Pathogenesis, Diagnosis, and Management of Cytokine Release Syndrome in Patients with Cancer: Focus on Infectious Disease Considerations
Abstract
1. Introduction
2. Methodology
3. Results
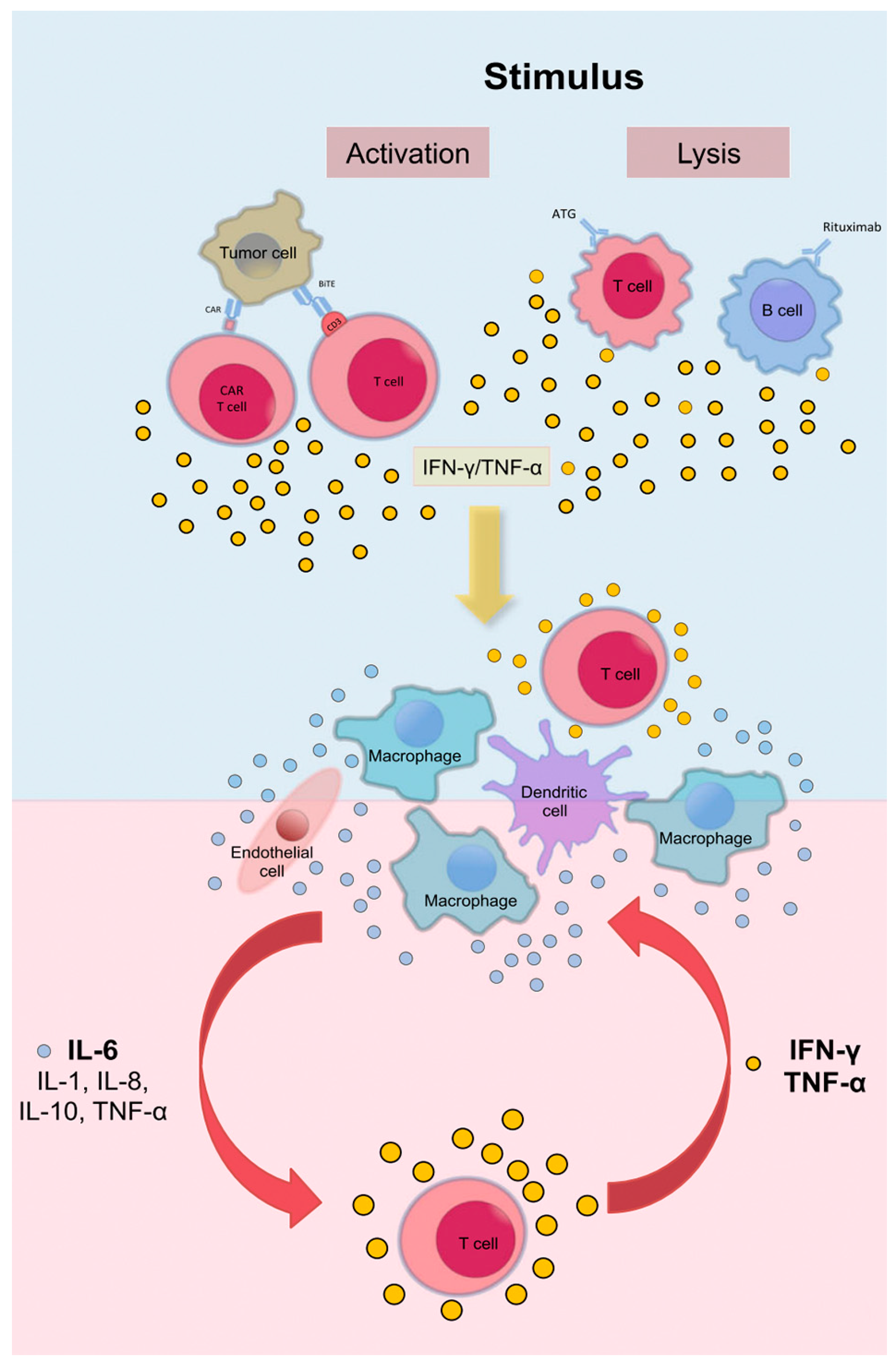
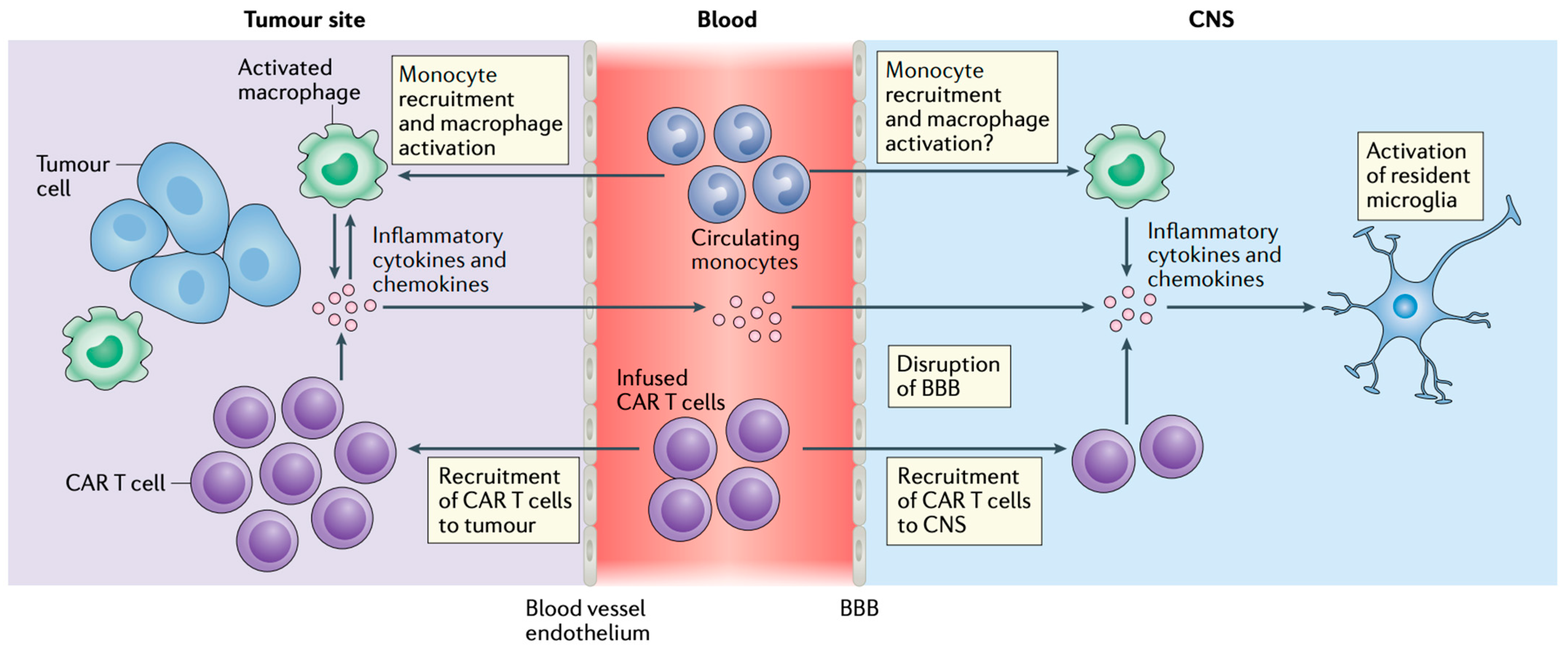
3.1. Pathogenesis
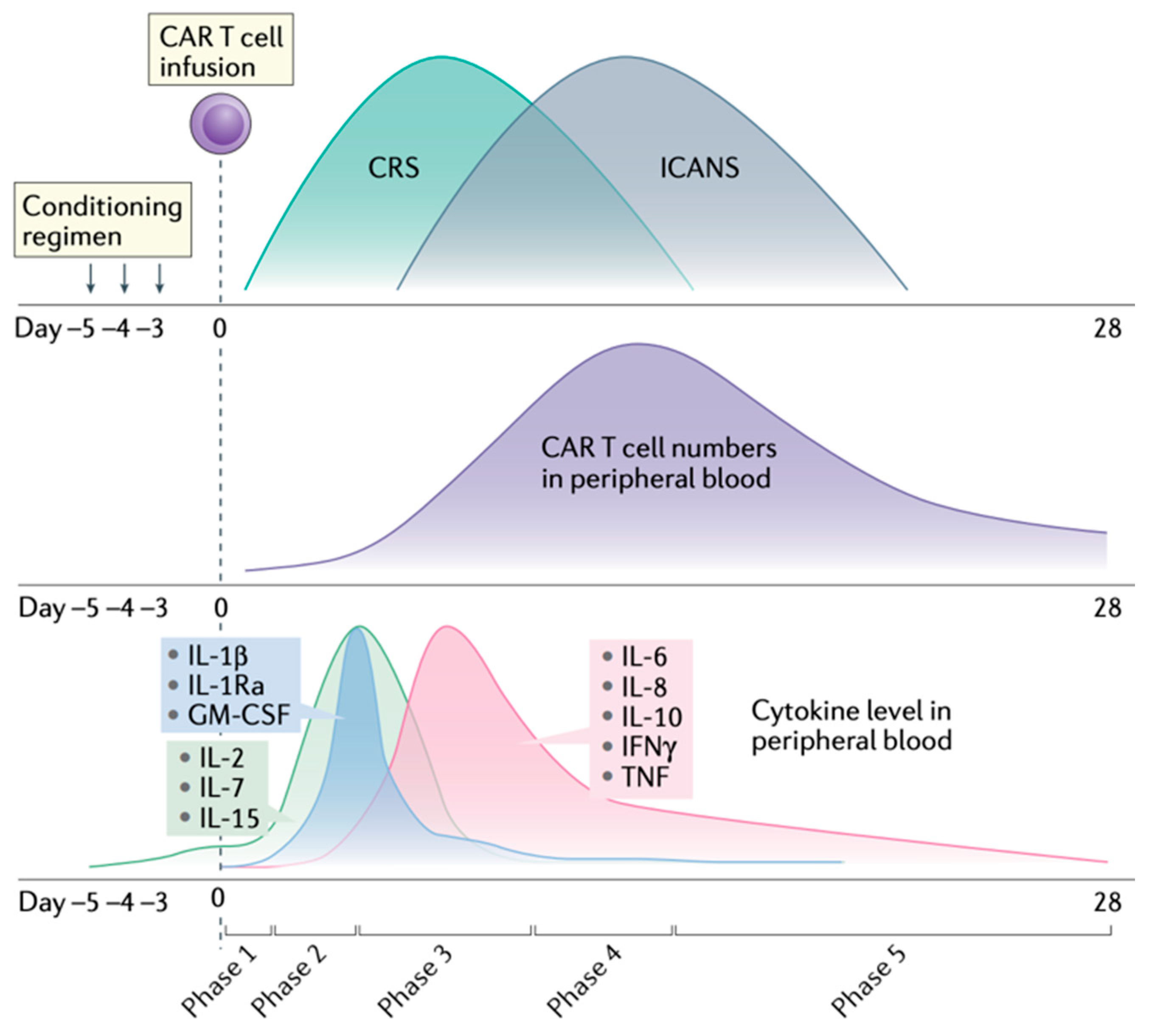
3.2. Clinical Presentation
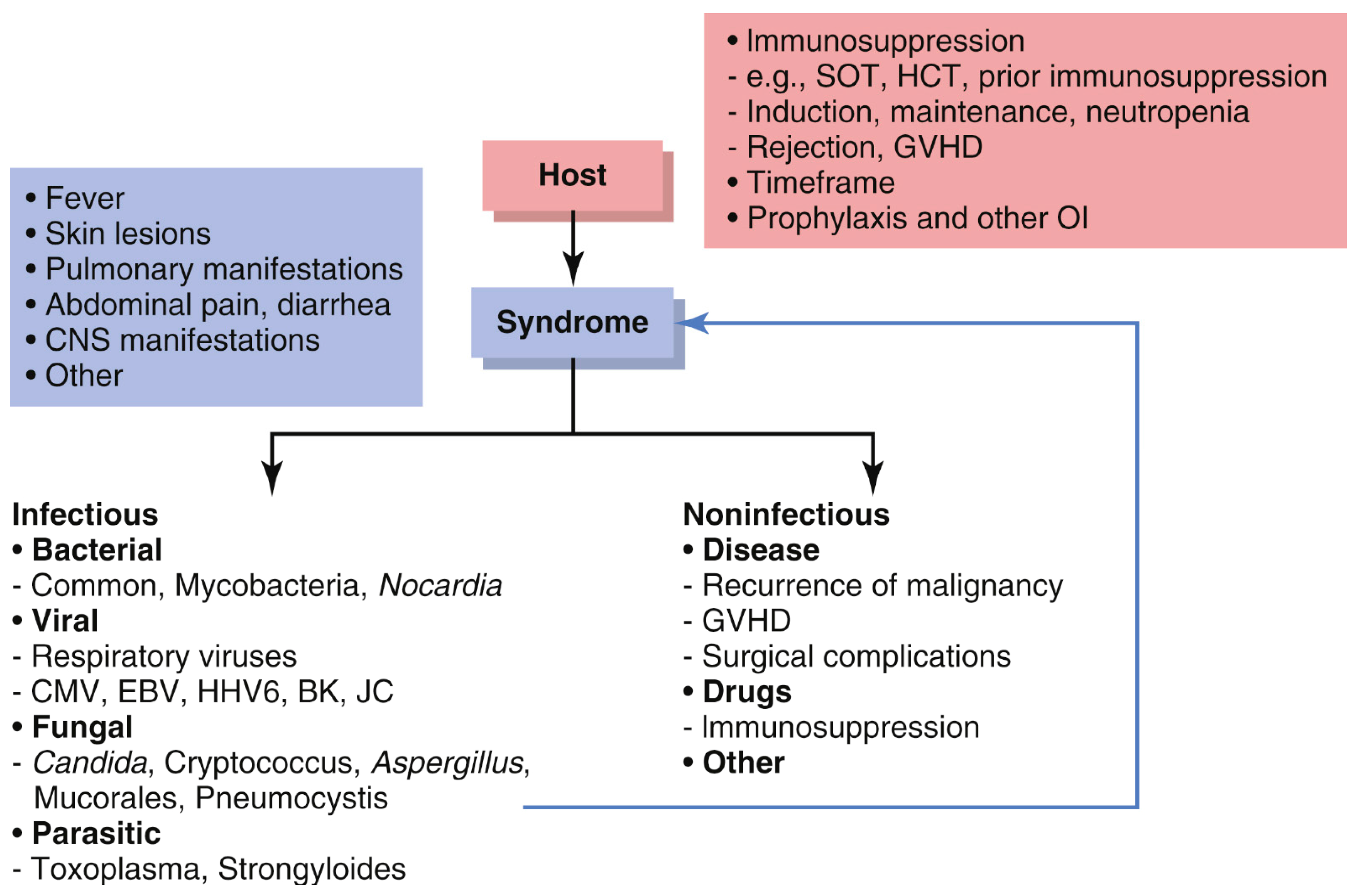
3.3. Differential Diagnosis
3.4. Treatment and Prophylaxis
4. Conclusions/Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Freyer, C.W.; Porter, D.L. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J. Allergy Clin. Immunol. 2020, 146, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Martin-Antonio, B. Editorial: Understanding the Cytokine Release Syndrome: Toward Improving Cancer Immunotherapy. Front. Immunol. 2021, 12, 666703. [Google Scholar] [CrossRef]
- Niimoto, T.; Todaka, T.; Kimura, H.; Suzuki, S.; Yoshino, S.; Hoashi, K.; Yamaguchi, H. Cytokine release syndrome following COVID-19 infection during treatment with nivolumab for cancer of esophagogastric junction carcinoma: A case report and review. Int. J. Emerg. Med. 2024, 17, 106. [Google Scholar] [CrossRef]
- Tay, S.H.; Toh, M.M.X.; Thian, Y.L.; Vellayappan, B.A.; Fairhurst, A.-M.; Chan, Y.H.; Aminkeng, F.; Bharwani, L.D.; Huang, Y.; Mak, A.; et al. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Front. Immunol. 2022, 13, 807050. [Google Scholar] [CrossRef]
- Aslan, A.T.; Akova, M.; Kontoyiannis, D.P. The Heterogeneous Syndrome of Noninfectious Causes of Persistent Fever in Neutropenic Patients With Hematologic Malignancy: Another Opportunity for Stewardship? Clin. Infect. Dis. 2024, 79, 1333–1337. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Khanam, R.; Faiman, B.; Batool, S.; Najmuddin, M.M.; Usman, R.; Kuriakose, K.; Ahmed, A.; Rehman, M.E.U.; Roksana, Z.; Syed, Z.; et al. Management of Adverse Reactions for BCMA-Directed Therapy in Relapsed Multiple Myeloma: A Focused Review. J. Clin. Med. 2023, 12, 5539. [Google Scholar] [CrossRef]
- Hughes, A.D.; Teachey, D.T.; Diorio, C. Riding the storm: Managing cytokine-related toxicities in CAR-T cell therapy. Semin. Immunopathol. 2024, 46, 5. [Google Scholar] [CrossRef]
- Arvanitis, P.; Farmakiotis, D.; Pelcovits, A. Progressive Multifocal Leukoencephalopathy Unmasked by Teclistamab in a Refractory Multiple Myeloma Patient. Curr. Oncol. 2024, 31, 2670–2678. [Google Scholar] [CrossRef]
- Maloney, D.G. Preclinical and phase I and II trials of rituximab. Semin. Oncol. 1999, 26, 74–78. [Google Scholar] [PubMed]
- Kulkarni, H.S.; Kasi, P.M. Rituximab and Cytokine Release Syndrome. Case Rep. Oncol. 2012, 5, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.H.; Kremer, J.M.; Jahreis, A.; Vernon, E.; Isaacs, J.D.; van Vollenhoven, R.F. Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 2011, 13, R141. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Frey, N.; Wood, P.A.; Weng, Y.; Grupp, S.A. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 2018, 11, 35. [Google Scholar] [CrossRef]
- Gagelmann, N.; Bishop, M.; Ayuk, F.; Bethge, W.; Glass, B.; Sureda, A.; Pasquini, M.C.; Kröger, N. Axicabtagene Ciloleucel versus Tisagenlecleucel for Relapsed or Refractory Large B Cell Lymphoma: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2024, 30, 584.e1–584.e13. [Google Scholar] [CrossRef]
- Abramson, J.S.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: Primary analysis of the phase 3 TRANSFORM study. Blood 2023, 141, 1675–1684. [Google Scholar] [CrossRef]
- Abebe, E.C.; Shiferaw, M.Y.; Admasu, F.T.; Dejenie, T.A. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front. Immunol. 2022, 13, 991092. [Google Scholar] [CrossRef]
- Ceschi, A.; Noseda, R.; Palin, K.; Verhamme, K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Z.; Yan, C. Cytokine release syndrome induced by pembrolizumab: A case report. Medicine 2022, 101, e31998. [Google Scholar] [CrossRef]
- Menakuru, S.R.; Azeem, Q.; Priscu, A.; Khan, I.; Beirat, A. Stage 4 Cytokine Release Syndrome Caused by the First Dose of Nivolumab and Ipilimumab Combination Therapy in a Patient with Metastatic Melanoma Successfully Treated with Methylprednisolone, Tocilizumab, and Etanercept. Case Rep. Oncol. 2022, 15, 648–653. [Google Scholar] [CrossRef]
- Ko, B.; Takebe, N.; Andrews, O.; Makena, M.R.; Chen, A.P. Rethinking Oncologic Treatment Strategies with Interleukin-2. Cells 2023, 12, 1316. [Google Scholar] [CrossRef] [PubMed]
- Rokade, S.; Damani, A.M.; Oft, M.; Emmerich, J. IL-2 based cancer immunotherapies: An evolving paradigm. Front. Immunol. 2024, 15, 1433989. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Ding, F.; Saul, M.; Sander, C.; Tarhini, A.A.; Kirkwood, J.M.; Tawbi, H.A. High-dose interleukin-2 (HD IL-2) for advanced melanoma: A single center experience from the University of Pittsburgh Cancer Institute. J. Immunother. Cancer 2017, 5, 74. [Google Scholar] [CrossRef]
- Denstaedt, S.J.; Zemans, R.L. Interferon with Dogma in Cytokine Release Syndrome and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2023, 68, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Mateos, M.V.; Nooka, A.; Banerjee, A.; Kobos, R.; Pei, L.; Qi, M.; Verona, R.; Doyle, M.; Smit, J.; et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer 2023, 129, 2035–2046. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.J.; Firestone, R.S.; Wang, A.X.; Korde, N.; Hultcrantz, M.L.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; et al. Patterns of CRS with teclistamab in relapsed/refractory multiple myeloma with or without prior T-cell redirection therapy. Blood Adv. 2024, 8, 3038–3044. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion–Mutated Non–Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Assouline, S.; Giri, P.; Schuster, S.J.; Cheah, C.Y.; Matasar, M.; Gregory, G.P.; Yoon, D.H.; Shadman, M.; Fay, K.; et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2023, 7, 4926–4935. [Google Scholar] [CrossRef]
- van de Donk, N.W.; O’neill, C.; de Ruijter, M.E.; Verkleij, C.P.; Zweegman, S. T-cell redirecting bispecific and trispecific antibodies in multiple myeloma beyond BCMA. Curr. Opin. Oncol. 2023, 35, 601–611. [Google Scholar] [CrossRef]
- Pan, D.; Richter, J. Management of Toxicities Associated with BCMA, GPRC5D, and FcRH5-Targeting Bispecific Antibodies in Multiple Myeloma. Curr. Hematol. Malig. Rep. 2024, 19, 237–245. [Google Scholar] [CrossRef]
- Gazeau, N.; Liang, E.C.; Wu, Q.; Voutsinas, J.M.; Barba, P.; Iacoboni, G.; Kwon, M.; Ortega, J.L.R.; López-Corral, L.; Hernani, R.; et al. Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2023, 29, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.F.; Bril, V.; Vu, T.; Karam, C.; Peric, S.; Margania, T.; Murai, H.; Bilinska, M.; Shakarishvili, R.; Smilowski, M.; et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): A multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021, 20, 526–536. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K.; Chen, C.I.; Howson-Jan, K.; White, D.; Roy, J.; Kovacs, M.J.; Shustik, C.; Sadura, A.; Shepherd, L.; Ding, K.; et al. Results of a Multicenter Randomized Phase II Trial of Thalidomide and Prednisone Maintenance Therapy for Multiple Myeloma after Autologous Stem Cell Transplant. Clin. Cancer Res. 2004, 10, 8170–8176. [Google Scholar] [CrossRef][Green Version]
- Zhou, L.; Fu, W.; Wu, S.; Xu, K.; Qiu, L.; Xu, Y.; Yan, X.; Zhang, Q.; Zhang, M.; Wang, L.; et al. Derivation and validation of a novel score for early prediction of severe CRS after CAR-T therapy in haematological malignancy patients: A multi-centre study. Br. J. Haematol. 2023, 202, 517–524. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Geng, Y.; Gao, L.; Xu, L.; Tang, G.; Ni, X.; Chen, L.; Chen, J.; Wang, T.; et al. The risk factors and early predictive model of hematotoxicity after CD19 chimeric antigen receptor T cell therapy. Front. Oncol. 2022, 12, 987965. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Bindal, P.; Trottier, C.A.; Elavalakanar, P.; Dodge, L.E.; Kim, S.; Logan, E.; Ma, S.; Liegel, J.; Arnason, J.; Alonso, C.D. Early versus late infectious complications following chimeric antigen receptor-modified T-cell therapy. Leuk. Lymphoma 2024, 1–11. [Google Scholar] [CrossRef]
- Xiao, X.; He, X.; Li, Q.; Zhang, H.; Meng, J.; Jiang, Y.; Deng, Q.; Zhao, M. Plasma Exchange Can Be an Alternative Therapeutic Modality for Severe Cytokine Release Syndrome after Chimeric Antigen Receptor-T Cell Infusion: A Case Report. Clin. Cancer Res. 2019, 25, 29–34. [Google Scholar] [CrossRef]
- Cobb, D.A.; Lee, D.W. Cytokine Release Syndrome Biology and Management. Cancer J. 2021, 27, 119–125. [Google Scholar] [CrossRef]
- Bonaldo, G.; Montanaro, N.; Vaccheri, A.; Motola, D. Safety profile of chimeric antigen receptor T-cell immunotherapies (CAR-T) in clinical practice. Eur. J. Clin. Pharmacol. 2021, 77, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Roex, G.; Timmers, M.; Wouters, K.; Campillo-Davo, D.; Flumens, D.; Schroyens, W.; Chu, Y.; Berneman, Z.N.; Lion, E.; Luo, F.; et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J. Hematol. Oncol. 2020, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Ni, F.; Yang, Z.; Gui, X.; Bao, Z.; Zhao, H.; Wei, G.; Wang, Y.; Zhang, M.; et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat. Commun. 2022, 13, 5313. [Google Scholar] [CrossRef]
- Sesques, P.; Kirkwood, A.A.; Kwon, M.; Rejeski, K.; Jain, M.D.; Di Blasi, R.; Brisou, G.; Gros, F.-X.; le Bras, F.; Bories, P.; et al. Novel prognostic scoring systems for severe CRS and ICANS after anti-CD19 CAR T cells in large B-cell lymphoma. J. Hematol. Oncol. 2024, 17, 61. [Google Scholar] [CrossRef]
- Westin, J.R.; Locke, F.L.; Dickinson, M.; Ghobadi, A.; Elsawy, M.; van Meerten, T.; Miklos, D.B.; Ulrickson, M.L.; Perales, M.-A.; Farooq, U.; et al. Safety and Efficacy of Axicabtagene Ciloleucel versus Standard of Care in Patients 65 Years of Age or Older with Relapsed/Refractory Large B-Cell Lymphoma. Clin. Cancer Res. 2023, 29, 1894–1905. [Google Scholar] [CrossRef]
- Afrough, A.; Abraham, P.R.; Turer, L.; Kaur, G.; Sannareddy, A.; Hansen, D.K.; Anderson, L.D., Jr. Toxicity of CAR T-Cell Therapy for Multiple Myeloma. Acta Haematol. 2024, 1–15. [Google Scholar] [CrossRef]
- Arcanjo, A.; Pinto, K.G.; Logullo, J.; Leite, P.E.C.; Menezes, C.C.B.; Freire-De-Lima, L.; Diniz-Lima, I.; Decoté-Ricardo, D.; Rodrigues-Da-Silva, R.N.; Freire-De-Lima, C.G.; et al. Critically Ill Coronavirus Disease 2019 Patients Exhibit Hyperactive Cytokine Responses Associated With Effector Exhausted Senescent T Cells in Acute Infection. J. Infect. Dis. 2021, 224, 1672–1683. [Google Scholar] [CrossRef]
- Jensen, I.J.; Winborn, C.S.; Fosdick, M.G.; Shao, P.; Tremblay, M.M.; Shan, Q.; Tripathy, S.K.; Snyder, C.M.; Xue, H.-H.; Griffith, T.S.; et al. Polymicrobial sepsis influences NK-cell-mediated immunity by diminishing NK-cell-intrinsic receptor-mediated effector responses to viral ligands or infections. PLoS Pathog. 2018, 14, e1007405. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar]
- Kazandjian, D.; Kowalski, A.; Landgren, O. T cell redirecting bispecific antibodies for multiple myeloma: Emerging therapeutic strategies in a changing treatment landscape. Leuk. Lymphoma 2022, 63, 3032–3043. [Google Scholar] [CrossRef]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2021, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions Related to CAR-T Cell Therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Kampouri, E.; Little, J.S.; Rejeski, K.; Manuel, O.; Hammond, S.P.; Hill, J.A. Infections after chimeric antigen receptor (CAR)-T-cell therapy for hematologic malignancies. Transpl. Infect. Dis. 2023, 25, e14157. [Google Scholar] [CrossRef]
- Raje, N.; Anderson, K.; Einsele, H.; Efebera, Y.; Gay, F.; Hammond, S.P.; Lesokhin, A.M.; Lonial, S.; Ludwig, H.; Moreau, P.; et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: Consensus recommendations from an expert panel. Blood Cancer J. 2023, 13, 116. [Google Scholar] [CrossRef]
- Cao, J.-X.; Wang, H.; Gao, W.-J.; You, J.; Wu, L.-H.; Wang, Z.-X. The incidence of cytokine release syndrome and neurotoxicity of CD19 chimeric antigen receptor-T cell therapy in the patient with acute lymphoblastic leukemia and lymphoma. Cytotherapy 2020, 22, 214–226. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Cao, J.; Zhang, C.; Liu, H.; Huang, H.; Cheng, H.; Qiao, J.; Wang, Y.; Wang, Y.; et al. Characteristics and Risk Factors of Cytokine Release Syndrome in Chimeric Antigen Receptor T Cell Treatment. Front. Immunol. 2021, 12, 611366. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Butt, O.H.; Zhou, A.Y.; Caimi, P.F.; Luckett, P.H.; Wisch, J.K.; Derenoncourt, P.-R.; Lee, K.; Wu, G.F.; de Lima, M.J.G.; Campian, J.L.; et al. Assessment of Pretreatment and Posttreatment Evolution of Neurofilament Light Chain Levels in Patients Who Develop Immune Effector Cell–Associated Neurotoxicity Syndrome. JAMA Oncol. 2022, 8, 1652–1657. [Google Scholar] [CrossRef]
- Hernani, R.; Aiko, M.; Victorio, R.; Benzaquén, A.; Pérez, A.; Piñana, J.L.; Hernández-Boluda, J.C.; Amat, P.; Pastor-Galán, I.; Remigia, M.J.; et al. EEG before chimeric antigen receptor T-cell therapy and early after onset of immune effector cell-associated neurotoxicity syndrome. Clin. Neurophysiol. 2024, 163, 132–142. [Google Scholar] [CrossRef]
- Tang, J.P.; Peters, C.W.; Quiros, C.; Wang, X.; Klomhaus, A.M.; Yamada, R.E.; Timmerman, J.M.; Moore, T.B.; Nowicki, T.S. Hypophosphatemia Due to Increased Effector Cell Metabolic Activity Is Associated with Neurotoxicity Symptoms in CD19-Targeted CAR T-cell Therapy. Cancer Immunol. Res. 2022, 10, 1433–1440. [Google Scholar] [CrossRef]
- Belin, C.; Devic, P.; Ayrignac, X.; Dos Santos, A.; Paix, A.; Sirven-Villaros, L.; Simard, C.; Lamure, S.; Gastinne, T.; Ursu, R.; et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci. Rep. 2020, 10, 18997. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Khaled, S.K.; Budde, E.; Stein, A.S. Cytokine Release Syndrome With the Novel Treatments of Acute Lymphoblastic Leukemia: Pathophysiology, Prevention, and Treatment. Curr. Oncol. Rep. 2019, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef]
- Lee, P.-I.; Hsueh, P.-R. Multisystem inflammatory syndrome in children: A dysregulated autoimmune disorder following COVID-19. J. Microbiol. Immunol. Infect. 2023, 56, 236–245. [Google Scholar] [CrossRef]
- Riegler, L.L.; Jones, G.P.; Lee, D.W. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther. Clin. Risk Manag. 2019, 15, 323–335. [Google Scholar] [CrossRef]
- Shin, Y.H.; Tian, X.; Park, J.J.; Kim, G.Y.; Aboujaoude, E.; Sturgill, M.G. Management of chimeric antigen receptor T-cell induced cytokine release syndrome: Current and emerging approaches. J. Oncol. Pharm. Pract. 2021, 28, 159–174. [Google Scholar] [CrossRef]
- Li, J.Y. Unexpected parvovirus B19 infection in a patient with multiple myeloma. Blood 2023, 142, 1026. [Google Scholar] [CrossRef]
- Bo, Y.; Tianbo, J.; Delong, L.A. BCMA-Targeted Bispecific Antibody Is Active in Multiple Myeloma. Cancer Discov. 2021, 11, 2366. [Google Scholar]
- Kurver, L.; Seers, T.; van Dorp, S.; van Crevel, R.; Pollara, G.; van Laarhoven, A. Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: Diagnostic Challenges and Determinants of Outcome. Open Forum Infect. Dis. 2024, 11, ofad697. [Google Scholar] [CrossRef]
- Gur, I.; Petersiel, N.; Karban, A.; Zuckerman, T.; Oren, I.; Stern, A. Immune reconstitution inflammatory syndrome (IRIS) in a patient with neuro pulmonary nocardiosis following hematopoietic cells transplantation (HCT). J. Infect. Chemother. 2021, 28, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Silversides, J.A.; Lappin, E.; Ferguson, A.J. Staphylococcal Toxic Shock Syndrome: Mechanisms and Management. Curr. Infect. Dis. Rep. 2010, 12, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L. Streptococcal Toxic-Shock Syndrome: Spectrum of Disease, Pathogenesis, and New Concepts in Treatment. Emerg. Infect. Dis. 1995, 1, 69–78. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Wang, B.; Liu, B. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J. Intern. Med. 2020, 289, 147–161. [Google Scholar] [CrossRef]
- Marsh, R.A. Epstein-Barr Virus and Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2017, 8, 1902. [Google Scholar]
- Gomez, R.; Maakaron, J.; Baiocchi, R. Macrophage Activation Syndrome Versus Hemophagocytic Lymphohistiocytosis: A Diagnostic Dilemma in a Patient With Still’s Disease and Epstein-Barr Virus Viremia. J. Hematol. 2019, 8, 68–70. [Google Scholar] [CrossRef]
- Wei, F.; Gao, C.; Wang, Y. The role of influenza A virus-induced hypercytokinemia. Crit. Rev. Microbiol. 2021, 48, 240–256. [Google Scholar] [CrossRef]
- Jayashree, K.; Rao, S.; Kamath, N. Influenza B virus triggering macrophage activation syndrome in an infant. Indian J. Crit. Care Med. 2017, 21, 802–803. [Google Scholar] [CrossRef]
- Rajapakse, S. Dengue shock. J. Emerg. Trauma Shock 2011, 4, 120–127. [Google Scholar]
- Ab-Rahman, H.A.; Rahim, H.; AbuBakar, S.; Wong, P.-F. Macrophage Activation Syndrome-Associated Markers in Severe Dengue. Int. J. Med Sci. 2016, 13, 179–186. [Google Scholar] [CrossRef]
- Reynard, S.; Gloaguen, E.; Baillet, N.; Madelain, V.; Guedj, J.; Raoul, H.; de Lamballerie, X.; Mullaert, J.; Baize, S. Early control of viral load by favipiravir promotes survival to Ebola virus challenge and prevents cytokine storm in non-human primates. PLoS Neglected Trop. Dis. 2021, 15, e0009300. [Google Scholar] [CrossRef]
- Tabaja, H.; Kanj, A.; El Zein, S.; Comba, I.Y.; Chehab, O.; Mahmood, M. A Review of Hemophagocytic Lymphohistiocytosis in Patients With HIV. Open Forum Infect. Dis. 2022, 9, ofac071. [Google Scholar] [CrossRef]
- Jung, J.; Hong, H.-L.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.-H. Immune reconstitution inflammatory syndrome in neutropenic patients with invasive pulmonary aspergillosis. J. Infect. 2015, 70, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, J.M.; Myers, R.A.; Hua, K.; Johnson, M.D.; Seidelman, J.L.; Tsalik, E.L.; Henao, R.; Ginsburg, G.S.; Woods, C.W.; Alexander, B.D.; et al. The host transcriptional response to Candidemia is dominated by neutrophil activation and heme biosynthesis and supports novel diagnostic approaches. Genome Med. 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Boulware, D.R. Cryptococcus-Related Immune Reconstitution Inflammatory Syndrome (IRIS): Pathogenesis and its Clinical Implications. Curr. Fungal Infect. Rep. 2011, 5, 252–261. [Google Scholar] [CrossRef]
- Kassalik, M.; Mönkemüller, K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol. Hepatol. 2011, 7, 766–768. [Google Scholar]
- Marra, C.M. Central nervous system infection with Toxoplasma gondii. Handb. Clin. Neurol. 2018, 152, 117–122. [Google Scholar]
- Neycheva, S.; Oparanov, B.; Kamburova, A.; Karalilova, R.; Stoeva, V. Hemophagocytic Lymphohistiocytosis Triggered by Leishmaniasis: A Case Report and Literature Review. Am. J. Case Rep. 2021, 22, e933012-1–e933012-6. [Google Scholar] [CrossRef]
- Wing, E.J.; Schiffman, F.J. Cecil Essentials of Medicine E-Book: Cecil Essentials of Medicine E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Schroeder, T.; Martens, T.; Fransecky, L.; Valerius, T.; Schub, N.; Pott, C.; Baldus, C.; Stölzel, F. Management of chimeric antigen receptor T (CAR-T) cell-associated toxicities. Intensiv. Care Med. 2024, 50, 1459–1469. [Google Scholar] [CrossRef]
- El Haddad, H.; Chaftari, A.-M.; Hachem, R.; Chaftari, P.; I Raad, I. Biomarkers of Sepsis and Bloodstream Infections: The Role of Procalcitonin and Proadrenomedullin With Emphasis in Patients With Cancer. Clin. Infect. Dis. 2018, 67, 971–977. [Google Scholar] [CrossRef]
- El Haddad, H.; Chaftari, A.-M.; Hachem, R.; Michael, M.; Jiang, Y.; Yousif, A.; Raad, S.; Jordan, M.; Chaftari, P.; Raad, I. Author Correction: Procalcitonin Guiding Antimicrobial Therapy Duration in Febrile Cancer Patients with Documented Infection or Neutropenia. Sci. Rep. 2018, 8, 6258. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Free, R.J.; Thompson, G.R.; Baddley, J.W.; Pappas, P.G.; Benedict, K.; Gold, J.A.W.; Endemic Mycoses Diagnostic Algorithm Subject Matter Expert Group; Tushla, L.A.; Chiller, T.; et al. Clinical Testing Guidance for Coccidioidomycosis, Histoplasmosis, and Blastomycosis in Patients With Community-Acquired Pneumonia for Primary and Urgent Care Providers. Clin. Infect. Dis. 2024, 78, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Zehr, B.; Brannock, K.; Wyma, R.; Kahwash, S.B. Differentiating fulminant EBV infection complicated by HLH from Lymphoma: Report of a case and a brief literature review. Diagn. Pathol. 2023, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, G.; Guan, E.; Song, L.; Song, A.; Liu, X.; Yi, Z.; Sun, L.-R. Treatment outcomes and prognostic factors for non-malignancy associated secondary hemophagocytic lymphohistiocytosis in children. BMC Pediatr. 2020, 20, 288. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Jin, F.; Dehoedt, C.; Rao, J.; Zhou, Y.; Li, P.; Yang, G.; Wang, M.; Zhang, R.; et al. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: A retrospective study of increasing awareness of a disease from a single-center in China. Orphanet J. Rare Dis. 2015, 10, 20. [Google Scholar] [CrossRef]
- Assari, R.; Ziaee, V.; Mirmohammadsadeghi, A.; Moradinejad, M.-H. Dynamic Changes, Cut-Off Points, Sensitivity, and Specificity of Laboratory Data to Differentiate Macrophage Activation Syndrome from Active Disease. Dis. Markers 2015, 2015, 424381. [Google Scholar] [CrossRef]
- Goubran, M.; Spaner, C.; Stukas, S.; Zoref-Lorenz, A.; Shojania, K.; Beckett, M.; Li, A.; Peterson, E.; Sekhon, M.; Grey, R.; et al. The role of C-reactive protein and ferritin in the diagnosis of HLH, adult-onset still’s disease, and COVID-19 cytokine storm. Sci. Rep. 2024, 14, 31306. [Google Scholar] [CrossRef]
- Naymagon, L.; Tremblay, D.; Mascarenhas, J. Reevaluating the role of ferritin in the diagnosis of adult secondary hemophagocytic lymphohistiocytosis. Eur. J. Haematol. 2020, 104, 344–351. [Google Scholar] [CrossRef]
- Sen, E.S.; Steward, C.G.; Ramanan, A.V. Diagnosing haemophagocytic syndrome. Arch. Dis. Child. 2017, 102, 279–284. [Google Scholar] [CrossRef]
- De Togni, E.; Wan, F.; Slade, M.; Plach, K.; Abboud, R. The impact of tocilizumab treatment for cytokine release syndrome on the incidence of early blood stream infections after peripheral blood haploidentical hematopoietic cell transplantation. Leuk. Lymphoma 2022, 63, 2975–2981. [Google Scholar] [CrossRef]
- Banerjee, R.; Fakhri, B.; Shah, N. Toci or not toci: Innovations in the diagnosis, prevention, and early management of cytokine release syndrome. Leuk. Lymphoma 2021, 62, 2600–2611. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2017, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Garner, W.; Hill, J.A. Infections after anti-CD19 chimeric antigen receptor T-cell therapy for hematologic malignancies: Timeline, prevention, and uncertainties. Curr. Opin. Infect. Dis. 2020, 33, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S.; Tandon, M.; Hong, J.S.; Nadeem, O.; Sperling, A.S.; Raje, N.; Munshi, N.; Frigault, M.; Barmettler, S.; Hammond, S.P. Respiratory infections predominate after day 100 following B-cell maturation antigen–directed CAR T-cell therapy. Blood Adv. 2023, 7, 5485–5495. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, R.; Wang, H.; Yi, Q.Y.; Yantao, L.; Zhong, Y.; Sun, M. Peri Cruiser CAR-T: An innovative platform to reduce on-target off-tumor toxicity of CAR-T therapy. J. Clin. Oncol. 2023, 41, 2539. [Google Scholar] [CrossRef]

| Drug | Indications | Mechanism of Action | CRS Incidence | Severe CRS Incidence | References |
|---|---|---|---|---|---|
| Monoclonal Antibodies | |||||
| Rituximab | B-cell non-Hodgkin lymphoma, chronic lymphocytic leukemia, autoimmune diseases. | Anti-CD20 monoclonal antibody | 1–2% (during first infusion) | Rare (<1%) | Shimabukuro-Vornhagen et al. [1], Maloney et al. [11], Kulkarni et al. [12] |
| Alemtuzumab | Chronic lymphocytic leukemia, multiple sclerosis | Anti-CD52 monoclonal antibody | ~10% (initial doses) | <5% | Shimabukuro-Vornhagen et al. [1] |
| Tocilizumab | Rheumatoid arthritis, systemic juvenile idiopathic arthritis, CRS treatment. | Anti-IL-6R monoclonal antibody | Rare for CRS (<1%). | Rare (<1%) | Schiff et al. [13] |
| Blinatumomab | Relapsed/refractory B-cell acute lymphoblastic leukemia (ALL). | CD19 × CD3 BiTE | ~60–70% | ~10–15% | Shimabukuro-Vornhagen et al. [1] |
| CAR T-cell Therapies | |||||
| Tisagenlecleucel | B-cell ALL, diffuse large B-cell lymphoma (DLBCL). | Anti-CD19 CAR T | ~37–49% | 15–23% | Shimabukuro-Vornhagen et al. [1], Porter et al. [14] |
| Axicabtagene Ciloleucel | Relapsed/refractory large B-cell lymphoma. | Anti-CD19 CAR T | ~80% | ~20% | Shimabukuro-Vornhagen et al. [1], Gagelmann et al. [15] |
| Lisocabtagene Maraleucel | Relapsed/refractory large B-cell lymphoma. | Anti-CD19 CAR T | ~42–46% | 2–3% | Shimabukuro-Vornhagen et al. [1], Abramson et al. [16] |
| Ciltacabtagene Autoleucel | Relapsed/refractory multiple myeloma. | Anti-BCMA CAR T | ~95% | ~5–10% | Shimabukuro-Vornhagen et al. [1], Abebe et al. [17] |
| Immune Checkpoint Inhibitors | |||||
| Nivolumab | Melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma. | Anti-PD-1 monoclonal antibody | Rare (<1%) | Very rare (<1%) | Ceschi et al. [18] |
| Pembrolizumab | Advanced melanoma, NSCLC, head and neck cancer, and other solid tumors. | Anti-PD-1 monoclonal antibody | Rare (<1%) | Very rare (1%) | Zhang et al. [19], Ceschi et al. [18] |
| Ipilimumab | Advanced melanoma, renal cell carcinoma, colorectal cancer. | Anti-CTLA-4 monoclonal antibody | Rare (2%) | Rare (1%) | Menakuru et al. [20], Ceschi et al. [18] |
| Cytokines | |||||
| Interleukin-2 | Metastatic renal cell carcinoma, metastatic melanoma. | IL-2 receptor agonist | ~30–50% | 5–10% | Shimabukuro-Vornhagen et al. [1], Ko et al. [21], Rokade et al. [22], Davar et al. [23] |
| Interferons | Hepatitis C, Multiple Sclerosis, and some cancers. | Type I interferon | Low (<10%) | <1% | Denstaedt et al. [24] |
| Bispecific Antibodies | |||||
| Teclistamab | Relapsed/refractory multiple myeloma. | BCMA × CD3 BiTE | ~72% | 1–2% | Martin et al. [25], Hamadeh et al. [26] |
| Amivantamab | NSCLC with EGFR exon 20 insertion mutations. | EGFR × MET BiTE | 10–15% | Rare (<1%) | Park et al. [27] |
| Mosunetuzumab | Relapsed or refractory B-cell non-Hodgkin lymphoma. | CD20 × CD3 BiTE | ~40–50% | <5% | Bartlett et al. [28] |
| Cevostamab | Relapsed or refractory multiple myeloma. | FcRH5 × CD3 BiTE | ~50–70% | ~5–10% | van de Donk et al. [29], Pan et al. [30] |
| Other Biologic Agents | |||||
| Anakinra | Rheumatoid arthritis, cytokine-driven inflammatory diseases, CRS treatment. | IL-1 receptor antagonist | Rare (<5%) | Rare (<1%) | Gazeau et al. [31] |
| Efgartigimod | Myasthenia Gravis. | Neonatal Fc receptor antagonist | Rare (<10%) | <1% | Howard et al. [32] |
| Small Molecule Inhibitors | |||||
| Lenalidomide | Multiple Myeloma, Myelodysplastic syndrome, Mantle Cell lymphoma | Immunomodulatory drug (IMiD); enhances T/NK-cell function, Cereblon modulator degrading Ikaros/Aiolos TFs | Rare (<10%) | <1% | McCarthy et al. [33] |
| Thalidomide | Multiple Myeloma, Erythema Nodosum Leprosum. | Immunomodulatory drug (IMiD); inhibits TNF-α production, modulates cereblon, suppresses angiogenesis | Rare (<10%) | <1% | Stewart et al. [34] |
| Kingdom | Pathogen | Clinical Manifestations | Micro Related CRS-like Syndrome | References |
|---|---|---|---|---|
| Bacterial | ||||
| Mycobacterium tuberculosis | Fever, hepatosplenomegaly, cytopenia, hyperferritinemia, lymphadenopathy | Hemophagocytic Lymphohistiocytosis | Kurver et al. [70] | |
| Nocardia spp. | Fever, lung nodules, brain abscesses, skin infections, systemic inflammation | Cytokine Storm Syndrome, Immune reconstitution inflammatory syndrome | Gur et al. [71] | |
| Staphylococcus aureus | High fever, hypotension, rash, multi-organ failure | Toxic Shock Syndrome | Silversides et al. [72] | |
| Streptococcus pyogenes | High fever, hypotension, rash, multiorgan failure, necrotizing fasciitis | Streptococcal TSS | Stevens et al. [73] | |
| Viral | ||||
| SARS-CoV-2 | Fever, respiratory distress, shock, hyperinflammation, multiorgan failure | Cytokine Storm Syndrome, Multisystem Inflammatory Syndrome in Children | Gao et al. [74], Lee et al. [65] | |
| EBV | Fever, hepatosplenomegaly, lymphadenopathy, pancytopenia, hyperferritinemia | Hemophagocytic Lymphohistiocytosis, Macrophage Activation Syndrome | Marsh et al. [75], Gomez et al. [76] | |
| Influenza | High fever, myalgia, hypotension, respiratory distress, ARDS | Hypercytokinemia, Macrophage Activation Syndrome (MAS) | Wei et al. [77], Jayashree et al. [78] | |
| Dengue | Fever, hypotension, vascular leakage, thrombocytopenia, hemorrhage | Dengue Shock Syndrome, Macrophage Activation Syndrome (MAS), Hemophagocytic Lymphohistiocytosis | Rajapakse et al. [79], Ab-Rahman et al. [80] | |
| Ebola | Fever, hemorrhage, hypotension, multi-organ failure | Post-Ebola Syndrome Cytokine Storm | Reynard et al. [81] | |
| CMV | Fever, hepatosplenomegaly, lymphadenopathy, cytopenia | Hemophagocytic Lymphohistiocytosis | Kampouri et al. [53] | |
| HIV | Persistent fever, pancytopenia, hepatosplenomegaly, immune dysregulation | Hemophagocytic Lymphohistiocytosis | Tabaja et al. [82] | |
| Fungal | ||||
| Aspergillus spp. | Fever, respiratory distress, lung nodules, CNS involvement (in severe cases) | Invasive Apsergillosis IRIS | Jung et al. [83] | |
| Candida spp. | Fever, sepsis-like shock, multi-organ dysfunction in severe invasive cases | Candidemia-Associated Hyperinflammatory Response | Steinbrick et al. [84] | |
| Cryptococcus spp. | Fever, headache, altered mental status, meningitis, CNS involvement | Cryptococcal IRIS | Wiesner et al. [85] | |
| Parasitic | ||||
| Strongyloides stercoralis | Fever, sepsis-like shock, eosinophilia, multi-organ dysfunction | Hyperinfection Syndrome | Kassalik et al. [86] | |
| Toxoplasma gondii | Fever, headache, seizures, encephalitis, chorioretinitis | Toxoplasma encephalitis | Marra et al. [87] | |
| Leishmania spp. | Fever, hepatosplenomegaly, pancytopenia, hyperferritinemia | Hemophagocytic Lymphohistiocytosis | Neycheva et al. [88] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvanitis, P.; Tziotis, A.; Papadimatos, S.; Farmakiotis, D. Pathogenesis, Diagnosis, and Management of Cytokine Release Syndrome in Patients with Cancer: Focus on Infectious Disease Considerations. Curr. Oncol. 2025, 32, 198. https://doi.org/10.3390/curroncol32040198
Arvanitis P, Tziotis A, Papadimatos S, Farmakiotis D. Pathogenesis, Diagnosis, and Management of Cytokine Release Syndrome in Patients with Cancer: Focus on Infectious Disease Considerations. Current Oncology. 2025; 32(4):198. https://doi.org/10.3390/curroncol32040198
Chicago/Turabian StyleArvanitis, Panos, Andreas Tziotis, Spyridon Papadimatos, and Dimitrios Farmakiotis. 2025. "Pathogenesis, Diagnosis, and Management of Cytokine Release Syndrome in Patients with Cancer: Focus on Infectious Disease Considerations" Current Oncology 32, no. 4: 198. https://doi.org/10.3390/curroncol32040198
APA StyleArvanitis, P., Tziotis, A., Papadimatos, S., & Farmakiotis, D. (2025). Pathogenesis, Diagnosis, and Management of Cytokine Release Syndrome in Patients with Cancer: Focus on Infectious Disease Considerations. Current Oncology, 32(4), 198. https://doi.org/10.3390/curroncol32040198





