assessment issues and personal risk factors evaluation. Support. Care Cancer 2020, 28, 5343–5351. [Google Scholar] [CrossRef]
- Nasu, I.; Shimano, R.; Kawazoe, H.; Nakamura, T.; Miura, Y.; Takano, T.; Hayashi, M. Patient-related Risk Factors for Nausea and Vomiting With Standard Antiemetics in Patients With Cancer Receiving Carboplatin: A Retrospective Study. Clin. Ther. 2020, 42, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Simino, G.P.R.; Reis, I.A.; Acurcio, F.A.; Andrade, E.I.G.; Brazil, N.M.L.; Cherchiglia, M.L. Risk factors associated with antineoplastic chemotherapy-induced nausea and vomiting. Rev. Saúde Pública 2020, 54, 106. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Ishibe, A.; Watanabe, J.; Watanabe, K.; Suwa, Y.; Suzuki, S.; Nakagawa, K.; Suwa, H.; Ota, M.; Ichikawa, Y.; et al. Risk factors of chemotherapy-induced nausea and vomiting in patients with metastatic colorectal cancer: A prospective cohort study (YCOG1301). Int. J. Color. Dis. 2020, 35, 2323–2329. [Google Scholar] [CrossRef]
- Zou, X.; Yang, X.; Lin, D.; Lin, Y.; Lan, J. Influencing factors and nursing measures of nausea and vomiting in patients with malignant tumor undergoing chemotherapy. Int. J. Nurs. 2020, 39, 2541–2545. [Google Scholar]
- Huang, X.; Li, X.; Li, J.; Luo, L.; Chen, H.; Tan, Y.; Wei, T.; Li, X.; Guo, L.; Cheng, J. Chemotherapy-Induced Nausea and Vomiting in Breast Cancer Patients: A Multicenter Prospective Observational Study. Asia-Pac. J. Oncol. Nurs. 2021, 8, 433–437. [Google Scholar] [CrossRef]
- Huijie, Z. A Real-World Survey of Chemotherapy-Related Nausea and Vomiting in Cancer Patients and Analysis of Risk Factors. Master’s Thesis, Sichuan University, Chengdu, China, 2021. [Google Scholar]
- Shimokawa, M.; Hayashi, T.; Nishimura, J.; Satoh, T.; Fukunaga, M.; Matsui, R.; Tsuji, Y.; Mizuki, F.; Kogawa, T. Pooled analysis of combination antiemetic therapy for chemotherapy-induced nausea and vomiting in patients with colorectal cancer treated with oxaliplatin-based chemotherapy of moderate emetic risk. BMC Cancer 2021, 21, 1111. [Google Scholar] [CrossRef]
- Deng, B.; Chen, Y.; Bian, Z.; Ju, J.; Zhou, Y.; Zhang, H.; Zhang, X.; Yand, R.; Xu, Z. Construction of a predictive model for the risk of chemotherapy-associated nausea and vomiting. China Nurs. Manag. 2022, 22, 1384–1390. [Google Scholar] [CrossRef]
- Bo, S.; Xun, L.; Huipin, W.; Shufang, L.; Erfeng, Z.; Changqing, M.; Pan, L.; Danna, L. Gender differences in risk factors for chemotherapy-related nausea and vomiting. Cent. South Pharm. 2022, 20, 1176–1182. [Google Scholar]
- Zhe, Y.; Yuan, L.; Jing, Z.; Zhaoyu, T.; Lin, C. Logistic regression analysis of risk factors associated with nausea and vomiting. J. Xinjiang Med. Univ. 2022, 45, 505–510. [Google Scholar]
- Ng, B.; Astari, Y.K.; Adrian Wiranata, J.; Leo, B.; Hutajulu, S.H.; Hardianti, M.S.; Taroeno-Hariadi, K.W.; Kurnianda, J.; Purwanto, I. Chemotherapy-Induced Nausea and Vomiting in Patients With Breast Cancer: Risk Factor and Predictive Model Using Classification and Regression Tree (CART). Cureus 2023, 15, e44438. [Google Scholar] [CrossRef]
- Yuqing, Z.; Chen, S.W.; Liping, W. Analysis of influencing factors of chemotherapy-related nausea and vomiting and construction of nomogram model. Med. High. Vocat. Educ. Mod. Nurs. 2023, 6, 346–351. [Google Scholar]
- Zhao, Y.; Zhao, B.; Chen, G.; Chen, Y.; Liao, Z.; Zhang, H.; Feng, W.; Li, Y.; Weng, H.; Li, W.; et al. Validation of different personalized risk models of chemotherapy-induced nausea and vomiting: Results of a randomized, double-blind, phase III trial of fosaprepitant for cancer patients treated with high-dose cisplatin. Cancer Commun. 2023, 43, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, V.; Ramaswamy, A.; Mandavkar, S.; Bhargava, P.; Naughane, D.; Sunn, S.F.; Srinivas, S.; Kapoor, A.; Mishra, B.K.; Gupta, A.; et al. Olanzapine as Antiemetic Prophylaxis in Moderately Emetogenic Chemotherapy: A Phase 3 Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2426076. [Google Scholar] [CrossRef] [PubMed]
- Wei, X. Analysis on the Occurrence of Vomiting during Chemotherapy and Its Influencing Factors in Patients with Breast Cancer. Med. Innov. China 2024, 21, 142–145. [Google Scholar] [CrossRef]
- Zhou, M.; Popovic, M.; Pasetka, M.; Pulenzas, N.; Ahrari, S.; Chow, E.; DeAngelis, C. Update on the management of chemotherapy-induced nausea and vomiting—focus on palonosetron. Ther. Clin. Risk Manag. 2015, 11, 713–729. [Google Scholar] [CrossRef][Green Version]
- Navari, R.M.; Nelson, W.W.; Shoaib, S.; Singh, R.; Zhang, W.; Bailey, W.L. Real-World Treatment Outcomes, Healthcare Resource Use, and Costs Associated with Antiemetics Among Cancer Patients on Cisplatin-Based Chemotherapy. Adv. Ther. 2023, 40, 3217–3226. [Google Scholar] [CrossRef]
- Hammer, C.; Fasching, P.A.; Loehberg, C.R.; Rauh, C.; Ekici, A.B.; Jud, S.M.; Bani, M.R.; Beckmann, M.W.; Strick, R.; Niesler, B. Polymorphism in HTR3D shows different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. Pharmacogenomics 2010, 11, 943–950. [Google Scholar] [CrossRef]
- Frazier, K.; Kambal, A.; Zale, E.A.; Pierre, J.F.; Hubert, N.; Miyoshi, S.; Miyoshi, J.; Ringus, D.L.; Harris, D.; Yang, K.; et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe 2022, 30, 809–823.E6. [Google Scholar] [CrossRef]
- Molassiotis, A.; Aapro, M.; Dicato, M.; Gascon, P.; Novoa, S.A.; Isambert, N.; Burke, T.A.; Gu, A.; Roila, F. Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: Results from a European prospective observational study. J. Pain Symptom Manag. 2014, 47, 839–848.E4. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, E.; Kim, I.; Lee, K.W.; Kim, T.M.; Lee, S.H.; Kim, D.W.; Heo, D.S. Cisplatin-Based Chemotherapy is a Strong Risk Factor for Thromboembolic Events in Small-Cell Lung Cancer. Cancer Res. Treat. 2015, 47, 670–675. [Google Scholar] [CrossRef]
- Hesketh, P.J. Chemotherapy-induced nausea and vomiting. New Engl. J. Med. 2008, 358, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Chinese Guidelines for the Prevention and Treatment of Chemotherapy-Induced Nausea and Vomiting in Antitumor Therapy (2023 Edition). Chin. J. Oncol. 2024, 46, 481–501. [Google Scholar]
- Chic, N.; Schettini, F.; Brasó-Maristany, F.; Sanfeliu, E.; Adamo, B.; Vidal, M.; Martínez, D.; Galván, P.; González-Farré, B.; Cortés, J. Oestrogen receptor activity in hormone-dependent breast cancer during chemotherapy. EBioMedicine 2021, 69, 103451. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.; Grunberg, S.; Herrstedt, J.; de Wit, R.; Gralla, R.J.; Carides, A.; Taylor, A.; Evans, J.; Horgan, K. Combined data from two phase III trials of the NK 1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: Effect of gender on treatment response. Support. Care Cancer 2006, 14, 354–360. [Google Scholar] [CrossRef]
- Mehlsen, M. Anticipatory nausea: The role of individual differences related to sensory perception and autonomic reactivity. Ann. Behav. Med. 2007, 33, 69–79. [Google Scholar]
- Shelke, A.R.; Mustian, K.M.; Morrow, G.R. The pathophysiology of treatment-related nausea and vomiting in cancer patients: Current models. Indian J. Physiol. Pharmacol. 2004, 48, 256–268. [Google Scholar]
- Baker, P.D.; Morzorati, S.L.; Ellett, M.L. The pathophysiology of chemotherapy-induced nausea and vomiting. Gastroenterol. Nurs. 2005, 28, 469–480. [Google Scholar] [CrossRef]
- McRonald, F.E.; Fleisher, D.R. Anticipatory nausea in cyclical vomiting. BMC Pediatr. 2005, 5, 3. [Google Scholar] [CrossRef]
- Aybar, D.O.; Kılıc, S.P.; Çınkır, H.Y. The effect of breathing exercise on nausea, vomiting and functional status in breast cancer patients undergoing chemotherapy. Complement. Ther. Clin. Pract. 2020, 40, 101213. [Google Scholar] [CrossRef]
- Molassiotis, A.; Yung, H.P.; Yam, B.M.; Chan, F.Y.; Mok, T. The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: A randomised controlled trial. Support. Care Cancer 2002, 10, 237–246. [Google Scholar] [CrossRef]
- Syrjala, K.L.; Cummings, C.; Donaldson, G.W. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: A controlled clinical trial. Pain 1992, 48, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Samami, E.; Shahhosseini, Z.; Hamzehgardeshi, Z.; Elyasi, F. Psychological interventions in chemotherapy-induced nausea and vomiting in women with breast cancer: A systematic review. Iran. J. Med. Sci. 2022, 47, 95. [Google Scholar] [PubMed]
- Venkatesan, T.; Levinthal, D.J.; Tarbell, S.E.; Jaradeh, S.S.; Hasler, W.L.; Issenman, R.M.; Adams, K.A.; Sarosiek, I.; Stave, C.D.; Sharaf, R.N. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterol. Motil. 2019, 31, e13604. [Google Scholar] [CrossRef]
- Kwak, S.M.; Choi, Y.S.; Yoon, H.M.; Kim, D.G.; Song, S.H.; Lee, Y.J.; Yeom, C.H.; Koh, S.J.; Park, J.; Lee, M.A. The relationship between interleukin-6, tumor necrosis factor-α, and fatigue in terminally ill cancer patients. Palliat. Med. 2012, 26, 275–282. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: Systematic review and meta-analysis of randomized controlled trials. BioMed Res. Int. 2015, 2015, 328636. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Higashijima, J.; Okitsu, H.; Miyake, H.; Yagi, T.; Miura, M.; Bando, Y.; Ando, T.; Hotchi, M.; Ishikawa, M. Effects of chemotherapy on quality of life and night-time sleep of colon cancer patients. J. Med. Investig. 2020, 67, 338–342. [Google Scholar] [CrossRef]
- Hosseini Koukamari, P.; Karimy, M.; Ghaffari, M.; Milajerdi, A. Effect of cognitive-behavioral therapy on fatigue in cancer patients: A systematic review and meta-analysis. Front. Psychol. 2025, 15, 1435110. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. A receptor-based paradigm of nausea and vomiting. J. Cancer Pain Symptom Palliation 2005, 1, 11–23. [Google Scholar] [CrossRef]
- Wickham, R.J. Revisiting the physiology of nausea and vomiting—Challenging the paradigm. Support. Care Cancer 2020, 28, 13–21. [Google Scholar] [CrossRef]
- Zhong, W.; Shahbaz, O.; Teskey, G.; Beever, A.; Kachour, N.; Venketaraman, V.; Darmani, N.A. Mechanisms of nausea and vomiting: Current knowledge and recent advances in intracellular emetic signaling systems. Int. J. Mol. Sci. 2021, 22, 5797. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Y. Chinese expert consensus on the management of clinical pathway and adverse events of trastuzumab deruxtecan (2024 edition). Chin. J. Oncol. 2024, 46, 304–318. [Google Scholar]
- Hesketh, P.J.; Drews, R.E.; Vora, S.R. Prevention of Chemotherapy-Induced Nausea and Vomiting in Adults. 2024. Available online: https://www.uptodate.com/contents/prevention-of-chemotherapy-induced-nausea-and-vomiting-in-adults (accessed on 10 May 2025).
- Roila, F.; Molassiotis, A.; Herrstedt, J.; Aapro, M.; Gralla, R.J.; Bruera, E.; Clark-Snow, R.A.; Dupuis, L.L.; Einhorn, L.H.; Feyer, P.; et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, V119–V133. [Google Scholar] [CrossRef] [PubMed]
| First Author | Publication Year | Study Region | Study Design | Sample (Total-Male-Female) | Incidence of Nausea (%) | Incidence of Vomiting (%) | Average Age, y | Tumor Type | Chemotherapeutic Regimen | Influencing Factor |
|---|---|---|---|---|---|---|---|---|---|---|
| Liu [26] | 2011 | China | Cross-sectional study | 459-286-173 | 40.52% | 40.52% | 62.2 | Lung cancer | GP (Gemcitabine + Cisplatin), DP (Docetaxel + Cisplatin), TP (Paclitaxel + Cisplatin), NP (Vincristine + Cisplatin), or EP (Etoposide + Cisplatin) | Gender, age, alcohol consumption, platinum dosage |
| Zhang [27] | 2011 | China | Cross-sectional study | 831-338-493 | 77.10% | 62.30% | 53 | Small-cell lung cancer | Cisplatin(≥50 mg/m2), Carboplatin, Oxaliplatin(≥50 mg/m2) | Chemotherapy regimen, history of vomiting during pregnancy, anxiety, combined use of other antiemetic drugs, digestive system tumors |
| Chan [28] | 2012 | Singapore | Cohort study | 156-88-68 | 50.00% | 16.70% | 60 | Lower gastrointestinal malignancies | XELOX (Capecitabine + Oxaliplatin) | Oxaliplatin (<mg/m2), fewer than three risk factors |
| Hilarius [29] | 2012 | Japan | Cohort study | 275-85-790 | 68.00% | 23.00% | 56 | Breast cancer, lung cancer, colorectal cancer | Carboplatin-based chemotherapy regimens | Use of antiemetic treatment |
| Wei [30] | 2012 | China | Cross-sectional study | 400-235-165 | 39.80% | 39.80% | 61.5 ± 2.5 | Lung cancer | NP, TP, DP (Docetaxel + Cisplatin), GP or EN (Etoposide + Cisplatin) | Age, female sex, alcohol consumption, dosage of platinum/cisplatin |
| Poon [31] | 2013 | Singapore | Cohort study | 473-197-276 | 69.10% | 52.70% | 55 | Gastrointestinal cancer, breast cancer, head and neck cancer | XELOX, Cisplatin-based regimens | Fatigue |
| lv [32] | 2015 | China | Cross-sectional study | 345-178-167 | 60.57% | 60.57% | 54 ± 4.5 | Colorectal cancer, pancreatic cancer, gastric cancer, esophageal cancer, breast cancer, bladder cancer, liver cancer, colon cancer | Platin-based chemotherapy regimens | Age, female, history of CINV, anxiety, pain, chemotherapy cycles |
| Hu [33] | 2016 | China | Cohort study | 881-472-390 | 40.00% | 40.00% | 56 | Lung cancer, breast cancer, head and neck squamous cell carcinoma, colon cancer, gynecologic cancer, stomach cancer, and others | Carboplatin, Cisplatin | Femal sex, age, alcohol consumption, history of motion sickness, BSA, emetogenicity of chemotherapy, antiemetic regimens |
| Mukoyama [34] | 2016 | Japan | Cohort study | 55-33-22 | NR | NR | 61.8 ± 8.6 | Ovarian cancer, cervical cancer, endometrial cancer, small-cell lung cancer, non-small-cell lung cancer, malignant pleural mesothelioma | Carboplatin + Paclitaxel, Carboplatin + Docetaxel, Cisplatin + 5-Fluorouracil, Cisplatin + Pemetrexed, Cisplatin + Gemcitabine, Cisplatin + Etoposide, Cisplatin + Vinorelbine, Cisplatin + Tegafur, Gimeracil, and Oteracil, Carboplatin + Pemetrexed, Carboplatin + Gemcitabine, Carboplatin + Etoposide, Carboplatin + Paclitaxel + Bevacizumab | Female, use of non-steroidal anti-inflammatory drugs, susceptibility to motion sickness, anxiety |
| Baba [35] | 2017 | Japan | Cohort study | 192-164-28 | 43.80% | 15.80% | 66 | Esophageal cancer | Cisplatin + 5-Fluorouracil), Cisplatin + 5-Fluorouracil + Docetaxel, Cisplatin + 5-Fluorouracil + Adriamycin, Docetaxel + nedaplatin + 5-Fluorouracil, Docetaxel + nedaplatin+ S-1 (Tegafur, gimeracil, and oteracil), Nedaplatin + 5-Fluorouracil, Nedaplatin | Motion sickness, age, use of other antiemetics |
| Yao [36] | 2017 | China | Cross-sectional study | 112-71-41 | 39.05% | 31.10% | 62.8 | Gastric cancer, colon cancer, rectal cancer | Oxaliplatin + Fluorouracil | Pylori infection |
| Yokoi [18] | 2018 | Japan | Cohort study | 156-98-58 | 73.00% | 73.00% | 63 | NR (Not reported) | Cisplatin-based regimens | ERCC1 8092AA and female sex |
| Chai [37] | 2019 | China | Cohort study | 643-345-298 | 55.20% | 55.20% | 56 ± 10 | Respiratory system tumors, digestive system tumors, breast tumors | Cisplatin + Adriamycin + Cyclophosphamide, Oxaliplatin | Age, female sex, alcohol consumption |
| Meissner [38] | 2019 | Germany | Cohort study | 121-0-121 | 65.00% | NR | 53 | Breast cancer | Platinum-based chemotherapy regimens | Age, cancer type, history of nausea, state and trait anxiety, lower quality of life |
| Tsuji [39] | 2019 | Japan | Cohort study | 825-615-210 | NR | NR | 64 | Lung cancer, esophageal cancer, stomach cancer, head and neck cancer | Cisplatin | Sex, age, cisplatin dose, granisetron use |
| Yoshida [40] | 2019 | Japan | Cohort study | 312-148-164 | NR | NR | NR | Digestive organ cancers, lung cancers, gynecological cancers | Oxaliplatin, Carboplatin | Elevation of serum creatinine, fatigue, performance status, elevation in alanine aminotransferase |
| Di Mattei [41] | 2020 | Italy | Cohort study | 81-0-81 | 82.10% | NR | 58 | Ovarian cancer, uterine cancer, vulvar tumor | TP (Paclitaxel + Carboplatin) | Age, anticipatory nausea, patient medium-high expectations of chemotherapy-induced nausea and parity emerged |
| Nasu [42] | 2020 | Japan | Cohort study | 314-141-173 | 43.70% | 15.00% | 67 | NR | Carboplatin | Age and total dexamethasone dose |
| Simino [43] | 2020 | Brasil | Cohort study | 269-80-188 | 58.00% | 32.70% | 55.2 | Breast cancer, colorectal cancer, cervical cancer, head and neck cancer, lung cancer, esophagus cancer, gastric cancer, ovarian cancer, lymphoma, and others | Cisplatin, CDDP-P (Cisplatin + Paclitaxel), Carboplatin + Paclitaxel, Cisplatin + Fluouracil, EP | Age, tobacco use, high emetogenic chemotherapy |
| Takei [44] | 2020 | Japan | Cohort study | 179-113-66 | NR | NR | 68 | Colorectal cancer | FOLFOX (5-Fluorouracil/Leucovorin + Oxaliplatin), XELOX | BMI, female sex |
| Zou [45] | 2020 | China | Cohort study | 200-116-84 | NR | NR | NR | Lung cancer, intestinal cancer, breast cancer, lymphoma, cervical cancer, osteocarcinoma | GP, FOLFOX | Abdominal distension, cycles of chemotherapy, chemotherapy regimens, tumor category, physical condition, tumor metastasis |
| Huang [46] | 2021 | China | Cohort study | 400-0-400 | 53.30% | 53.30% | 52.65 | Breast cancer | Cisplatin | Pain/insomnia, history of CINV, chemotherapy regimens, history of motion sickness, history of CINV, chemotherapy cycles, incidence of acute CINV |
| Zhou [47] | 2021 | China | Cohort study | 1000-642-468 | 36.00% | 36.00% | 56.67 ± 10.95 | Lung cancer, breast cancer, aasopharyngeal carcinoma, esophageal cancer, gastric cancer, colorectal cancer, cervical cancer, ovarian cancer, lymphoma, gallbladder cancer, pancreatic cancer, soft tissue sarcoma | Oxaliplatin | Antiemetic drugs, chemotherapy cycles, age, combined radiotherapy |
| Cisplatin, Carboplatin | ||||||||||
| Shimokawa [48] | 2021 | Japan | Cohort study | 661-391-270 | 37.72% | 10.13% | 64 | Colorectal cancer | Oxaliplatin | Female sex, antiemetic drugs |
| Deng [49] | 2022 | China | Cohort study | 2215-1297-918 | 28.80% | 28.80% | 55.79 ± 12.08 | Lung cancer, gastrointestinal tumors, gynecological tumors, breast tumors, and others | Platinum-based chemotherapy regimens | Chemotherapy regimens, chemotherapy regimens including AC (Anthracycline + Cyclophosphamide) drugs, 1 day before chemotherapy, night sleep duration <7 h, prodromal CINV symptoms, moderate to severe anxiety before chemotherapy, pain, age, alcohol consumption, fatigue, previous history of CINV, chemotherapy psychological expectations |
| Sun [50] | 2022 | China | Cohort study | 871-448-423 | NR | NR | 63.03 ± 10.33 | Lung cancer, liver cancer, melanoma, colorectal cancer, breast cancer, gastroesophageal cancer | Platinum-based chemotherapy regimens | Men with larger body surface areas, opioids, and first chemotherapy; women with anxiety and tension |
| Yin [51] | 2022 | China | Cohort study | 110--59-51 | 100.00% | 100.00% | 52.5 | Gastric cancer | Oxaliplatin | Psychological state, chemotherapy cycles, NRS score and dietary quality |
| Ng [52] | 2023 | Indonesia | Cross-sectional study | 137-0-137 | 43.10% | 43.10% | 52.1 | Breast cancer | Cisplatin, Carboplatin | BMI |
| Zhang [53] | 2023 | China | Cohort study | 320-NR-NR | 40.94% | 29.38% | NR | Breast cancer, lung cancer, stomach cancer, colorectal cancer, esophageal cancer, lymphoma, ovarian cancer, and others | Oxaliplatin | Age, gender, history of alcohol consumption, PSQI score, previous history of CINV, history of pregnancy and vomiting, number of chemotherapy sessions, types of antiemetic drugs, participation in social work, motion sickness, use of non-steroidal anti-inflammatory drugs, psychological expectation of CINV, anxiety |
| Zhao [54] | 2023 | China | Cohort study | 720-524-182 | 48.70% | 22.10% | NR | Lung cancer, urogenital cancer, gastrointestinal cancer, and others | Cisplatin-based chemotherapy regimens | Female cancer patients without a history of alcohol consumption, with larger BSA and received high-dose cisplatin |
| Ostwal [55] | 2024 | China | Cohort study | 560-259-301 | 68.00% | 23.00% | 56 | Colorectal cancer, gastric cancer, gastroesophageal cancer, non-small-cell lung cancer, carcinoma, biliary tract carcinoma, urinary bladder cancer, and others | Oxaliplatin, Carboplatin | Use of olanzapine |
| Wei [56] | 2024 | China | Cohort study | 500-0-500 | 47.20% | 47.20% | NR | Breast cancer | Platinum-based chemotherapy regimens | Anxiety, electrolyte imbalance, motion sickness history, gastrointestinal disease history, nausea and vomiting situation at 24 h before chemotherapy, chemotherapy regimens containing anthracycline or platinum, multidrug combination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, K.; Lin, X.; Xia, X.; Ye, H.; Yang, D.; Fan, Y.; Sun, Q.; Wang, R. Incidence and Risk Factors of Platinum-Based Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis. Curr. Oncol. 2025, 32, 325. https://doi.org/10.3390/curroncol32060325
Jin K, Lin X, Xia X, Ye H, Yang D, Fan Y, Sun Q, Wang R. Incidence and Risk Factors of Platinum-Based Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis. Current Oncology. 2025; 32(6):325. https://doi.org/10.3390/curroncol32060325
Chicago/Turabian StyleJin, Kaili, Xianlei Lin, Xiaoting Xia, Huiling Ye, Dan Yang, Ying Fan, Qiuhua Sun, and Rongyun Wang. 2025. "Incidence and Risk Factors of Platinum-Based Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis" Current Oncology 32, no. 6: 325. https://doi.org/10.3390/curroncol32060325
APA StyleJin, K., Lin, X., Xia, X., Ye, H., Yang, D., Fan, Y., Sun, Q., & Wang, R. (2025). Incidence and Risk Factors of Platinum-Based Chemotherapy-Induced Nausea and Vomiting: A Systematic Review and Meta-Analysis. Current Oncology, 32(6), 325. https://doi.org/10.3390/curroncol32060325





