Molecular Prognostic Factors in Uterine Serous Carcinomas: A Systematic Review
Abstract
1. Introduction
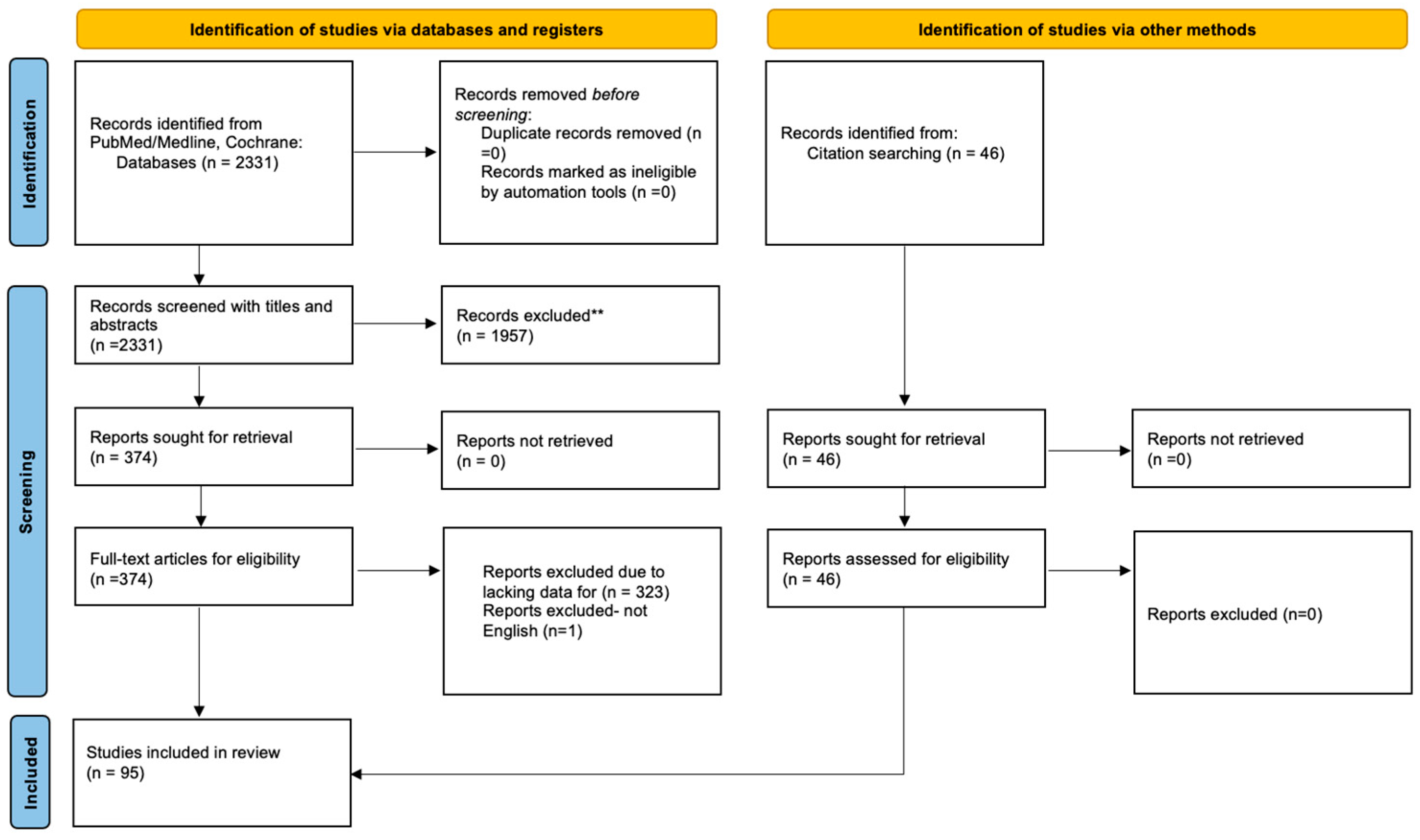
2. Materials and Methods
3. Results
3.1. DNA Repair
3.1.1. BRCA1/2
3.1.2. HRR Status
3.1.3. TP53
| Prognostic Factor | Bibliography | Method | No. Patients/ No. USC Patients | Result |
|---|---|---|---|---|
| P53 | Inoue et al. [10] | IHC | 139/12 | Five-year survival rate p53 overexpressed 60% vs. p53 not-overexpressed 87% |
| Reinartz et al. [13] | IHC | 128/11 | Not an independent prognostic factor of survival | |
| King et al. [14] | IHC | 22/22 | Significantly shorter survival (p < 0.022) | |
| Hamel et al. [16] | IHC | 221/8 | Associated with compromised PFS (p < 0.001), independent prognostic factor for PFS | |
| Bancher-Todesca et al. [17] | IHC | 23/23 | Significantly shorter survival than those whose tumors did not (p = 0.033) | |
| Geisler et al. [18] | IHC | 137/14 | Independent prognostic factor of worse five-year survival in multivariate analysis (p = 0.0028) | |
| Salvesen et al. [20] | IHC | 142/3 | Independent prognostic impact (p < or =0.05) | |
| Coronado et al. [22] | IHC | 114/27 non-endometrioid | p53 (p < 0.001) overexpression had a positive correlation with a high risk of recurrence, independent prognostic indicator of recurrence | |
| Lundgren et al. [24] | IHC | 358/40 non-endometrioid | Significant predictor of relapse (p < 0.001), in multivariate analysis lost its prognostic capability | |
| Alkushi et al. [27] | IHC | 200/13 | Prognostic significance in the subset of patients with endometrioid carcinomas (p = 0.02), but not in patients with clear cell or papillary serous carcinomas | |
| Daniilidou et al. [48] | IHC | 61/12 | Not correlated with stage (p = 0.466) | |
| Vandenput et al. [43] | IHC | 149/92 USC and clear cell | In metastatic or recurent patients correlated with survival (p = 0.01) | |
| Winder et al. [66] | IHC | 313/313 | Associated with worse overall survival (OS) HR, 4.20 [95% CI, 1.54–11.45]; p16: HR, 1.95 [95% CI, 1.01–3.75] and progression free survival (PFS) HR, 2.16 [95% CI, 1.09–4.27] | |
| Jia et al. [73] | IHC | 212/77 | p53 abnormalities correlated with worse disease-free survival (DFS) (p = 0.025). p53 (HR: 2.270, 95% CI: 1.124–4.586, p = 0.022) independently predicted DFS in non-EEC patients, not OS | |
| p53 isoform γ | Bischof et al. [67] | quantitative Real-Time PCRs (RT-qPCR) | 37/37 | Relative p53γ expression to be associated with reduced PFS |
| BRCA | Bruchim et al. [39] | differential restriction | 31/31 | mOS 25 months, no significant differences in the mOS, two-year survival, or PFS between the mutation carriers and the noncarriers |
| Kadan et al. [69] | DNA sequencing | 64/64 | mOS (25 vs. 37 months; p = 0.442), mPFS (37 vs. 29 months; p = 0.536), and mDSS (60 vs. 39 months; p = 0.316) were similar between the carrier and noncarrier groups | |
| BRCA1 | Amichay et al. [44] | IHC | 52/52 | Did not correlate to survival |
| Beirne et al. [47] | IHC | 72/72 | Statistically significant decreased PFS when exhibiting tumor cell nuclei staining of 76% or greater (p = 0.0023) | |
| HRD | Jönsson et al. [79] | OncoScan SNP array | 19/19 | No significant correlation with OS |
| Dong et al. [81] | next-generation sequencing | 60/60 | Similar PFS (HR, 0.500; 95% CI, 0.203–1.232; p = 0.132), but significantly longer DSS in the tHRRmt patients than in the tHRRwt patients (HR, 0.176; 95% CI, 0.050–0.626; p = 0.007), when p53abn: both PFS and DSS were significantly longer in the tHRRmt (p = 0.040 and p = 0.008) |
3.2. Membrane Receptors
3.2.1. ErbB-2/HER2/Neu
3.2.2. EGFR
| Prognostic Factor | Bibliography | Method | No. Patients/No. USC Patients | Result |
|---|---|---|---|---|
| EGFR | Khalifa et al. [11] | IHC—overexpression | 69/16 | Significantly correlated with histologic grade (p < 0.001), metastasis (p < 0.001), cell type (p < 0.01), myometrial invasion (p < 0.01), independent prognostic factor when controlling for cell type |
| Khalifa et al. [12] | IHC—positivity | 69/16 | Significantly correlated with nonendometrioid cell types and tumor metastases, survival from 86 to 27% (p < 0.03) | |
| Reinartz et al. [13] | IHC—positivity | 128/11 | Did not correlate with length of survival or known prognostic variables | |
| Konecny et al. [37] | IHC | 279/134 non-endometrioid | Significantly associated with poor overall survival in patients with type II EC (EGFR, median survival 20 vs. 33 months, p = 0.028), retained prognostic independence when adjusting for histology, stage, grade, and age (p = 0.0197) | |
| HER2 | Khalifa et al. [12] | IHC—overexpression | 69/16 | Significantly associated with depth of myometrial invasion |
| Saffari et al. [15] | gene amplification by FISH | 92 | Shorter overall survival than women whose endometrial cancer lacked amplification (p = 0.018) | |
| IHC—moderate/high expression | 92 | Lower cumulative overall survival by log rank analysis (p < 0.0001), independent predictor of overall survival (p = 0.0163) | ||
| Hamel et al. [16] | IHC | 221/8 | Independent prognostic factor for worse PFS | |
| Rolitsky et al. [19] | gene amplification by FISH | 72/7 | Significant negative predictive value beyond stage, grade, and cell type (p = 0.002) | |
| Coronado et al. [22] | IHC | 316 | HER-2/neu (p = 0.018) overexpression had a positive correlation with a high risk of recurrence, not an independent prognostic indicator of recurrence | |
| Slomovitz et al. [28] | IHC | 68/68 | Overexpression associated with a poorer OS (p = 0.008) | |
| Santin et al. [29] | IHC | 27/27 | Short survival associated significantly with heavy HER2/neu expression (p = 0.02) | |
| Santin et al. [30] | gene amplification by FISH | 30/30 | Significantly shorter survival time from diagnosis to disease-related death | |
| Diaz-Montes et al. [31] | IHC HercepTest (DAKO) | 25/25 | Overexpression significantly associated with a worse survival outcome (HR = 6.58, 95%CI: 1.36–31.89, p = 0.02) | |
| Morrison et al. [32] | IHC | 483/58 | OS significantly shorter (p = 0.0001) in overexpression (median, 5.2 years) versus those that did not (median of all cases, 13 years) | |
| gene amplification by FISH | 483/58 | OS was significantly shorter (p = 0.0001) in amplification of HER-2 (median, 3.5 years) versus those that did not (median of all cases, 13 years) | ||
| Odicino et al. [34] | IHC | 12/12 | Overexpression associated with a poorer OS and a very low relapse-free survival time | |
| Villella et al. [128] | IHC | 26/26 | Correlated with lower OS (p = 0.01) | |
| Singh et al. [34] | IHC | 45/45 | Did not reach statistical significance in OS and PFS, but had a hazard ratio (HR) of 1.5 in RFS | |
| Ren et al. [42] | IHC | 36/36 | Expression 2 + ~3 + significantly associated with advanced surgical stage and worse OS (p = 0.03 and p = 0.0023, resp.) | |
| Konecny et al. [37] | gene amplification by FISH | 279/134 non-endometrioid | Not significantly associated with poor OS in patients with type II EC (HER2, median survival 18 vs. 29 months, p = 0.113) | |
| Togami et al. [46] | IHC | 71/71 | correlated with lower OS (p = 0.01), independent prognostic indicators for RFS (p = 0.022) | |
| Zhang et al. [58] | IHC | Meta-analysis | Correlated with worse outcome with a HR of 1.98 (95% CI, 1.49–2.62) for OS, and a HR of 2.26 (95% CI, 1.57–3.25) for PFS | |
| Chen et al. [62] | IHC | 52/52 | Not assosiated with prognosis | |
| Jamieson et al. [75] | sWGS, targeted panel sequencing, IHC | 187/187 p53abn | Associated with worse outcomes | |
| Erickson et al. [88] | IHC | 169/169 | significantly more recurrences in the HER2-positive cohort (50.0% vs. 16.8%, p < 0.001), associated with worse PFS and OS (p < 0.001, p = 0.024), multivariate analysis: HER2 (+)associated with inferior PFS (aHR 3.50, 95%CI 1.84–6.67; p < 0.001) and OS (aHR 2.00, 95%CI 1.04–3.88; p = 0.039) | |
| Shao et al. [100] | IHC, FISH | 77/77 | amplification significantly associated with deep myometrial invasion (>1/2), and increased intra-epithelial and stromal density of CD20 + or CD8 + TIL (all p < 0.05), associated with poor OS and PFS only in univariate analysis |
3.3. Hormone Receptors
| Prognostic Factor | Bibliography | Method | No. Patients/No. USC Patients | Result |
|---|---|---|---|---|
| Hormone Receptors | Engelsen et al. [33] | IHC | 200/100 non-endometrioid | Loss of hormone receptors significantly correlated with aggressive phenotype and poor prognosis |
| Togami et al. [46] | IHC | 71/71 | Correlated with higher OS (p = 0.008), independent prognostic indicators for RFS (p = 0 p = 0.01), independent factor associated with OS (p = 0.044) | |
| Zhang et al. [58] | IHC | Meta-analysis | Pooled hazard ratios (HRs) of ER for OS, CSS, and PFS were 0.75 (95% CI, 0.68–0.83), 0.45 (95% CI, 0.33–0.62), and 0.66 (95% CI, 0.52–0.85). Combined HRs of PR for OS, CSS, and PFS reached 0.63 (95% CI, 0.56–0.71), 0.62 (95% CI, 0.42–0.93), and 0.45 (95% CI, 0.30–0.68) | |
| Przewoźny et al. [83] | IHC | 103/15 | Loss of ER and PgR expression connected with a poor prognosis. | |
| ERa | Sho et al. [56] | IHC | 33/33 | Cancer-specific five-year survival rates without an expression 54.5% vs. with an expression 0.0% (p = 0.04); significant prognostic indicator in patients with USC (p < 0.05) |
| ER | Kobel et al. [59] | IHC | 192/192 | Not significantly associated with OS |
| Karnezis et al. [64] | IHC | 460/104 | Associated with improved DSS | |
| PR | Kobel et al. [59] | IHC | 192/192 | Significantly associated with favorable OS (log rank, p = 0.0024). PR expression was significantly associated with favorable OS independent of age, stage, center and lymph-vascular invasion in stage I and II USC (hazard ratio = 0.266, 95% CI 0.094–0.750, p = 0.0123) |
| Karnezis et al. [64] | IHC | 460/104 | Assosiated with favourable outcomes [HR (CI) 0.39 (0.25–0.62) for DSS, p < 0.0001] |
3.4. Adhesion Molecules
3.4.1. L1CAM
3.4.2. CD44
| Prognostic Factor | Bibliography | Method | No. Patients/No. USC Patients | Result |
|---|---|---|---|---|
| CD44 | Hosford et al. [25] | IHC | 32/32 | No correlation to known prognostic features |
| L1CAM | Van Gool et al. [61] | IHC | 116/30 non-endometrioid | Significant association with rate of distant metastasis (p = 0.018) |
| Karnezis et al. [64] | IHC | 460/104 | Associated with poor outcomes (hazard ratio (HR) 3.35 [2.10–5.23] DSS, p < 0.0001) |
3.5. Cell Cycle and Intracellular Signaling Pathways
3.5.1. Cyclin D1
3.5.2. P16
3.5.3. Synuclein-γ
3.5.4. PTEN-PI3K-AKT-FBXW7 Pathway
3.5.5. Tubulin-β-III
| Prognostic Factor | Bibliography | Method | No. Patients/No. USC Patients | Result |
|---|---|---|---|---|
| P21 | Salvesen et al. [20] | IHC | 142/3 | Influenced survival in univariate analyses (p < or =0.05), not independent prognostic impact |
| P16 | Salvesen et al. [21] | IHC | 316 | Five-year survival of 47% for absent/minimal nuclear p16 expression vs. 81% for moderate/high nuclear p16 expression (p < 0.0001), independent prognostic factor |
| Winder et al. [66] | IHC | 313/313 | associated with worse OS p16: HR, 1.95 [95% CI, 1.01–3.75] and PFS HR, 1.53 [95% CI, 0.87–2.69] compared with low levels | |
| Synuclein-gamma (SNCG) | Morgan et al. [38] | IHC | 20/20 | Correlated with advanced stage and decreased PFS |
| Winder et al. [66] | IHC | 313/313 | PFS rate at 5 years worse for high SNCG expression, at 40% vs. 56% for low SNCG expression (log-rank p = 0.0081; HR, 1.36; 95% CI, 0.96–1.92 in adjusted Cox model) | |
| class III beta-tubulin | Vandenput et al. [43] | IHC | 149/92 USC and clear cell | No correlation with recurrence or survival |
| Roque et al. [51] | real-time PCR | 48/48 | Overexpression stratified patients by OS (copy number ≤ 400: 615 days; copy number > 400: 165 days, p = 0.049) | |
| PTEN | Daniilidou et al. [48] | IHC | 61/12 | Not correlated with stage (p = 0.267) |
| Karnezis et al. [64] | IHC | 460/104 | Not assosiated with outcomes | |
| CyclinD1 | Liang et al. [50] | IHC | 201/21 | High CyclinD1 expression associated with poor prognosis vs. patients without CyclinD1 staining (p < 0.05) |
| PIK3CA | McIntyre et al. [55] | Sanger sequencing of DNA | 99/26 | Not associated with shorter disease-specific survival (p = 0.57) |
| Lemetre et al. [60] | RNA-sequencing | 323/52 | Not correlated with prognosis | |
| Holst et al. [70] | FISH | 188 | PIK3CA amplifications were associated with disease-specific mortality | |
| Jamieson et al. [75] | sWGS, targeted panel sequencing | 187/187 p53abn | Associated with worse OS | |
| FBXW7 | Chen et al. [62] | IHC | 52/52 | Not assosiated with prognosis |
| Dinoi et al. [74] | IHC | 36/36 | Associated with a decreased risk of progression, after adjusting for stage | |
| PPP2R1A | Taskin et al. [65] | IHC | 78/17 | Significantly related to poor prognosis only in univariate analysis |
| Hong et al. [87] | NGS | 263/41 | PPP2R1A mutations had significantly shorter survival than did those without mutations (p = 0.005 and p < 0.001) | |
| PPP2R1B | Dinoi et al. [74] | IHC | 36/36 | Associated with a decreased risk of progression, after adjusting for stage |
3.6. Cancer Signatures
3.7. Immunogenicity
| Prognostic Factor | Bibliography | Method | No. Patients/No. USC Patients | Result |
|---|---|---|---|---|
| PDL1-PD1 | Mamat et al. [80] | Meta-analysis | 516/84 | Survival outcomes of PD-L1 high expression had a significant association with worse OS in immune cells (IC) but not in tumor cells |
| Zong et al. [91] | IHC–TPS-CPS | 833/113 | PD-L1 TPS (+) but not in ICs or CPS, was associated with a favorable prognosis | |
| Chen et al. [94] | IHC–TPS-CPS | 99/99 | PD-L1 CPS (+) associated with improved OS (p = 0.038), no association between PD-L1 expression and survival was found using TPS | |
| Kucukgoz et al. [102] | IHC | 53/17 | Expression of PD-1 and PD-L1 expressions in tumor area associated with shorter survival (p = 0.006 and 0.001), PD-1 and PD-L1 expressions in microenvironment were not found to be related with survival | |
| Pasanen et al. [103] | Multiplex IHC | 842/29 | Advanced cancers showed more frequent Ca-PD-L1 positivity (p = 0.016), and CPS (p = 0.029) and IC ≥ 1% (p = 0.037) positivity compared with early disease | |
| Engerud et al. [104] | IHC | 689/65 | PD-L1 and PD-1 expression showed no impact on survival | |
| TIM-3 | Chen et al. [94] | IHC–TPS-CPS | 99/99 | No association with survival |
| B7-H3 | Chen et al. [94] | IHC–TPS-CPS | 99/99 | No association with survival |
| lymphocyte-activation gene 3 (LAG-3) | Chen et al. [95] | IHC | 94/94 | High levels of LAG-3 expression associated with better PFS and OS than those with lower levels of expression (PFS, p = 0.03, OS, p = 0.04), multivariate analysis: high TIGIT expression had an independent prognostic value for better OS |
| T-cell immunoglobulin and ITIM domain (TIGIT) | Chen et al. [95] | IHC | 94/94 | High levels of TIGIT expression associated with better PFS and OS than those with lower levels of expression (PFS, p = 0.01, OS, p = 0.009) |
| V-domain immunoglobulin (Ig) suppressor of T-cell activation (VISTA) | Chen et al. [95] | IHC | 94/94 | No significant association with survival |
| CD40 | Zhao et al. [98] | IHC | 68/23 | Correlated with worse OS (p < 0.05) in non-endometroid histologies |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| USC | Uterine Serous Carcinoma |
| dMMR | Deficient Mismatch repair |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| DFS | Disease-Free Survival |
| EC | Endometrial Cancer |
| IHC | Immunohistochemistry |
| HBOC | Hereditary Breast-Ovarian Cancer |
| HRR | Homologous Recombination Repair |
| HRD | Homologous Recombination Repair Deficient |
| LVSI | Lymph-vascular space invasion |
| TCGA | The Cancer Genome Atlas |
| SNCG | Synuclein-gamma |
| HGSOC | high-grade serous ovarian cancer |
| IO | Immunotherapy |
| TPS | Tumor proportion score |
| CPS | Combined positive score |
| TILs | Tumor-infiltrating lymphocytes |
References
- Scarfone, G.; Secomandi, R.; Parazzini, F.; Vigano, R.; Mangili, G.; Frigerio, L.; Villa, A.; Tateo, S.; Ricci, E.; Bolis, G. Clear cell and papillary serous endometrial carcinomas: Survival in a series of 128 cases. Arch. Gynecol. Obstet. 2013, 287, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Tang, Y.H.; Chiang, Y.C.; Wang, K.L.; Fu, H.C.; Ke, Y.M.; Lau, H.Y.; Hsu, K.F.; Wu, C.H.; Cheng, W.F. Impact of management on the prognosis of pure uterine papillary serous cancer—A Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol. Oncol. 2014, 133, 221–228. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Schettino, C.; Arenare, L.; Pisano, C.; Lombardi, D.; De Giorgi, U.; Andreetta, C.; Cinieri, S.; De Angelis, C. Carboplatin and paclitaxel plus avelumab compared with carboplatin and paclitaxel in advanced or recurrent endometrial cancer (MITO END-3): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2023, 24, 286–296. [Google Scholar] [CrossRef]
- Powell, M.; Bjørge, L.; Willmott, L.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.; Gropp-Meier, M. Overall survival in patients with endometrial cancer treated with dostarlimab plus carboplatin-paclitaxel in the randomized ENGOT-EN6/GOG-3031/RUBY trial. Ann. Oncol. 2024, 35, 728–738. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Inoue, M.; Okayama, A.; Fujita, M.; Enomoto, T.; Sakata, M.; Tanizawa, O.; Ueshima, H. Clinicopathological characteristics of p53 overexpression in endometrial cancers. Int. J. Cancer 1994, 58, 14–19. [Google Scholar] [CrossRef]
- Khalifa, M.A.; Abdoh, A.A.; Mannel, R.S.; Haraway, S.D.; Walker, J.L.; Min, K.W. Prognostic utility of epidermal growth factor receptor overexpression in endometrial adenocarcinoma. Cancer 1994, 73, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.A.; Mannel, R.S.; Haraway, S.D.; Walker, J.; Min, K.W. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol. Oncol. 1994, 53, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, J.J.; George, E.; Lindgren, B.R.; Niehans, G.A. Expression of p53, transforming growth factor alpha, epidermal growth factor receptor, and c-erbB-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Hum. Pathol. 1994, 25, 1075–1083. [Google Scholar] [CrossRef]
- King, S.A.; Adas, A.A.; LiVolsi, V.A.; Takahashi, H.; Behbakht, K.; McGovern, P.; Benjamin, I.; Rubin, S.C.; Boyd, J. Expression and mutation analysis of the p53 gene in uterine papillary serous carcinoma. Cancer 1995, 75, 2700–2705. [Google Scholar] [CrossRef]
- Saffari, B.; Jones, L.A.; el-Naggar, A.; Felix, J.C.; George, J.; Press, M.F. Amplification and overexpression of HER-2/neu (c-erbB2) in endometrial cancers: Correlation with overall survival. Cancer Res. 1995, 55, 5693–5698. [Google Scholar]
- Hamel, N.W.; Sebo, T.J.; Wilson, T.O.; Keeney, G.L.; Roche, P.C.; Suman, V.J.; Hu, T.C.; Podratz, K.C. Prognostic value of p53 and proliferating cell nuclear antigen expression in endometrial carcinoma. Gynecol. Oncol. 1996, 62, 192–198. [Google Scholar] [CrossRef]
- Bancher-Todesca, D.; Gitsch, G.; Williams, K.E.; Kohlberger, P.; Neunteufel, W.; Obermair, A.; Heinze, G.; Breitenecker, G.; Hacker, N.F. p53 protein overexpression: A strong prognostic factor in uterine papillary serous carcinoma. Gynecol. Oncol. 1998, 71, 59–63. [Google Scholar] [CrossRef]
- Geisler, J.P.; Geisler, H.E.; Wiemann, M.C.; Zhou, Z.; Miller, G.A.; Crabtree, W. p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol. Oncol. 1999, 74, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Rolitsky, C.D.; Theil, K.S.; McGaughy, V.R.; Copeland, L.J.; Niemann, T.H. HER-2/neu amplification and overexpression in endometrial carcinoma. Int. J. Gynecol. Pathol. 1999, 18, 138–143. [Google Scholar] [CrossRef]
- Salvesen, H.B.; Iversen, O.E.; Akslen, L.A. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: A population-based endometrial carcinoma study. J. Clin. Oncol. 1999, 17, 1382–1390. [Google Scholar] [CrossRef]
- Salvesen, H.B.; Das, S.; Akslen, L.A. Loss of nuclear p16 protein expression is not associated with promoter methylation but defines a subgroup of aggressive endometrial carcinomas with poor prognosis. Clin. Cancer Res. 2000, 6, 153–159. [Google Scholar] [PubMed]
- Coronado, P.J.; Vidart, J.A.; Lopez-asenjo, J.A.; Fasero, M.; Furio-bacete, V.; Magrina, J.; Escudero, M. P53 overexpression predicts endometrial carcinoma recurrence better than HER-2/neu overexpression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 103–108. [Google Scholar] [CrossRef]
- Al Kushi, A.; Lim, P.; Aquino-Parsons, C.; Gilks, C.B. Markers of proliferative activity are predictors of patient outcome for low-grade endometrioid adenocarcinoma but not papillary serous carcinoma of endometrium. Mod. Pathol. 2002, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, C.; Auer, G.; Frankendal, B.; Moberger, B.; Nilsson, B.; Nordström, B. Nuclear DNA content, proliferative activity, and p53 expression related to clinical and histopathologic features in endometrial carcinoma. Int. J. Gynecol. Cancer 2002, 12, 110–118. [Google Scholar] [CrossRef]
- Hosford, S.; Elliott, J.; Ma, Z.W.; Majeste, R.; Dubeshter, B. CD44 expression in papillary serous endometrial carcinoma. Int. J. Gynecol. Cancer 2003, 13, 480–484. [Google Scholar] [CrossRef]
- Acs, G.; Xu, X.; Chu, C.; Acs, P.; Verma, A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer 2004, 100, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.; Lim, P.; Coldman, A.; Huntsman, D.; Miller, D.; Gilks, C.B. Interpretation of p53 immunoreactivity in endometrial carcinoma: Establishing a clinically relevant cut-off level. Int. J. Gynecol. Pathol. 2004, 23, 129–137. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Broaddus, R.R.; Burke, T.W.; Sneige, N.; Soliman, P.T.; Wu, W.; Sun, C.C.; Munsell, M.F.; Gershenson, D.M.; Lu, K.H. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004, 22, 3126–3132. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Siegel, E.R.; Palmieri, M.; Thomas, M.; Cannon, M.J.; Kay, H.H.; Roman, J.J.; Burnett, A.; Pecorelli, S. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): A major prognostic indicator in uterine serous papillary cancer. Am. J. Obstet. Gynecol. 2005, 192, 813–818. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Van Stedum, S.; Bushen, W.; Palmieri, M.; Siegel, E.R.; De Las Casas, L.E.; Roman, J.J.; Burnett, A.; Pecorelli, S. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer 2005, 104, 1391–1397. [Google Scholar] [CrossRef]
- Diaz-Montes, T.P.; Ji, H.; Smith Sehdev, A.E.; Zahurak, M.L.; Kurman, R.J.; Armstrong, D.K.; Bristow, R.E. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol. Oncol. 2006, 100, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Copeland, L.; Maxwell, G.L.; Fowler, J.M. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006, 24, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, I.B.; Stefansson, I.M.; Akslen, L.A.; Salvesen, H.B. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am. J. Obstet. Gynecol. 2008, 199, 543.E1–543.E7. [Google Scholar] [CrossRef] [PubMed]
- Odicino, F.E.; Bignotti, E.; Rossi, E.; Pasinetti, B.; Tassi, R.A.; Donzelli, C.; Falchetti, M.; Fontana, P.; Grigolato, P.G.; Pecorelli, S. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int. J. Gynecol. Cancer 2008, 18, 14–21. [Google Scholar] [CrossRef]
- Singh, P.; Smith, C.L.; Cheetham, G.; Dodd, T.J.; Davy, M.L. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: Its significance and clinical correlation. Int. J. Gynecol. Cancer 2008, 18, 1344–1351. [Google Scholar] [CrossRef]
- Cocco, E.; Bellone, S.; El-Sahwi, K.; Cargnelutti, M.; Casagrande, F.; Buza, N.; Tavassoli, F.A.; Siegel, E.R.; Visintin, I.; Ratner, E.; et al. Serum amyloid A (SAA): A novel biomarker for uterine serous papillary cancer. Br. J. Cancer 2009, 101, 335–341. [Google Scholar] [CrossRef]
- Konecny, G.E.; Santos, L.; Winterhoff, B.; Hatmal, M.; Keeney, G.L.; Mariani, A.; Jones, M.; Neuper, C.; Thomas, B.; Muderspach, L.; et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br. J. Cancer 2009, 100, 89–95. [Google Scholar] [CrossRef]
- Morgan, J.; Hoekstra, A.V.; Chapman-Davis, E.; Hardt, J.L.; Kim, J.J.; Buttin, B.M. Synuclein-gamma (SNCG) may be a novel prognostic biomarker in uterine papillary serous carcinoma. Gynecol. Oncol. 2009, 114, 293–298. [Google Scholar] [CrossRef]
- Bruchim, I.; Amichay, K.; Kidron, D.; Attias, Z.; Biron-Shental, T.; Drucker, L.; Friedman, E.; Werner, H.; Fishman, A. BRCA1/2 germline mutations in Jewish patients with uterine serous carcinoma. Int. J. Gynecol. Cancer 2010, 20, 1148–1153. [Google Scholar] [CrossRef]
- Hiroki, E.; Akahira, J.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010, 101, 241–249. [Google Scholar] [CrossRef]
- Bishop, E.A.; Lengyel, E.R.; Yamada, S.D.; Montag, A.; Temkin, S.M. The expression of hepatocyte growth factor (HGF) and c-Met in uterine serous carcinoma. Gynecol. Oncol. 2011, 121, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, H.; Zhou, X.; Yang, W.; Huang, X.; Lu, Y.; Shi, D. Clinicopathological characteristics and her-2/neu status in chinese patients with uterine papillary serous carcinoma. ISRN Obstet. Gynecol. 2011, 2011, 575327. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, I.; Capoen, A.; Coenegrachts, L.; Verbist, G.; Moerman, P.; Vergote, I.; Amant, F. Expression of ERCC1, p53, and class III beta-tubulin do not reveal chemoresistance in endometrial cancer: Results from an immunohistochemical study. Int. J. Gynecol. Cancer 2011, 21, 1071–1077. [Google Scholar] [CrossRef]
- Amichay, K.; Kidron, D.; Attias-Geva, Z.; Schayek, H.; Sarfstein, R.; Fishman, A.; Werner, H.; Bruchim, I. BRCA1 is expressed in uterine serous carcinoma (USC) and controls insulin-like growth factor I receptor (IGF-IR) gene expression in USC cell lines. Int. J. Gynecol. Cancer 2012, 22, 748–754. [Google Scholar] [CrossRef]
- Pradhan, M.; Davidson, B.; Abeler, V.M.; Danielsen, H.E.; Trope, C.G.; Kristensen, G.B.; Risberg, B.A. DNA ploidy may be a prognostic marker in stage I and II serous adenocarcinoma of the endometrium. Virchows Arch. 2012, 461, 291–298. [Google Scholar] [CrossRef]
- Togami, S.; Sasajima, Y.; Oi, T.; Ishikawa, M.; Onda, T.; Ikeda, S.; Kato, T.; Tsuda, H.; Kasamatsu, T. Clinicopathological and prognostic impact of human epidermal growth factor receptor type 2 (HER2) and hormone receptor expression in uterine papillary serous carcinoma. Cancer Sci. 2012, 103, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Beirne, J.P.; Quinn, J.E.; Maxwell, P.; Kalloger, S.E.; McAlpine, J.; Gilks, C.B.; Harley, I.J.; McCluggage, W.G. BRCA1 immunohistochemical staining as a prognostic indicator in uterine serous carcinoma. Int. J. Gynecol. Cancer 2013, 23, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Daniilidou, K.; Frangou-Plemenou, M.; Grammatikakis, J.; Grigoriou, O.; Vitoratos, N.; Kondi-Pafiti, A. 2013 Prognostic significance and diagnostic value of PTEN and p53 expression in endometrial carcinoma. A retrospective clinicopathological and immunohistochemical study. J. BUON 2013, 18, 195–201. [Google Scholar]
- González-Rodilla, I.; Aller, L.; Llorca, J.; Muñoz, A.B.; Verna, V.; Estévez, J.; Schneider, J. The E-Cadherin expression vs. tumor cell proliferation paradox in endometrial cancer. Anticancer Res. 2013, 33, 5091–5095. [Google Scholar]
- Liang, S.; Mu, K.; Wang, Y.; Zhou, Z.; Zhang, J.; Sheng, Y.; Zhang, T. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn. Pathol. 2013, 8, 138. [Google Scholar] [CrossRef]
- Roque, D.M.; Bellone, S.; English, D.P.; Buza, N.; Cocco, E.; Gasparrini, S.; Bortolomai, I.; Ratner, E.; Silasi, D.A.; Azodi, M.; et al. Tubulin-beta-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer 2013, 119, 2582–2592. [Google Scholar] [CrossRef]
- Suzuki, F.; Nagase, S.; Suzuki, K.; Oba, E.; Hiroki, E.; Matsuda, Y.; Akahira, J.; Nishigori, H.; Sugiyama, T.; Otsuki, T.; et al. Decreased expression of 14-3-3sigma is predictive of poor prognosis for patients with human uterine papillary serous carcinoma. Tohoku J. Exp. Med. 2013, 231, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Allo, G.; Bernardini, M.Q.; Wu, R.C.; Shih Ie, M.; Kalloger, S.; Pollett, A.; Gilks, C.B.; Clarke, B.A. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod. Pathol. 2014, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hedley, C.; Sriraksa, R.; Showeil, R.; Van Noorden, S.; El-Bahrawy, M. The frequency and significance of WT-1 expression in serous endometrial carcinoma. Hum. Pathol. 2014, 45, 1879–1884. [Google Scholar] [CrossRef]
- McIntyre, J.B.; Nelson, G.S.; Ghatage, P.; Morris, D.; Duggan, M.A.; Lee, C.H.; Doll, C.M.; Kobel, M. PIK3CA missense mutation is associated with unfavorable outcome in grade 3 endometrioid carcinoma but not in serous endometrial carcinoma. Gynecol. Oncol. 2014, 132, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Hachisuga, T.; Nguyen, T.T.; Urabe, R.; Kurita, T.; Kagami, S.; Kawagoe, T.; Matsuura, Y.; Shimajiri, S. Expression of estrogen receptor-alpha as a prognostic factor in patients with uterine serous carcinoma. Int. J. Gynecol. Cancer 2014, 24, 102–106. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Centritto, F.; Schlessinger, J.; Lifton, R. Improved survival of patients with hypermutation in uterine serous carcinoma. Gynecol. Oncol. Rep. 2015, 12, 3–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Gong, C.; Zhang, F.; He, J.; Zhang, W.; Zhao, Y.; Sun, J. Prognostic role of hormone receptors in endometrial cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2015, 13, 208. [Google Scholar] [CrossRef]
- Kobel, M.; Atenafu, E.G.; Rambau, P.F.; Ferguson, S.E.; Nelson, G.S.; Ho, T.C.; Panzarella, T.; McAlpine, J.N.; Gilks, C.B.; Clarke, B.A.; et al. Progesterone receptor expression is associated with longer overall survival within high-grade histotypes of endometrial carcinoma: A Canadian high risk endometrial cancer consortium (CHREC) study. Gynecol. Oncol. 2016, 141, 559–563. [Google Scholar] [CrossRef]
- Lemetre, C.; Vieites, B.; Ng, C.K.; Piscuoglio, S.; Schultheis, A.M.; Marchio, C.; Murali, R.; Lopez-Garcia, M.A.; Palacios, J.C.; Jungbluth, A.A.; et al. RNASeq analysis reveals biological processes governing the clinical behaviour of endometrioid and serous endometrial cancers. Eur. J. Cancer 2016, 64, 149–158. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Stelloo, E.; Nout, R.A.; Nijman, H.W.; Edmondson, R.J.; Church, D.N.; MacKay, H.J.; Leary, A.; Powell, M.E.; Mileshkin, L.; et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol. 2016, 29, 174–181. [Google Scholar] [CrossRef]
- Chen, W.; Husain, A.; Nelson, G.S.; Rambau, P.F.; Liu, S.; Lee, C.H.; Lee, S.; Duggan, M.A.; Kobel, M. Immunohistochemical Profiling of Endometrial Serous Carcinoma. Int. J. Gynecol. Pathol. 2017, 36, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Lin, D.I. Amplification of the NSD3-BRD4-CHD8 pathway in pelvic high-grade serous carcinomas of tubo-ovarian and endometrial origin. Mol. Clin. Oncol. 2017, 7, 301–307. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Leung, S.; Magrill, J.; McConechy, M.K.; Yang, W.; Chow, C.; Kobel, M.; Lee, C.H.; Huntsman, D.G.; Talhouk, A.; et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J. Pathol. Clin. Res. 2017, 3, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Taskin, O.; Onder, S.; Topuz, S.; Sozen, H.; Sen, F.; Ilhan, R.; Yavuz, E. A Selected Immunohistochemical Panel Aids in Differential Diagnosis and Prognostic Stratification of Subtypes of High-grade Endometrial Carcinoma: A Clinicopathologic and Immunohistochemical Study at a Single Institution. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 696–702. [Google Scholar] [CrossRef]
- Winder, A.D.; Maniar, K.P.; Wei, J.J.; Liu, D.; Scholtens, D.M.; Lurain, J.R.; Schink, J.C.; Buttin, B.M.; Filiaci, V.L.; Lankes, H.A.; et al. Synuclein-gamma in uterine serous carcinoma impacts survival: An NRG Oncology/Gynecologic Oncology Group study. Cancer 2017, 123, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Bischof, K.; Knappskog, S.; Stefansson, I.; McCormack, E.M.; Trovik, J.; Werner, H.M.J.; Woie, K.; Gjertsen, B.T.; Bjorge, L. High expression of the p53 isoform gamma is associated with reduced progression-free survival in uterine serous carcinoma. BMC Cancer 2018, 18, 684. [Google Scholar] [CrossRef]
- Giordano, G.; Campanini, N.; Goldoni, M.; Rodolfi, A.M.; Brigati, F.; Merisio, C.; Berretta, R. Immunohistochemical Detection of Hematopoietic Cell-specific Protein-Tyrosine Phosphatase (Tyrosine Phosphatase SHP-1) in a Series of Endometrioid and Serous Endometrial Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 468–477. [Google Scholar] [CrossRef]
- Kadan, Y.; Raviv, O.; Segev, Y.; Lavie, O.; Bruchim, I.; Fishman, A.; Michaelson, R.; Beller, U.; Helpman, L. Impact of BRCA mutations on outcomes among patients with serous endometrial cancer. Int. J. Gynecol. Obstet. 2018, 142, 91–96. [Google Scholar] [CrossRef]
- Holst, F.; Werner, H.M.J.; Mjos, S.; Hoivik, E.A.; Kusonmano, K.; Wik, E.; Berg, A.; Birkeland, E.; Gibson, W.J.; Halle, M.K.; et al. PIK3CA Amplification Associates with Aggressive Phenotype but Not Markers of AKT-MTOR Signaling in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 334–345. [Google Scholar] [CrossRef]
- Kakimoto, S.; Miyamoto, M.; Einama, T.; Matsuura, H.; Iwahashi, H.; Ishibashi, H.; Sakamoto, T.; Hada, T.; Takano, M. Co-Expression of Mesothelin and CA125 Is Associated with the Poor Prognosis of Endometrial Serous Carcinoma and Mixed Carcinomas Including Serous Carcinoma. Pathol. Oncol. Res. 2020, 26, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Hussein, S.; Abdalsalam, M.M.; Sameh, H.; Waley, A.B.; Ebian, H.F.; Sakr, M.M.H.; Attia, R.N.; Sameh, R. Prognostic implication of CD47 and CTLA-4 expressions in endometrial carcinoma. Hum. Immunol. 2025, 86, 111210. [Google Scholar] [CrossRef]
- Jia, H.; Wu, S.; Ma, G.; Yang, P.; Li, X.; Zeng, M.; Ji, X.; Xing, X. p53 Immunohistochemistry staining patterns and prognosis significance in 212 cases of non-endometrioid endometrial cancer. Pathol. Res. Pract. 2024, 263, 155595. [Google Scholar] [CrossRef] [PubMed]
- Dinoi, G.; Mariani, A.; Martinelli, E.; Ciucci, A.; Zannoni, G.F.; Weaver, A.L.; Keeney, G.L.; Vasmatzis, G.; Anastasiadis, P.Z.; Fanfani, F.; et al. In search for biomarkers and potential drug targets for uterine serous endometrial cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 1647–1658. [Google Scholar] [CrossRef]
- Jamieson, A.; Sobral de Barros, J.; Cochrane, D.R.; Douglas, J.M.; Shankar, S.; Lynch, B.J.; Leung, S.; Martin, S.; Senz, J.; Lum, A.; et al. Targeted and Shallow Whole-Genome Sequencing Identifies Therapeutic Opportunities in p53abn Endometrial Cancers. Clin. Cancer Res. 2024, 30, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Qin, P.; Xiong, H.; Chen, R.; Zhang, M.; Jiang, Q. A 4-gene signature predicts prognosis of uterine serous carcinoma. BMC Cancer 2021, 21, 154. [Google Scholar] [CrossRef]
- Matoba, Y.; Zarrella, D.T.; Pooladanda, V.; Azimi Mohammadabadi, M.; Kim, E.; Kumar, S.; Xu, M.; Qin, X.; Ray, L.J.; Devins, K.M.; et al. Targeting Galectin 3 illuminates its contributions to the pathology of uterine serous carcinoma. Br. J. Cancer 2024, 130, 1463–1476. [Google Scholar] [CrossRef]
- Govorov, I.; Attarha, S.; Kovalevska, L.; Andersson, E.; Kashuba, E.; Mints, M. STK4 protein expression pattern follows different trends in endometrioid and serous endometrial adenocarcinoma upon tumor progression. Sci. Rep. 2022, 12, 22154. [Google Scholar] [CrossRef]
- Jönsson, J.M.; Bååth, M.; Björnheden, I.; Sahin, I.D.; Måsbäck, A.; Hedenfalk, I. Homologous Recombination Repair Mechanisms in Serous Endometrial Cancer. Cancers 2021, 13, 254. [Google Scholar] [CrossRef]
- Mamat Yusof, M.N.; Chew, K.T.; Kampan, N.; Abd Aziz, N.H.; Md Zin, R.R.; Tan, G.C.; Shafiee, M.N. PD-L1 Expression in Endometrial Cancer and Its Association with Clinicopathological Features: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3911. [Google Scholar] [CrossRef]
- Dong, L.; Wang, T.; Li, N.; Yao, H.; Ying, J.; Wu, L.; Yuan, G. Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma. Cells 2022, 11, 3563. [Google Scholar] [CrossRef] [PubMed]
- Munakata, S.; Ito, T.; Asano, T.; Yamashita, T. Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis. Diagnostics 2023, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Przewoźny, S.; Rogaliński, J.; de Mezer, M.; Markowska, A.; Markowska, J.; Żurawski, J. Estrogen Receptor (ER) and Progesterone Receptor (PgR) Expression in Endometrial Cancer-An Immunohistochemical Assessment. Diagnostics 2024, 14, 322. [Google Scholar] [CrossRef]
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef]
- Richter, C.; Mayhew, D.; Rennhack, J.P.; So, J.; Stover, E.H.; Hwang, J.H.; Szczesna-Cordary, D. Genomic Amplification and Functional Dependency of the Gamma Actin Gene ACTG1 in Uterine Cancer. Int. J. Mol. Sci. 2020, 21, 8690. [Google Scholar] [CrossRef]
- Ucuncu Kefeli, A.; Yaprak Bayrak, B.; Betul Tunce, E.; Vural, C.; Suyusal, I.H.; Kefeli, U.; Aksu, M.G. Expression of netrin-1 in uterine serous carcinoma and its association with prognosis. Int. J. Gynecol. Obstet. 2024, 166, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Cho, H.W.; Ouh, Y.T.; Lee, J.K.; Chun, Y.; Gim, J.A. Genomic landscape of advanced endometrial cancer analyzed by targeted next-generation sequencing and the cancer genome atlas (TCGA) dataset. J. Gynecol. Oncol. 2022, 33, e29. [Google Scholar] [CrossRef]
- Erickson, B.K.; Najjar, O.; Damast, S.; Blakaj, A.; Tymon-Rosario, J.; Shahi, M.; Santin, A.; Klein, M.; Dolan, M.; Cimino-Mathews, A.; et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: A multi-institutional cohort study. Gynecol. Oncol. 2020, 159, 17–22. [Google Scholar] [CrossRef]
- Schatz-Siemers, N.; Chen, Y.T.; Chen, Z.; Wang, D.; Ellenson, L.H.; Du, Y.N. Expression of the Receptor for Hyaluronic Acid-Mediated Motility (RHAMM) in Endometrial Cancer is Associated With Adverse Histologic Parameters and Tumor Progression. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 453–459. [Google Scholar] [CrossRef]
- Tran, L.K.H.; Tran, P.M.H.; Mysona, D.P.; Purohit, S.B.; Myers, E.; Lee, W.S.; Dun, B.; Xu, D.; Liu, H.; Hopkins, D.; et al. A 73-gene proliferative transcriptomic signature predicts uterine serous carcinoma patient survival and response to primary therapy. Gynecol. Oncol. 2020, 157, 340–347. [Google Scholar] [CrossRef]
- Zong, L.; Sun, Z.; Mo, S.; Lu, Z.; Yu, S.; Xiang, Y.; Chen, J. PD-L1 expression in tumor cells is associated with a favorable prognosis in patients with high-risk endometrial cancer. Gynecol. Oncol. 2021, 162, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Howitt, B.; Cheng, T.; King, M.; Stawiski, K.; Fendler, W.; Chowdhury, D.; Matulonis, U.; Konstantinopoulos, P.A. MicroRNA profiling in a case-control study of African American women with uterine serous carcinoma. Gynecol. Oncol. 2021, 163, 453–458. [Google Scholar] [CrossRef]
- Egan, D.; Moran, B.; Wilkinson, M.; Pinyol, M.; Guerra, E.; Gatius, S.; Matias-Guiu, X.; Kolch, W.; le Roux, C.W.; Brennan, D.J. CRABP2—A novel biomarker for high-risk endometrial cancer. Gynecol. Oncol. 2022, 167, 314–322. [Google Scholar] [CrossRef]
- Chen, H.; Molberg, K.; Carrick, K.; Niu, S.; Rivera Colon, G.; Gwin, K.; Lewis, C.; Zheng, W.; Castrillon, D.H.; Lucas, E. Prevalence and prognostic significance of PD-L1, TIM-3 and B7-H3 expression in endometrial serous carcinoma. Mod. Pathol. 2022, 35, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Molberg, K.; Carrick, K.; Niu, S.; Rivera Colon, G.; Gwin, K.; Lewis, C.; Lea, J.; Panwar, V.; Zheng, W.; et al. Expression and Prognostic Significance of LAG-3, TIGIT, VISTA, and IDO1 in Endometrial Serous Carcinoma. Mod. Pathol. 2024, 37, 100532. [Google Scholar] [CrossRef]
- Takeuchi, H.; Miyamoto, T.; Fuseya, C.; Asaka, R.; Ida, K.; Ono, M.; Tanaka, Y.; Shinagawa, M.; Ando, H.; Asaka, S.; et al. PIM1 is a Poor Prognostic Factor for and Potential Therapeutic Target in Serous Carcinoma of the Endometrium. Int. J. Gynecol. Pathol. 2023, 42, 282–292. [Google Scholar] [CrossRef]
- Saglam, O.; Tang, Z.; Tang, G.; Medeiros, L.J.; Toruner, G.A. KAT6A amplifications are associated with shorter progression-free survival and overall survival in patients with endometrial serous carcinoma. PLoS ONE 2020, 15, e0238477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Sun, B.; Cheng, Y.; Wang, J. Heterogeneity of CD40 Expression in Different Types of High-Risk Endometrial Cancer Affects Discordant Prognostic Outcomes. Ther. Clin. Risk Manag. 2023, 19, 549–556. [Google Scholar] [CrossRef]
- Kurimchak, A.M.; Kumar, V.; Herrera-Montávez, C.; Johnson, K.J.; Srivastava, N.; Davarajan, K.; Peri, S.; Cai, K.Q.; Mantia-Smaldone, G.M.; Duncan, J.S. Kinome profiling of primary endometrial tumors using multiplexed inhibitor beads and mass spectrometry identifies SRPK1 as candidate therapeutic target. Mol. Cell. Proteom. 2020, 19, 2068–2090. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, R.; Shi, H.; Ye, L.; Wang, H.; Lu, B. Human epidermal growth factor 2 (HER2) amplification in uterine serous carcinoma: An analysis of prognosis and immune microenvironment. Virchows Arch. 2024. [Google Scholar] [CrossRef]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Gilks, C.B.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoz Gulec, U.; Kilic Bagir, E.; Paydas, S.; Guzel, A.B.; Gumurdulu, D.; Vardar, M.A. Programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) expressions in type 2 endometrial cancer. Arch. Gynecol. Obstet. 2019, 300, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.; Ahvenainen, T.; Pellinen, T.; Vahteristo, P.; Loukovaara, M.; Bützow, R. PD-L1 Expression in Endometrial Carcinoma Cells and Intratumoral Immune Cells: Differences Across Histologic and TCGA-based Molecular Subgroups. Am. J. Surg. Pathol. 2020, 44, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Engerud, H.; Berg, H.F.; Myrvold, M.; Halle, M.K.; Bjorge, L.; Haldorsen, I.S.; Hoivik, E.A.; Trovik, J.; Krakstad, C. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol. Oncol. 2020, 157, 260–267. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Lavie, O.; Hornreich, G.; Ben Arie, A.; Renbaum, P.; Levy-Lahad, E.; Beller, U. BRCA1 germline mutations in women with uterine serous papillary carcinoma. Obstet. Gynecol. 2000, 96, 28–32. [Google Scholar] [CrossRef]
- Lavie, O.; Ben-Arie, A.; Pilip, A.; Rennert, G.; Cohen, Y.; Feiner, B.; Auslnader, R. BRCA2 germline mutation in a woman with uterine serous papillary carcinoma--case report. Gynecol. Oncol. 2005, 99, 486–488. [Google Scholar] [CrossRef]
- Lavie, O.; Hornreich, G.; Ben-Arie, A.; Rennert, G.; Cohen, Y.; Keidar, R.; Sagi, S.; Lahad, E.L.; Auslander, R.; Beller, U. BRCA germline mutations in Jewish women with uterine serous papillary carcinoma. Gynecol. Oncol. 2004, 92, 521–524. [Google Scholar] [CrossRef]
- Wallbillich, J.J.; Morris, R.T.; Ali-Fehmi, R. Comparing mutation frequencies for homologous recombination genes in uterine serous and high-grade serous ovarian carcinomas: A case for homologous recombination deficiency testing in uterine serous carcinoma. Gynecol. Oncol. 2020, 159, 381–386. [Google Scholar] [CrossRef]
- Goshen, R.; Chu, W.; Elit, L.; Pal, T.; Hakimi, J.; Ackerman, I.; Fyles, A.; Mitchell, M.; Narod, S.A. Is uterine papillary serous adenocarcinoma a manifestation of the hereditary breast-ovarian cancer syndrome? Gynecol. Oncol. 2000, 79, 477–481. [Google Scholar] [CrossRef]
- de Jonge, M.M.; Mooyaart, A.L.; Vreeswijk, M.P.; de Kroon, C.D.; van Wezel, T.; van Asperen, C.J.; Smit, V.T.; Dekkers, O.M.; Bosse, T. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: A meta-analysis and case report. Eur. J. Cancer 2017, 72, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hornreich, G.; Beller, U.; Lavie, O.; Renbaum, P.; Cohen, Y.; Levy-Lahad, E. Is uterine serous papillary carcinoma a BRCA1-related disease? Case report and review of the literature. Gynecol. Oncol. 1999, 75, 300–304. [Google Scholar] [CrossRef]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 339–367. [Google Scholar] [CrossRef]
- Koshiyama, M.; Konishi, I.; Wang, D.P.; Mandai, M.; Komatsu, T.; Yamamoto, S.; Nanbu, K.; Naito, M.F.; Mori, T. Immunohistochemical analysis of p53 protein over-expression in endometrial carcinomas: Inverse correlation with sex steroid receptor status. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 423, 265–271. [Google Scholar] [CrossRef]
- Moll, U.M.; Chalas, E.; Auguste, M.; Meaney, D.; Chumas, J. Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum. Pathol. 1996, 27, 1295–1300. [Google Scholar] [CrossRef]
- Williams, J.A., Jr.; Wang, Z.R.; Parrish, R.S.; Hazlett, L.J.; Smith, S.T.; Young, S.R. Fluorescence in situ hybridization analysis of HER-2/neu, c-myc, and p53 in endometrial cancer. Exp. Mol. Pathol. 1999, 67, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Peiro, G.; Mayr, D.; Hillemanns, P.; Lohrs, U.; Diebold, J. Analysis of HER-2/neu amplification in endometrial carcinoma by chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, p53 and Ki-67 protein expression, and outcome. Mod. Pathol. 2004, 17, 227–287. [Google Scholar] [CrossRef] [PubMed]
- Bur, M.E.; Perlman, C.; Edelmann, L.; Fey, E.; Rose, P.G. p53 expression in neoplasms of the uterine corpus. Am. J. Clin. Pathol. 1992, 98, 81–87. [Google Scholar] [CrossRef]
- Strang, P.; Nordstöm, B.; Nilsson, S.; Bergström, R.; Tribukait, B. Mutant p53 protein as a predictor of survival in endometrial carcinoma. Eur. J. Cancer 1996, 32, 598–602. [Google Scholar] [CrossRef]
- Engelsen, I.B.; Stefansson, I.; Akslen, L.A.; Salvesen, H.B. Pathologic expression of p53 or p16 in preoperative curettage specimens identifies high-risk endometrial carcinomas. Am. J. Obstet. Gynecol. 2006, 195, 979–986. [Google Scholar] [CrossRef]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Wang, X.; Marshall, D.S.; Leitao, M.; Hedvat, C.V.; Hummer, A.; Thaler, H.; O’Reilly, R.J.; Soslow, R.A. Wilms Tumor Gene (WT1) and p53 expression in endometrial carcinomas: A study of 130 cases using a tissue microarray. Gynecol. Oncol. 2004, 94, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Uberall, I.; Kolar, Z.; Trojanec, R.; Berkovcova, J.; Hajduch, M. The status and role of ErbB receptors in human cancer. Exp. Mol. Pathol. 2008, 84, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef]
- Elsahwi, K.S.; Santin, A.D. erbB2 Overexpression in Uterine Serous Cancer: A Molecular Target for Trastuzumab Therapy. Obstet. Gynecol. Int. 2011, 2011, 128295. [Google Scholar] [CrossRef]
- Androutsopoulos, G.; Adonakis, G.; Liava, A.; Ravazoula, P.; Decavalas, G. Expression and potential role of ErbB receptors in type II endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 204–208. [Google Scholar] [CrossRef]
- Sato, S.; Ito, K.; Ozawa, N.; Yajima, A.; Sasano, H. Expression of c-myc, epidermal growth factor receptor and c-erbB-2 in human endometrial carcinoma and cervical adenocarcinoma. Tohoku J. Exp. Med. 1991, 165, 137–145. [Google Scholar] [CrossRef]
- Villella, J.A.; Cohen, S.; Smith, D.H.; Hibshoosh, H.; Hershman, D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer 2006, 16, 1897–1902. [Google Scholar] [CrossRef]
- Creasman, W.T. Prognostic significance of hormone receptors in endometrial cancer. Cancer 1993, 71, 1467–1470. [Google Scholar] [CrossRef]
- Moos, M.; Tacke, R.; Scherer, H.; Teplow, D.; Früh, K.; Schachner, M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature 1988, 334, 701–703. [Google Scholar] [CrossRef]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef]
- Kamiguchi, H.; Hlavin, M.L.; Lemmon, V. Role of L1 in neural development: What the knockouts tell us. Mol. Cell. Neurosci. 1998, 12, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Smith, D.D.; Ouyang, C.; Warden, C.D.; Williams, J.C.; Han, E.S. L1CAM is an independent predictor of poor survival in endometrial cancer—An analysis of The Cancer Genome Atlas (TCGA). Gynecol. Oncol. 2016, 141, 336–340. [Google Scholar] [CrossRef]
- Geels, Y.P.; Pijnenborg, J.M.; Gordon, B.B.; Fogel, M.; Altevogt, P.; Masadah, R.; Bulten, J.; van Kempen, L.C.; Massuger, L.F. L1CAM Expression is Related to Non-Endometrioid Histology, and Prognostic for Poor Outcome in Endometrioid Endometrial Carcinoma. Pathol. Oncol. Res. 2016, 22, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Huszar, M.; Pfeifer, M.; Schirmer, U.; Kiefel, H.; Konecny, G.E.; Ben-Arie, A.; Edler, L.; Munch, M.; Muller-Holzner, E.; Jerabek-Klestil, S.; et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J. Pathol. 2010, 220, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, S.H.; Yeom, B.W.; Lee, K.W. Significance of CD44v6 expression in gynecologic malignancies. J. Obstet. Gynaecol. Res. 2006, 32, 379–386. [Google Scholar] [CrossRef]
- Kansu-Celik, H.; Gungor, M.; Ortac, F.; Kankaya, D.; Ensari, A. Expression of CD44 variant 6 and its prognostic value in benign and malignant endometrial tissue. Arch. Gynecol. Obstet. 2017, 296, 313–318. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Krawczyk, T.; Smigielski, J.; Malinowski, A. CD44 expression in curettage and postoperative specimens of endometrial cancer. Arch. Gynecol. Obstet. 2015, 291, 383–390. [Google Scholar] [CrossRef]
- Nikaido, T.; Li, S.F.; Shiozawa, T.; Fujii, S. Coabnormal expression of cyclin D1 and p53 protein in human uterine endometrial carcinomas. Cancer 1996, 78, 1248–1253. [Google Scholar] [CrossRef]
- Shih, H.C.; Shiozawa, T.; Kato, K.; Imai, T.; Miyamoto, T.; Uchikawa, J.; Nikaido, T.; Konishi, I. Immunohistochemical expression of cyclins, cyclin-dependent kinases, tumor-suppressor gene products, Ki-67, and sex steroid receptors in endometrial carcinoma: Positive staining for cyclin A as a poor prognostic indicator. Hum. Pathol. 2003, 34, 471–478. [Google Scholar] [CrossRef]
- Khabaz, M.N.; Abdelrahman, A.S.; Butt, N.S.; Al-Maghrabi, B.; Al-Maghrabi, J. Cyclin D1 is significantly associated with stage of tumor and predicts poor survival in endometrial carcinoma patients. Ann. Diagn. Pathol. 2017, 30, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.J.; Hendricks, D.T.; Farley, J.; Taylor, R.R.; Geradts, J.; Rose, G.S.; Birrer, M.J. p27 and cyclin D1 abnormalities in uterine papillary serous carcinoma. Gynecol. Oncol. 2000, 77, 439–445. [Google Scholar] [CrossRef]
- Soslow, R.A.; Shen, P.U.; Chung, M.H.; Isacson, C.; Baergen, R.N. Cyclin D1 expression in high-grade endometrial carcinomas--association with histologic subtype. Int. J. Gynecol. Pathol. 2000, 19, 329–334. [Google Scholar] [CrossRef]
- Salvesen, H.B.; Kumar, R.; Stefansson, I.; Angelini, S.; MacDonald, N.; Smeds, J.; Jacobs, I.J.; Hemminki, K.; Das, S.; Akslen, L.A. Low frequency of BRAF and CDKN2A mutations in endometrial cancer. Int. J. Cancer 2005, 115, 930–934. [Google Scholar] [CrossRef]
- Mhawech-Fauceglia, P.; Wang, D.; Syriac, S.; Godoy, H.; Dupont, N.; Liu, S.; Odunsi, K. Synuclein-gamma (SNCG) protein expression is associated with poor outcome in endometrial adenocarcinoma. Gynecol. Oncol. 2012, 124, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Mhawech-Fauceglia, P.; Wang, D.; Kesterson, J.; Syriac, S.; Clark, K.; Frederick, P.J.; Lele, S.; Liu, S. Gene expression profiles in stage I uterine serous carcinoma in comparison to grade 3 and grade 1 stage I endometrioid adenocarcinoma. PLoS ONE 2011, 6, e18066. [Google Scholar] [CrossRef] [PubMed]
- Bąkiewicz, A.; Michalak, J.; Sporny, S. Immunoexpression and clinical significance of the PTEN and MLH1 proteins in endometrial carcinomas. Pol. J. Pathol. 2010, 61, 185–191. [Google Scholar] [PubMed]
- Zhu, C.; Luo, J.; Shi, H.; Xie, X.; Ding, Z. Expression of tubulin, p53, ki67, receptors for estrogen, and progesterone in endometrial cancer. Eur. J. Gynaecol. Oncol. 2009, 30, 514–517. [Google Scholar]
- Weisz, L.; Damalas, A.; Liontos, M.; Karakaidos, P.; Fontemaggi, G.; Maor-Aloni, R.; Kalis, M.; Levrero, M.; Strano, S.; Gorgoulis, V.G.; et al. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007, 67, 2396–2401. [Google Scholar] [CrossRef]
- Bogani, G.; Betella, I.; Multinu, F.; Casarin, J.; GhezzI, F.; Sorbi, F.; VizziellI, G.; Petrillo, M.; Cianci, S.; Berretta, R. Characteristics and outcomes of surgically staged multiple classifier endometrial cancer. Eur. J. Surg. Oncol. 2024, 50, 107269. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020, 26, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.-Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The phase III DUO-E trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Koskas, M.; Depreeuw, J.; Moens, S.; Annibali, D.; Cuppens, T.; Moerman, P.; Lambrechts, D.; Amant, F. Genomic Characterisation and Response to Trastuzumab and Paclitaxel in Advanced or Recurrent HER2-positive Endometrial Carcinoma. Anticancer. Res. 2016, 36, 5381–5384. [Google Scholar] [CrossRef]
- Zhu, L.; Lopez, S.; Bellone, S.; Black, J.; Cocco, E.; Zigras, T.; Predolini, F.; Bonazzoli, E.; Bussi, B.; Stuhmer, Z.; et al. Dacomitinib (PF-00299804), a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor, demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro. Tumor Biol. 2015, 36, 5505–5513. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.N.; Cocco, E.; Bellone, S.; Anagnostou, V.K.; Brower, S.L.; Richter, C.E.; Siegel, E.R.; Schwartz, P.E.; Rutherford, T.J.; Santin, A.D. Differential sensitivity to platinum-based chemotherapy in primary uterine serous papillary carcinoma cell lines with high vs low HER-2/neu expression in vitro. Am. J. Obstet. Gynecol. 2010, 203, 162.E1–162.E8. [Google Scholar] [CrossRef]
- Jin, M.H.; Nam, A.R.; Park, J.E.; Bang, J.H.; Bang, Y.J.; Oh, D.Y. Resistance Mechanism against Trastuzumab in HER2-Positive Cancer Cells and Its Negation by Src Inhibition. Mol. Cancer Ther. 2017, 16, 1145–1154. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svarna, A.; Liontos, M.; Papatheodoridi, A.; Papanota, A.-M.; Zografos, E.; Kaparelou, M.; Zagouri, F.; Dimopoulos, M.-A. Molecular Prognostic Factors in Uterine Serous Carcinomas: A Systematic Review. Curr. Oncol. 2025, 32, 251. https://doi.org/10.3390/curroncol32050251
Svarna A, Liontos M, Papatheodoridi A, Papanota A-M, Zografos E, Kaparelou M, Zagouri F, Dimopoulos M-A. Molecular Prognostic Factors in Uterine Serous Carcinomas: A Systematic Review. Current Oncology. 2025; 32(5):251. https://doi.org/10.3390/curroncol32050251
Chicago/Turabian StyleSvarna, Anna, Michalis Liontos, Alkistis Papatheodoridi, Aristea-Maria Papanota, Eleni Zografos, Maria Kaparelou, Flora Zagouri, and Meletios-Athanasios Dimopoulos. 2025. "Molecular Prognostic Factors in Uterine Serous Carcinomas: A Systematic Review" Current Oncology 32, no. 5: 251. https://doi.org/10.3390/curroncol32050251
APA StyleSvarna, A., Liontos, M., Papatheodoridi, A., Papanota, A.-M., Zografos, E., Kaparelou, M., Zagouri, F., & Dimopoulos, M.-A. (2025). Molecular Prognostic Factors in Uterine Serous Carcinomas: A Systematic Review. Current Oncology, 32(5), 251. https://doi.org/10.3390/curroncol32050251






