Folliculin (FLCN) in Thyroid Tumors: Incidence, Significance, and Role as a Driver Gene and Secondary Alteration
Abstract
1. Introduction
2. Materials and Methods
2.1. Index Patients
2.2. Database Sources and Case Selection
2.3. Orien-Avatar Dataset
2.4. Publicly Available Datasets
3. Results
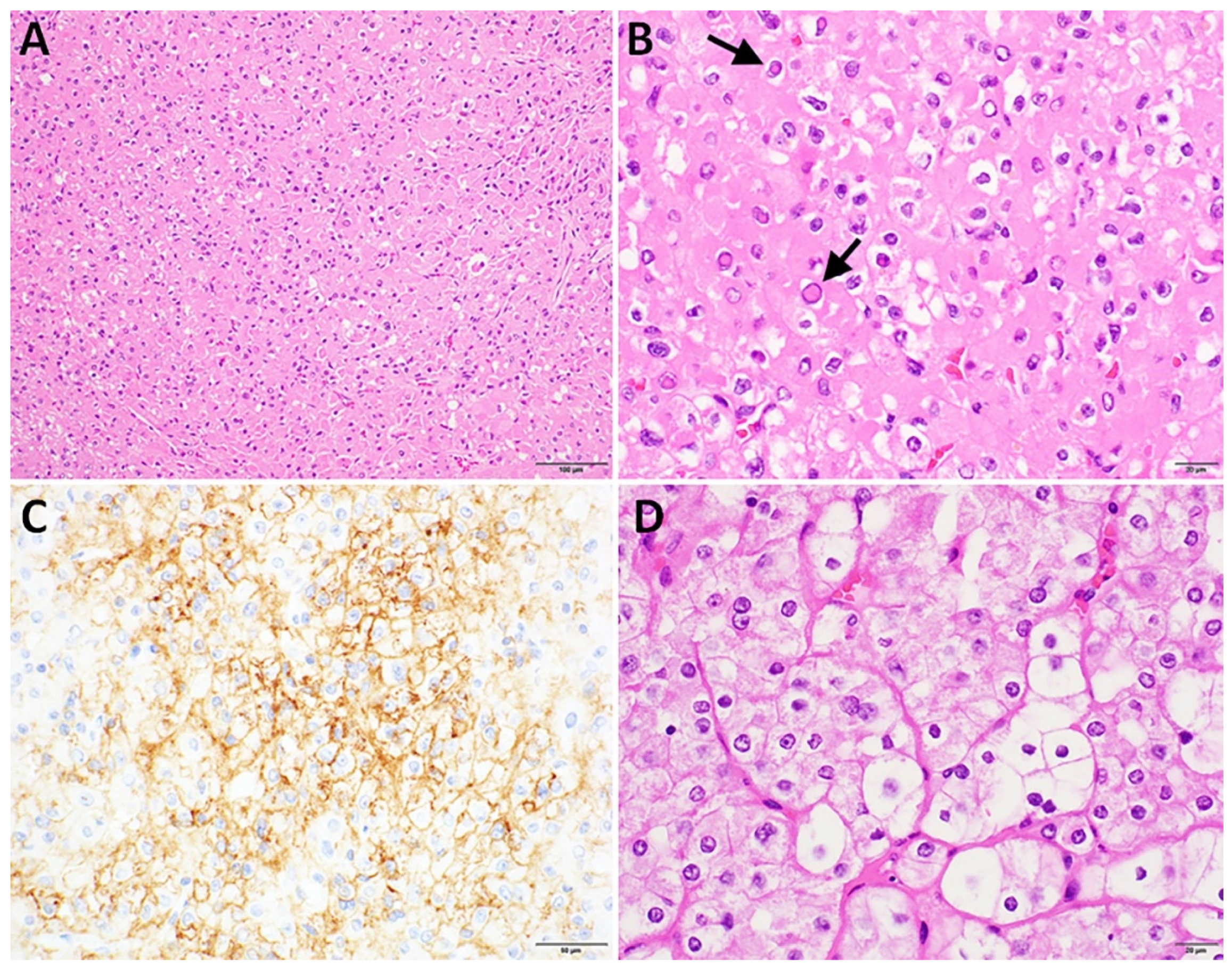
3.1. Patient 1
Clinicopathologic Findings
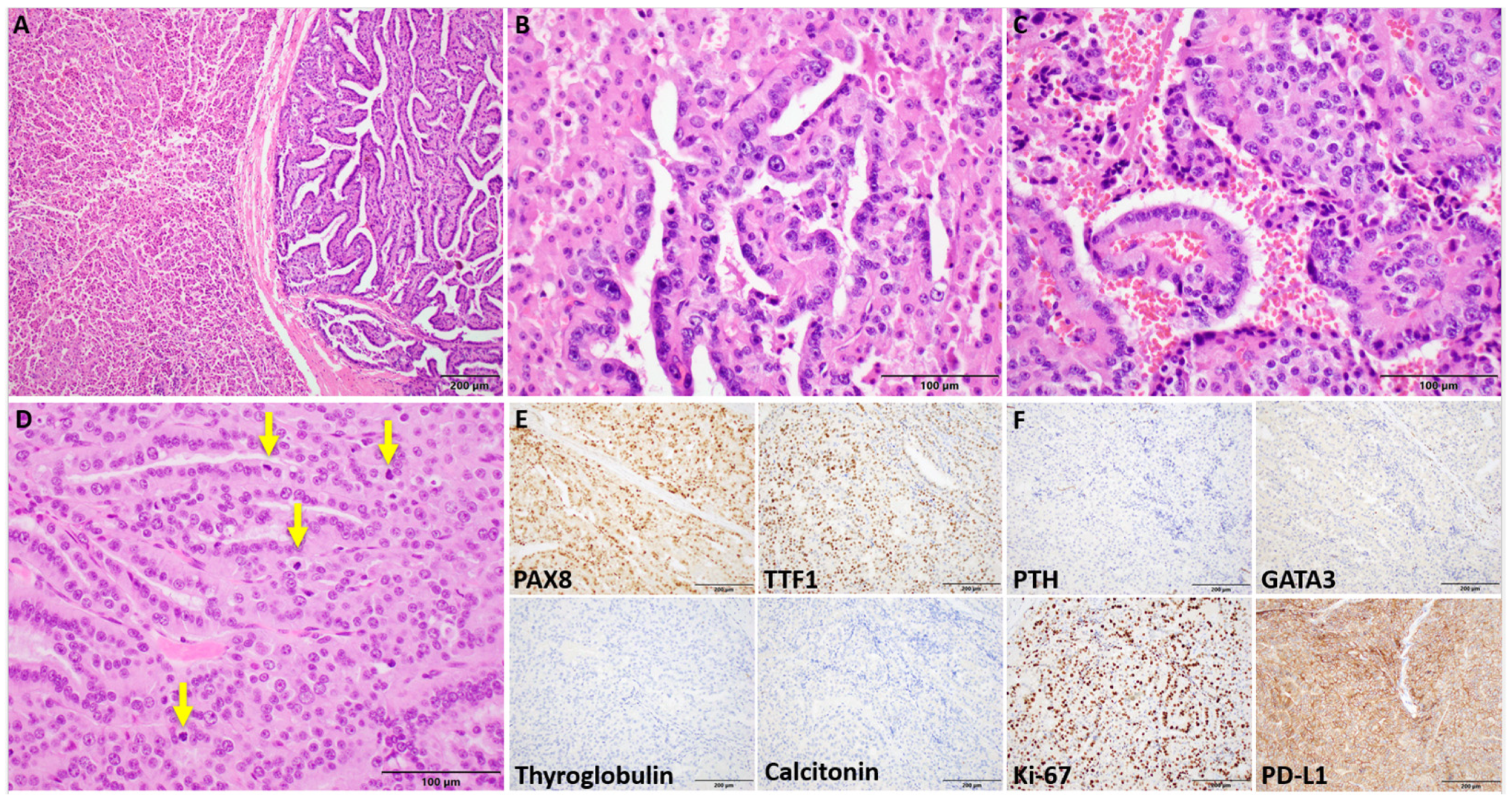
3.2. Patient 2
Clinicopathologic Findings
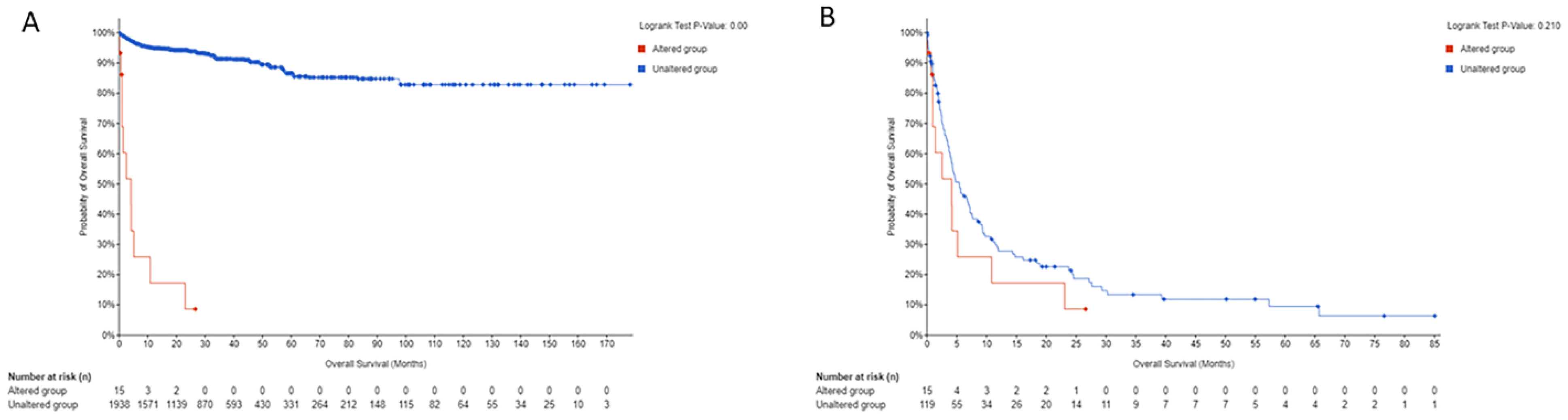
3.3. Orien Avatar Dataset
3.4. Publicly Available Datasets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameselle-Teijeiro, J.M.; Mete, O.; Asa, S.L.; LiVolsi, V. Inherited follicular epithelial-derived thyroid carcinomas: From molecular biology to histological correlates. Endocr. Pathol. 2021, 32, 77–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Krishnan, V.; Wirth, L.J.; Nucera, C.; Venturina, M.; Sadow, P.M.; Mita, A.; Sacks, W. Case report of ccdc149-alk fusion: A novel genetic alteration and a clinically relevant target in metastatic papillary thyroid carcinoma. Thyroid 2022, 32, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, J.; Nosé, V. Hereditary and familial thyroid tumours. Histopathology 2018, 72, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New therapies for advanced thyroid cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Ringel, M.D. New horizons: Emerging therapies and targets in thyroid cancer. J. Clin. Endocrinol. Metab. 2021, 106, e382–e388. [Google Scholar] [CrossRef]
- Birt, A.R.; Hogg, G.R.; Dubé, W.J. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch. Dermatol. 1977, 113, 1674–1677. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Molecular genetics and clinical features of birt-hogg-dubé syndrome. Nat. Rev. Urol. 2015, 12, 558–569. [Google Scholar] [CrossRef]
- Dong, L.; Gao, M.; Hao, W.J.; Zheng, X.Q.; Li, Y.G.; Li, X.L.; Yu, Y. Case report of birt-hogg-dubé syndrome: Germline mutations of flcn detected in patients with renal cancer and thyroid cancer. Medicine 2016, 95, e3695. [Google Scholar] [CrossRef]
- Jha, S.; Welch, J.; Tora, R.; Lack, J.; Warner, A.; Del Rivero, J.; Sadowski, S.M.; Nilubol, N.; Schmidt, L.S.; Linehan, W.M.; et al. Germline- and somatic-inactivating flcn variants in parathyroid cancer and atypical parathyroid tumors. J. Clin. Endocrinol. Metab. 2023, 108, 2686–2698. [Google Scholar] [CrossRef]
- Toro, J.R.; Wei, M.H.; Glenn, G.M.; Weinreich, M.; Toure, O.; Vocke, C.; Turner, M.; Choyke, P.; Merino, M.J.; Pinto, P.A.; et al. Bhd mutations, clinical and molecular genetic investigations of birt-hogg-dubé syndrome: A new series of 50 families and a review of published reports. J. Med. Genet. 2008, 45, 321–331. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Warren, M.B.; Nickerson, M.L.; Weirich, G.; Matrosova, V.; Toro, J.R.; Turner, M.L.; Duray, P.; Merino, M.; Hewitt, S.; et al. Birt-hogg-dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am. J. Hum. Genet. 2001, 69, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Rehal, P.K.; Nahorski, M.S.; Macdonald, F.; Claessens, T.; Van Geel, M.; Gijezen, L.; Gille, J.J.; Giraud, S.; Richard, S.; et al. A new locus-specific database (lsdb) for mutations in the folliculin (flcn) gene. Hum. Mutat. 2010, 31, E1043–E1051. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Hong, S.B.; Sharma, N.; Warren, M.B.; Nickerson, M.L.; Iwamatsu, A.; Esposito, D.; Gillette, W.K.; Hopkins, R.F., III; Hartley, J.L.; et al. Folliculin encoded by the bhd gene interacts with a binding protein, fnip1, and ampk, and is involved in ampk and mtor signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 15552–15557. [Google Scholar] [CrossRef] [PubMed]
- Gunji, Y.; Akiyoshi, T.; Sato, T.; Kurihara, M.; Tominaga, S.; Takahashi, K.; Seyama, K. Mutations of the birt hogg dube gene in patients with multiple lung cysts and recurrent pneumothorax. J. Med. Genet. 2007, 44, 588–593. [Google Scholar] [CrossRef]
- Benhammou, J.N.; Vocke, C.D.; Santani, A.; Schmidt, L.S.; Baba, M.; Seyama, K.; Wu, X.; Korolevich, S.; Nathanson, K.L.; Stolle, C.A.; et al. Identification of intragenic deletions and duplication in the flcn gene in birt-hogg-dubé syndrome. Genes Chromosomes Cancer 2011, 50, 466–477. [Google Scholar] [CrossRef]
- Iwabuchi, C.; Ebana, H.; Ishiko, A.; Negishi, A.; Mizobuchi, T.; Kumasaka, T.; Kurihara, M.; Seyama, K. Skin lesions of birt-hogg-dubé syndrome: Clinical and histopathological findings in 31 japanese patients who presented with pneumothorax and/or multiple lung cysts. J. Dermatol. Sci. 2018, 89, 77–84. [Google Scholar] [CrossRef]
- Peraza Labrador, A.; Umorin, M.; Shrestha, M.; Villacrez, C.A.; Wright, J. A possible association of salivary gland tumors and oral lesions with birt-hogg-dube syndrome: A systematic review. Head Neck Pathol. 2024, 18, 52. [Google Scholar] [CrossRef]
- El-Houjeiri, L.; Biondini, M.; Paquette, M.; Kuasne, H.; Pacis, A.; Park, M.; Siegel, P.M.; Pause, A. Folliculin impairs breast tumor growth by repressing tfe3-dependent induction of the warburg effect and angiogenesis. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- da Silva, N.F.; Gentle, D.; Hesson, L.B.; Morton, D.G.; Latif, F.; Maher, E.R. Analysis of the birt-hogg-dubé (bhd) tumour suppressor gene in sporadic renal cell carcinoma and colorectal cancer. J. Med. Genet 2003, 40, 820–824. [Google Scholar] [CrossRef]
- Nahorski, M.S.; Lim, D.H.; Martin, L.; Gille, J.J.; McKay, K.; Rehal, P.K.; Ploeger, H.M.; van Steensel, M.; Tomlinson, I.P.; Latif, F.; et al. Investigation of the birt-hogg-dube tumour suppressor gene (flcn) in familial and sporadic colorectal cancer. J. Med. Genet 2010, 47, 385–390. [Google Scholar] [CrossRef]
- Moguillansky, N.; Ataya, A. A 44-year-old woman with multiple neoplasms and cystic lung disease. Chest 2021, 159, e381–e384. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Yoshino, T.; Xiu, J.; Helmstetter, A.; Ribeiro, J.R.; Klimov, S.; Gilg, B.; Gao, J.J.; Elton, J.; Oberley, M.J.; et al. Real-world evidence provides clinical insights into tissue-agnostic therapeutic approvals. Nat. Commun. 2025, 16, 2646. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cbio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cbioportal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Zeng, P.Y.F.; Prokopec, S.D.; Lai, S.Y.; Pinto, N.; Chan-Seng-Yue, M.A.; Clifton-Bligh, R.; Williams, M.D.; Howlett, C.J.; Plantinga, P.; Cecchini, M.J.; et al. The genomic and evolutionary landscapes of anaplastic thyroid carcinoma. Cell Rep. 2024, 43, 113826. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Invest. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Flcn: The causative gene for birt-hogg-dubé syndrome. Gene 2018, 640, 28–42. [Google Scholar] [CrossRef]
- Park, H.; Staehling, K.; Tsang, M.; Appleby, M.W.; Brunkow, M.E.; Margineantu, D.; Hockenbery, D.M.; Habib, T.; Liggitt, H.D.; Carlson, G.; et al. Disruption of fnip1 reveals a metabolic checkpoint controlling b lymphocyte development. Immunity 2012, 36, 769–781. [Google Scholar] [CrossRef]
- Yoshimura, S.; Gerondopoulos, A.; Linford, A.; Rigden, D.J.; Barr, F.A. Family-wide characterization of the denn domain rab gdp-gtp exchange factors. J. Cell Biol. 2010, 191, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Hasumi, H.; Baba, M.; Hasumi, Y.; Huang, Y.; Oh, H.; Hughes, R.M.; Klein, M.E.; Takikita, S.; Nagashima, K.; Schmidt, L.S.; et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor flcn. J. Natl. Cancer Inst. 2012, 104, 1750–1764. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A tumor suppressor complex with gap activity for the rag gtpases that signal amino acid sufficiency to mtorc1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Endoh, M.; Ma, W.; Toyama, H.; Hirayama, A.; Nishikawa, K.; Takubo, K.; Hano, H.; Hasumi, H.; Umemoto, T.; et al. Folliculin regulates osteoclastogenesis through metabolic regulation. J. Bone Min. Res. 2018, 33, 1785–1798. [Google Scholar] [CrossRef]
- Baba, M.; Toyama, H.; Sun, L.; Takubo, K.; Suh, H.C.; Hasumi, H.; Nakamura-Ishizu, A.; Hasumi, Y.; Klarmann, K.D.; Nakagata, N.; et al. Loss of folliculin disrupts hematopoietic stem cell quiescence and homeostasis resulting in bone marrow failure. Stem. Cells 2016, 34, 1068–1082. [Google Scholar] [CrossRef]
- Collodet, C.; Foretz, M.; Deak, M.; Bultot, L.; Metairon, S.; Viollet, B.; Lefebvre, G.; Raymond, F.; Parisi, A.; Civiletto, G.; et al. Ampk promotes induction of the tumor suppressor flcn through activation of tfeb independently of mtor. Faseb J. 2019, 33, 12374–12391. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. Tfeb links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong, A.L.; Patange, S.; Raben, N.; Puertollano, R. The nutrient-responsive transcription factor tfe3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal 2014, 7, ra9. [Google Scholar] [CrossRef]
- Ramirez Reyes, J.M.J.; Cuesta, R.; Pause, A. Folliculin: A regulator of transcription through ampk and mtor signaling pathways. Front. Cell Dev. Biol. 2021, 9, 667311. [Google Scholar] [CrossRef]
- Yan, M.; Audet-Walsh, É.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic ampk activation via loss of flcn induces functional beige adipose tissue through pgc-1α/errα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The estrogen-related receptor alpha (erralpha) functions in ppargamma coactivator 1alpha (pgc-1alpha)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated genomic analysis of hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 2018, 34, 256–270.e5. Available online: https://www.sciencedirect.com/science/article/pii/S1535610818303076 (accessed on 1 August 2024). [CrossRef]
- Gopal, R.K.; Kübler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in hürthle cell carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic tert promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Gad, S.; Massard, C.; Carton, E.; Longchampt, E.; Faudot, T.; Lamoril, J.; Ferlicot, S. Case report: Expanding the tumour spectrum associated with the birt-hogg-dubé cancer susceptibility syndrome. F1000Research 2014, 3, 159. [Google Scholar] [CrossRef][Green Version]
- Vaid, S.; Klein, R.; Veeraraghavan, P.; Blakaj, D.M.; Agrawal, A.; Sipos, J.; Sherman, S.I.; Klubo-Gwiezdzinska, J.; Gubbi, S. Sat562 metastatic oncocytic (formerly hürthle cell) thyroid carcinoma in a patient with birt-hogg-dubé syndrome. J. Endocr. Soc. 2023, 7 (Suppl. S1), bvad114.2033. [Google Scholar] [CrossRef]
- Ababneh, E.; Nosé, V. The classic, the trendy, and the refashioned: A primer for pathologists on what is new in familial endocrine tumor syndromes. Adv. Anat. Pathol. 2023, 30, 69–78. [Google Scholar] [CrossRef]
- Kluger, N.; Giraud, S.; Coupier, I.; Avril, M.F.; Dereure, O.; Guillot, B.; Richard, S.; Bessis, D. Birt-hogg-dubé syndrome: Clinical and genetic studies of 10 french families. Br. J. Dermatol. 2010, 162, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Mannan, R.; Zhang, Y.; Chinnaiyan, A.; Rangaswamy, R.; Chugh, S.; Su, F.; Cao, X.; Wang, R.; Skala, S.L.; et al. Hybrid oncocytic tumors (hots) in birt-hogg-dubé syndrome patients-a tale of two cities: Sequencing analysis reveals dual lineage markers capturing the 2 cellular populations of hot. Am. J. Surg. Pathol. 2024, 48, 163–173. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. Ampk is a negative regulator of the warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013, 17, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. Tert promoter mutations and their association with braf v600e mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef]
- Alzumaili, B.; Sadow, P.M. Update on molecular diagnostics in thyroid pathology: A review. Genes 2023, 14, 1314. [Google Scholar] [CrossRef]
- Sura, G.H.; Thrall, M.J.; Rogers, J.; Hodjat, P.; Christensen, P.; Cubb, T.D.; Khadra, H.S.; Thomas, J.S.; Jacobi, E.M. A retrospective analysis of molecular testing in cytologically indeterminate thyroid nodules with histologic correlation: Experience at a heterogenous multihospital system. Diagn. Cytopathol. 2024, 52, 82–92. [Google Scholar] [CrossRef]
| Age at Dx | Sex | Pathologic Dx | FLCN Gene Coverage | Somatic vs. Germline FLCN Alteration | FLCN Alteration | VAF | Variant Reads | Reference Reads | Other Mutations | Family Cancer History | Overall Survival (Months) | Vital Status | T Stage at Dx | N Stage at Dx | Metastases |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 71.8 | M | Oncocytic carcinoma | Germline status only, FLCN not covered on tumor NGS panel | Germline | G319fs | 0.42 | 154 | 214 | Tumor sequenced (FLCN gene not on panel) | “Colon, NOS” | 25 | Living | T3 | N1b | Liver, lung, rib, sternum, clavicle and associated joints, and adrenal gland; lymph nodes of head, face, and neck |
| 43 | F | PTC, Follicular Variant | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF), ARID1A E1735* (likely oncogenic; likely LOF) | N/A | 59 | Living | T3 | N0 | N/A |
| 46 | M | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF) | Lung NOS, Thyroid gland | 62 | Living | T1a | N1a | Rib, sternum, and clavicle and associated joints; lymph nodes of head, face, and neck |
| 39 | F | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | No oncogenic point mutations | N/A | 113 | Living | T3 | N0 | N/A |
| 72 | M | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss in metastasis but not in primary | N/A | N/A | N/A | Primary and lymph node metastasis: BRAF V600E (known oncogenic; likely GOF) | Kidney, NOS, Skin, NOS, Thyroid gland | 51 | Living | T1b | N1b | Lymph nodes of head, face, and neck |
| 14 | F | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF) | N/A | 80 | Living | T3 | N1a | Lymph nodes of head, face, and neck |
| 44 | F | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF) | Skin, NOS | 84 | Living | T1b | N1b | Lymph nodes of head, face, and neck |
| 67 | M | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF) | N/A | 42 | Living | T3 | N1b | Lymph nodes of head, face, and neck |
| 41 | F | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss in metastasis but not in primary | N/A | N/A | N/A | Primary and lymph node metastasis: BRAF V600E (known oncogenic; likely GOF) | Thyroid Gland | 75 | Living | T3 | N1b | Lymph nodes of head, face, and neck |
| 23 | F | PTC, NOS | Germline and Tumor | Somatic | Biallelic loss | N/A | N/A | N/A | BRAF V600E (known oncogenic; likely GOF) | N/A | 20 | Living | T2 | N1a | Lymph nodes of head, face, and neck |
| Database | Age at Dx | Sex | Pathologic Dx | FLCN Gene Coverage | FLCN Alteration | VAF | Variant Reads | Reference Reads | Other Mutations | Overall Survival (Months) | Vital Status | T Stage at Dx | Metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC | Tumor | Q220* truncating mutation | 0.54 | 22 | 41 | BRAF V600E (oncogenic; gain of function), TP53 M246I (likely oncogenic; likely loss of function), NF2 Q453* (likely oncogenic; likely loss of function) | 4 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | TP53 K132N; likely oncogenic; likely loss of function | 1 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC arising in a background of PTC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic; gain of function) | 4 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic; gain of function), ASXL2 Q1013 (likely oncogenic; likely loss of function) | <1 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC arising in a background of PTC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic; gain of function), TP53 M246I (likely oncogenic; likely loss of function) | 3 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | TP53 Q331H; likely oncogenic; likely loss of function | 23 | Deceased | T4b | N/A |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | Not provided | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | No oncogenic point mutations | N/A | N/A | N/A | N/A |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS Q61R (oncogenic gain of function), TP53 C135W (likely oncogenic; likely loss of function) | 3 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | NF2 E186* (likely oncogenic; likely loss of function) | 11 | Deceased | N/A | Yes (Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic; gain of function) | 1 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | STK11 Q220* (likely oncogenic; likely loss of function) | 1 | Deceased | T4b | Yes (Nodal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS Q61K (oncogenic; gain of function) + PIK3CA Q546L (likely oncogenic; likely gain of function) | 1 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC arising in a background of onocytic carcinoma | Tumor | Biallelic loss | N/A | N/A | N/A | ATR X878_splice in anaplastic (splice; likely oncogenic; likely loss of function); EP400 X2778_splice in oncocytic carcinoma (splice; likely oncogenic; likely loss of function) | 1 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC arising in a background of PTC | Tumor | Biallelic loss | N/A | N/A | N/A | PTC component only: BRAF V600E (oncogenic; gain of function), TP53 E285K (likely oncogenic; likely loss of function), BTG1 Q82* (likely oncogenic; likely loss of function) | 3 | Living | N/A | N/A |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC arising in a background of oncocytic carcinoma | Tumor | Biallelic loss | N/A | N/A | N/A | Oncocytic carcinoma component only: PTEN W274* (likely oncogenic; likely loss of function) | 6 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC arising in a background of poorly differentiated thyroid carcinoma | Tumor | Biallelic loss | N/A | N/A | N/A | No oncogenic point mutations | 19 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS Q61R (oncogenic + gain of function), TP53 I195T (likely oncogenic; likely loss of function), EIF1AX G9R (likely oncogenic; likely gain of function) | 1 | Deceased | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS Q61R (oncogenic + gain of function) | <1 | Living | N/A | No |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic + gain of function), PTEN G129R (oncogenic + gain of function), PICK3CA E453K (oncogenic + gain of function), CHEK2 I157T (oncogenic + gain of function), MUTHY R233* (likely oncogenic; likely loss of function) | 5 | Deceased | T4b | Yes (Nodal + Distal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC likely arising out of a follicular carcinoma | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS Q61R (oncogenic + gain of function) | 1 | Living | T4a | Yes (Nodal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC | Tumor | Biallelic loss | N/A | N/A | N/A | BRAF V600E (oncogenic + gain of function), RAD51C P21A (likely oncogenic + known biological effect), NF2 Q178* (likely oncogenic; likely loss of function) | 27 | Living | T4b | Yes (Nodal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | F | ATC arising out of a PTC | Tumor | Biallelic loss | N/A | N/A | N/A | No oncogenic point mutations | 3 | Deceased | T4a | Yes (Nodal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | >=70 | M | ATC arising out of a PTC | Tumor | Biallelic loss | N/A | N/A | N/A | TP53 R273C (likely oncogenic; loss of function) in both anaplastic and PTC components | 5 | Deceased | T4a | Yes (Nodal) |
| Anaplastic Thyroid Cancers (GATCI, Cell Reports 2024) | <70 | M | ATC arising out of a follicular carcinoma | Tumor | Biallelic loss | N/A | N/A | N/A | NRAS G13R in anaplastic and PTC components (oncogenic + gain of function), EIF1AX X113_splice in follicular carcinoma component only (likely oncogenic + likely gain of function) | 3 | Deceased | T4b | Yes (Distal) |
| Thyroid Carcinoma (TCGA, PanCancer Atlas) | 36 | F | PTC with predominantly follicular architecture with oncocytic features and focal tall cell features | Tumor | Biallelic loss | N/A | N/A | N/A | Not profiled for point mutations | 40 | Living | T3 | Yes (Distal, Lung) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.A.; Slone, C.; McDonald, R.J.; Dueber, J.C.; Ashraf, A.M.; Windon, M.J.; Fackelmayer, O.J.; Lee, C.Y.; Bocklage, T.J.; Allison, D.B. Folliculin (FLCN) in Thyroid Tumors: Incidence, Significance, and Role as a Driver Gene and Secondary Alteration. Curr. Oncol. 2025, 32, 224. https://doi.org/10.3390/curroncol32040224
Hassan FA, Slone C, McDonald RJ, Dueber JC, Ashraf AM, Windon MJ, Fackelmayer OJ, Lee CY, Bocklage TJ, Allison DB. Folliculin (FLCN) in Thyroid Tumors: Incidence, Significance, and Role as a Driver Gene and Secondary Alteration. Current Oncology. 2025; 32(4):224. https://doi.org/10.3390/curroncol32040224
Chicago/Turabian StyleHassan, Faisal A., Camryn Slone, Robert J. McDonald, Julie C. Dueber, Adeel M. Ashraf, Melina J. Windon, Oliver J. Fackelmayer, Cortney Y. Lee, Therese J. Bocklage, and Derek B. Allison. 2025. "Folliculin (FLCN) in Thyroid Tumors: Incidence, Significance, and Role as a Driver Gene and Secondary Alteration" Current Oncology 32, no. 4: 224. https://doi.org/10.3390/curroncol32040224
APA StyleHassan, F. A., Slone, C., McDonald, R. J., Dueber, J. C., Ashraf, A. M., Windon, M. J., Fackelmayer, O. J., Lee, C. Y., Bocklage, T. J., & Allison, D. B. (2025). Folliculin (FLCN) in Thyroid Tumors: Incidence, Significance, and Role as a Driver Gene and Secondary Alteration. Current Oncology, 32(4), 224. https://doi.org/10.3390/curroncol32040224





