Extramedullary Multiple Myeloma: Challenges and Opportunities
Abstract
1. Introduction
2. Epidemiology
3. Pathogenesis of Multiple Myeloma Within the Context of the BM Milieu
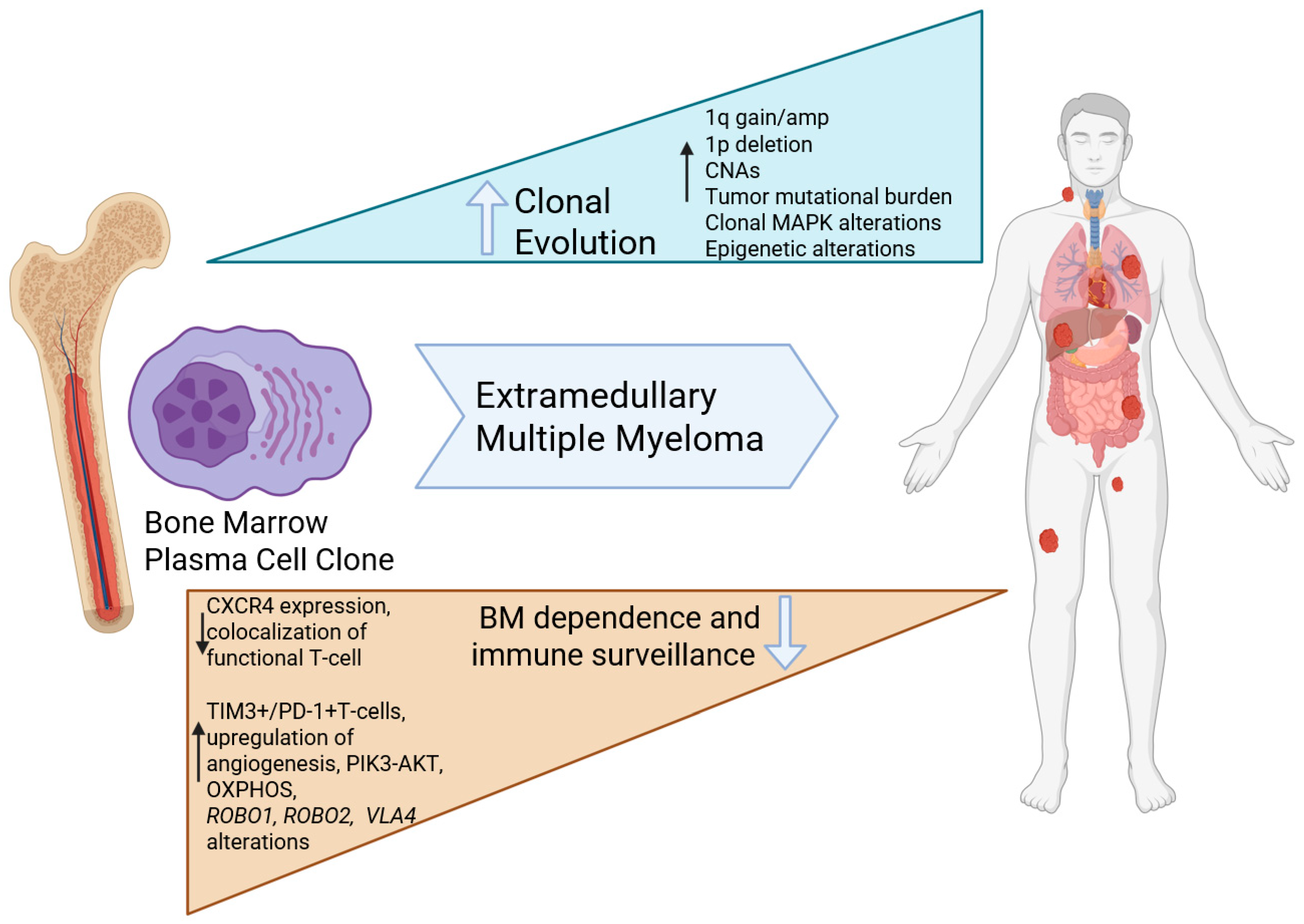
4. Pathophysiology of Extramedullary Multiple Myeloma
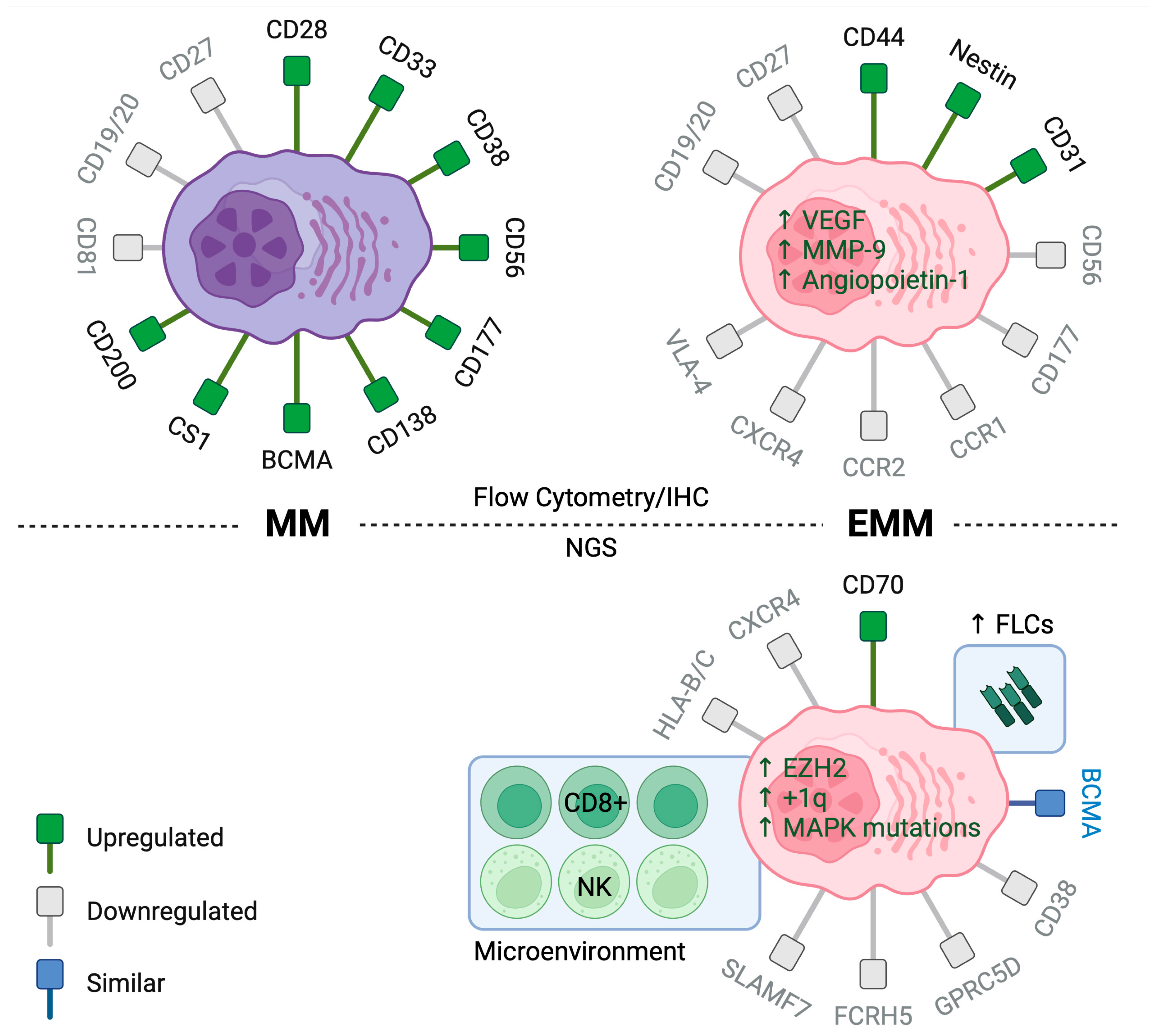
4.1. Insights from Immunophenotypic Studies
4.2. Insights from Molecular Studies
4.3. Insights from Immune Microenvironment Studies in EMM
5. Treatment and Prognosis of Multiple Myeloma and Extramedullary Multiple Myeloma in the Era of Novel Therapies
5.1. Outcomes of Primary EMM in the Era of Novel Therapies
5.2. Outcomes of Secondary EMM in the Era of Novel Therapies
6. Treatment and Prognosis of Multiple Myeloma and Extramedullary Multiple Myeloma in the Era of T-Cell-Redirecting Therapies
6.1. Outcomes of EMM in Patients Treated with Chimeric Antigen T-Cell Therapy (CART)
6.2. Predictors of Response to CART
6.3. Toxicities and Patterns of Relapse After CART
7. Outcomes of EMM in Patients Treated with Bispecific T-Cell Engagers (BiTEs)
8. Conclusions
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hideshima, T.; Mitsiades, C.; Tonon, G.; Richardson, P.G.; Anderson, K.C. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. Cancer 2007, 7, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Barwick, B.G.; Gupta, V.A.; Vertino, P.M.; Boise, L.H. Cell of Origin and Genetic Alterations in the Pathogenesis of Multiple Myeloma. Front. Immunol. 2019, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Moser-Katz, T.; Joseph, N.S.; Dhodapkar, M.V.; Lee, K.P.; Boise, L.H. Game of Bones: How Myeloma Manipulates Its Microenvironment. Front. Oncol. 2021, 10, 625199. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef]
- Fernández de Larrea, C.; Kyle, R.; Rosiñol, L.; Paiva, B.; Engelhardt, M.; Usmani, S.; Caers, J.; Gonsalves, W.; Schjesvold, F.; Merlini, G.; et al. Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021, 11, 192. [Google Scholar] [CrossRef]
- de Wergifosse, M.; Champagne, B.; Ito, S.; Fukuda, K.; Nakano, M. Challenging compounds for calculating molecular second hyperpolarizabilities: The triplet state of the trimethylenemethane diradical and two derivatives. Phys. Chem. Chem. Phys. 2016, 18, 6420–6429. [Google Scholar] [CrossRef]
- Fernandez de Larrea, C.; Kyle, R.A.; Durie, B.G.; Ludwig, H.; Usmani, S.; Vesole, D.H.; Hajek, R.; San Miguel, J.F.; Sezer, O.; Sonneveld, P.; et al. Plasma cell leukemia: Consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013, 27, 780–791. [Google Scholar] [CrossRef]
- Labopin, M.; Ruggeri, A.; Gorin, N.C.; Gluckman, E.; Blaise, D.; Mannone, L.; Milpied, N.; Yakoub-Agha, I.; Deconinck, E.; Michallet, M.; et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica 2014, 99, 535–540. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Heuck, C.; Mitchell, A.; Szymonifka, J.; Nair, B.; Hoering, A.; Alsayed, Y.; Waheed, S.; Haider, S.; Restrepo, A.; et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012, 97, 1761–1767. [Google Scholar] [CrossRef]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef]
- Wali, A.; Kumar, A.M.V.; Hinderaker, S.G.; Heldal, E.; Qadeer, E.; Fatima, R.; Ullah, A.; Safdar, N.; Yaqoob, A.; Anwar, K.; et al. Pre-treatment loss to follow-up among smear-positive TB patients in tertiary hospitals, Quetta, Pakistan. Public Health Action 2017, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.; Ghobrial, I.M. Extramedullary multiple myeloma. Leuk. Lymphoma 2013, 54, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Cicero, K.I.; Lee, S.S.; Cowan, A.J. Definers and drivers of functional high-risk multiple myeloma: Insights from genomic, transcriptomic, and immune profiling. Front. Oncol. 2023, 13, 1240966. [Google Scholar] [CrossRef] [PubMed]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef]
- Zanwar, S.; Sidana, S.; Shune, L.; Puglianini, O.C.; Pasvolsky, O.; Gonzalez, R.; Dima, D.; Afrough, A.; Kaur, G.; Davis, J.A.; et al. Impact of extramedullary multiple myeloma on outcomes with idecabtagene vicleucel. J. Hematol. Oncol. 2024, 17, 42. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.J.; Iacobelli, S.; Koster, L.; Nahi, H.; Stoppa, A.M.; Masszi, T.; Caillot, D.; Lenhoff, S.; Udvardy, M.; et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: A study from the Chronic Malignancies Working Party of the EBMT. Haematologica 2018, 103, 890–897. [Google Scholar] [CrossRef]
- Jiménez-Segura, R.; Rosiñol, L.; Cibeira, M.T.; Fernández de Larrea, C.; Tovar, N.; Rodríguez-Lobato, L.G.; Bladé, E.; Moreno, D.F.; Oliver-Caldés, A.; Bladé, J. Paraskeletal and extramedullary plasmacytomas in multiple myeloma at diagnosis and at first relapse: 50-years of experience from an academic institution. Blood Cancer J. 2022, 12, 135. [Google Scholar] [CrossRef]
- Montefusco, V.; Gay, F.; Spada, S.; De Paoli, L.; Di Raimondo, F.; Ribolla, R.; Musolino, C.; Patriarca, F.; Musto, P.; Galieni, P.; et al. Outcome of paraosseous extra-medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica 2020, 105, 193–200. [Google Scholar] [CrossRef]
- Beksac, M.; Seval, G.C.; Kanellias, N.; Coriu, D.; Rosinol, L.; Ozet, G.; Goranova-Marinova, V.; Unal, A.; Bila, J.; Ozsan, H.; et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: Analysis of parameters that improve outcome. Haematologica 2020, 105, 201–208. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.J.; Koster, L.; Caillot, D.; Pioltelli, P.; Lleonart, J.B.; Remenyi, P.; Blaise, D.; Schaap, N.; Trneny, M.; et al. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Batsukh, K.; Lee, S.E.; Min, G.J.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Kim, H.J.; et al. Distinct Clinical Outcomes between Paramedullary and Extramedullary Lesions in Newly Diagnosed Multiple Myeloma. Immune Netw. 2017, 17, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Gogi, R.; Patel, A.K.; Mookerjee, A.; Sahoo, R.K.; Malik, P.S.; Sharma, A.; Thulkar, S.; Kumar, R.; Biswas, A.; et al. Multiple myeloma with extramedullary disease: Impact of autologous stem cell transplantation on outcome. Bone Marrow Transplant. 2017, 52, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Mangiacavalli, S.; Pompa, A.; Ferretti, V.; Klersy, C.; Cocito, F.; Varettoni, M.; Cartia, C.S.; Cazzola, M.; Corso, A. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann. Hematol. 2017, 96, 73–80. [Google Scholar] [CrossRef]
- Ichinohe, T.; Kuroda, Y.; Okamoto, S.; Matsue, K.; Iida, S.; Sunami, K.; Komeno, T.; Suzuki, K.; Ando, K.; Taniwaki, M.; et al. A multicenter phase 2 study of pomalidomide plus dexamethasone in patients with relapsed and refractory multiple myeloma: The Japanese MM-011 trial. Exp. Hematol. Oncol. 2015, 5, 11. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; An, G.; Sui, W.; Zou, D.; Zhao, Y.; Qi, J.; Li, F.; Hao, M.; Qiu, L. Features of extramedullary disease of multiple myeloma: High frequency of p53 deletion and poor survival: A retrospective single-center study of 834 cases. Clin. Lymphoma Myeloma Leuk. 2015, 15, 286–291. [Google Scholar] [CrossRef]
- Weinstock, M.; Aljawai, Y.; Morgan, E.A.; Laubach, J.; Gannon, M.; Roccaro, A.M.; Varga, C.; Mitsiades, C.S.; Paba-Prada, C.; Schlossman, R.; et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br. J. Haematol. 2015, 169, 851–858. [Google Scholar] [CrossRef]
- Varga, C.; Xie, W.; Laubach, J.; Ghobrial, I.M.; O’Donnell, E.K.; Weinstock, M.; Paba-Prada, C.; Warren, D.; Maglio, M.E.; Schlossman, R.; et al. Development of extramedullary myeloma in the era of novel agents: No evidence of increased risk with lenalidomide-bortezomib combinations. Br. J. Haematol. 2015, 169, 843–850. [Google Scholar] [CrossRef]
- Pour, L.; Sevcikova, S.; Greslikova, H.; Kupska, R.; Majkova, P.; Zahradova, L.; Sandecka, V.; Adam, Z.; Krejci, M.; Kuglik, P.; et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica 2014, 99, 360–364. [Google Scholar] [CrossRef]
- Rasche, L.; Bernard, C.; Topp, M.S.; Kapp, M.; Duell, J.; Wesemeier, C.; Haralambieva, E.; Maeder, U.; Einsele, H.; Knop, S. Features of extramedullary myeloma relapse: High proliferation, minimal marrow involvement, adverse cytogenetics: A retrospective single-center study of 24 cases. Ann. Hematol. 2012, 91, 1031–1037. [Google Scholar] [CrossRef]
- Short, K.D.; Rajkumar, S.V.; Larson, D.; Buadi, F.; Hayman, S.; Dispenzieri, A.; Gertz, M.; Kumar, S.; Mikhael, J.; Roy, V.; et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 2011, 25, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Minnema, M.C.; van de Donk, N.W.; Zweegman, S.; Hegenbart, U.; Schonland, S.; Raymakers, R.; Zijlmans, J.M.; Kersten, M.J.; Bos, G.M.; Lokhorst, H.M. Extramedullary relapses after allogeneic non-myeloablative stem cell transplantation in multiple myeloma patients do not negatively affect treatment outcome. Bone Marrow Transplant. 2008, 41, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Perez-Simon, J.A.; Sureda, A.; Fernandez-Aviles, F.; Sampol, A.; Cabrera, J.R.; Caballero, D.; Martino, R.; Petit, J.; Tomas, J.F.; Moraleda, J.M.; et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia 2006, 20, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Katodritou, E.; Dalampira, D.; Delimpasi, S.; Ntanasis-Stathopoulos, I.; Karaolidou, F.; Gkioka, A.I.; Labropoulou, V.; Spanoudakis, E.; Triantafyllou, T.; Kotsopoulou, M.; et al. Update Analysis of Central Nervous System Multiple Myeloma Prognosis and Survival: A Real-World Multi-Institutional Study of the Greek Myeloma Study Group. Blood 2023, 142, 1981. [Google Scholar] [CrossRef]
- Oshima, K.; Kanda, Y.; Nannya, Y.; Kaneko, M.; Hamaki, T.; Suguro, M.; Yamamoto, R.; Chizuka, A.; Matsuyama, T.; Takezako, N.; et al. Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am. J. Hematol. 2001, 67, 1–5. [Google Scholar] [CrossRef]
- Liss, B.; Kutscher, K. An unusual case of a multiple myeloma with thyroid involvement at primary diagnosis. Blood 2023, 141, 1092. [Google Scholar] [CrossRef]
- You, W.S.; Bhuta, S. Myeloma of Laryngeal Cartilage: Literature Review and Case Study. Ear Nose Throat J. 2021, 100, NP114–NP119. [Google Scholar] [CrossRef]
- Saidi, I.; El Idrissi Tourane, L.o.; Ait Batahar, S.; Amro, L. A case of Multiple Myeloma with lung plasmacytoma. Respir. Med. Case Rep. 2022, 39, 101713. [Google Scholar] [CrossRef]
- Chim, C.S.; Wong, W.M.; Nicholls, J.; Chung, L.P.; Liang, R. Extramedullary sites of involvement in hematologic malignancies: Case 3. Hemorrhagic gastric plasmacytoma as the primary presentation in multiple myeloma. J. Clin. Oncol. 2002, 20, 344–347. [Google Scholar] [CrossRef]
- Abelman, W.; Virchis, A.; Yong, K. Extramedullary myeloma representing as a pericardial effusion with tamponade: Two case reports and a further review of 19 cases in the literature. Leuk. Lymphoma 2005, 46, 137–142. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Zhang, L. Multiple myeloma with onset of pancreas involvement: A case report. Medicine 2019, 98, e16567. [Google Scholar] [CrossRef]
- Yamashita, K.; Horiuchi, T.; Hayashida, A.; Tachibana, H.; Toki, D.; Kondo, T. Multiple myeloma with testicular involvement: A case report. Urol. Case Rep. 2019, 26, 100971. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.P.; Zhang, J.J.; Huang, X.N. Multiple myeloma with rupture of ovarian plasmacytoma. Chin. Med. J. 2012, 125, 2948–2950. [Google Scholar] [PubMed]
- Requena, L.; Kutzner, H.; Palmedo, G.; Calonje, E.; Requena, C.; Pérez, G.; Pastor, M.A.; Sangueza, O.P. Cutaneous Involvement in Multiple Myeloma: A Clinicopathologic, Immunohistochemical, and Cytogenetic Study of 8 Cases. Arch. Dermatol. 2003, 139, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Aslaner Ak, M.; Erdemir, R.U. Multiple muscle involvement in relapsed multiple myeloma: A rare case. J. Cancer Res. Ther. 2022, 18, 1165–1167. [Google Scholar] [CrossRef]
- Mathew, J.; Lubitz, S.; Zaidan, J. SUN-928 Adrenal Plasmacytoma in Multiple Myeloma Patient-An Unusual Presentation. J. Endocr. Soc. 2020, 4, SUN-928. [Google Scholar] [CrossRef]
- Zanwar, S.; Ho, M.; Lin, Y.; Kapoor, P.; Binder, M.; Buadi, F.K.; Dispenzieri, A.; Dingli, D.; Fonder, A.; Gertz, M.A.; et al. Natural history, predictors of development of extramedullary disease, and treatment outcomes for patients with extramedullary multiple myeloma. Am. J. Hematol. 2023, 98, 1540–1549. [Google Scholar] [CrossRef]
- Bergsagel, P.L.; Kuehl, W.M. Chromosome translocations in multiple myeloma. Oncogene 2001, 20, 5611–5622. [Google Scholar] [CrossRef]
- Samur, M.K.; Aktas Samur, A.; Shah, P.; Park, J.; Fulciniti, M.; Shammas, M.A.; Corre, J.; Anderson, K.C.; Parmigiani, G.; Avet-Loiseau, H.; et al. Development of hyperdiploidy starts at an early age and takes a decade to complete. Blood 2024, 145, 520–525. [Google Scholar] [CrossRef]
- Ho, M.; Patel, A.; Goh, C.Y.; Moscvin, M.; Zhang, L.; Bianchi, G. Changing paradigms in diagnosis and treatment of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Leukemia 2020, 34, 3111–3125. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Rodríguez-García, Y.; Encinas, J.; Maroto-Martín, E.; Castellano, E.; Teixidó, J.; Martínez-López, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef]
- Singh, P.; Mohammad, K.S.; Pelus, L.M. CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells 2020, 38, 849–859. [Google Scholar] [CrossRef]
- Alsayed, Y.; Ngo, H.; Runnels, J.; Leleu, X.; Singha, U.K.; Pitsillides, C.M.; Spencer, J.A.; Kimlinger, T.; Ghobrial, J.M.; Jia, X.; et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 2007, 109, 2708–2717. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Liu, C.-J.; Zavidij, O.; Azab, A.K.; Baz, R.; Laubach, J.P.; Mishima, Y.; Armand, P.; Munshi, N.C.; Basile, F.; et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. Am. J. Hematol. 2019, 94, 1244–1253. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ghobrial, I.M.; Roodman, G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006, 34, 1289–1295. [Google Scholar] [CrossRef]
- Sahin, A.O.; Buitenhuis, M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adhes. Migr. 2012, 6, 39–48. [Google Scholar] [CrossRef]
- Cerny, J.; Fadare, O.; Hutchinson, L.; Wang, S.A. Clinicopathological features of extramedullary recurrence/relapse of multiple myeloma. Eur. J. Haematol. 2008, 81, 65–69. [Google Scholar] [CrossRef]
- Chang, H.; Bartlett, E.S.; Patterson, B.; Chen, C.I.; Yi, Q.L. The absence of CD56 on malignant plasma cells in the cerebrospinal fluid is the hallmark of multiple myeloma involving central nervous system. Br. J. Haematol. 2005, 129, 539–541. [Google Scholar] [CrossRef]
- Dahl, I.M.; Rasmussen, T.; Kauric, G.; Husebekk, A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br. J. Haematol. 2002, 116, 273–277. [Google Scholar] [CrossRef]
- Sahara, N.; Takeshita, A.; Shigeno, K.; Fujisawa, S.; Takeshita, K.; Naito, K.; Ihara, M.; Ono, T.; Tamashima, S.; Nara, K.; et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br. J. Haematol. 2002, 117, 882–885. [Google Scholar] [CrossRef]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Reme, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef]
- Garayoa, M.; Garcia, J.L.; Santamaria, C.; Garcia-Gomez, A.; Blanco, J.F.; Pandiella, A.; Hernandez, J.M.; Sanchez-Guijo, F.M.; del Canizo, M.C.; Gutierrez, N.C.; et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia 2009, 23, 1515–1527. [Google Scholar] [CrossRef]
- Binder, M.; Szalat, R.E.; Talluri, S.; Fulciniti, M.; Avet-Loiseau, H.; Parmigiani, G.; Samur, M.K.; Munshi, N.C. Bone marrow stromal cells induce chromatin remodeling in multiple myeloma cells leading to transcriptional changes. Nat. Commun. 2024, 15, 4139. [Google Scholar] [CrossRef]
- Rajan, A.M.; Rajkumar, S.V. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015, 5, e365. [Google Scholar] [CrossRef]
- Vande Broek, I.; Vanderkerken, K.; Van Camp, B.; Van Riet, I. Extravasation and homing mechanisms in multiple myeloma. Clin. Exp. Metastasis 2008, 25, 325–334. [Google Scholar] [CrossRef]
- Garcés, J.J.; San-Miguel, J.; Paiva, B. Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma. Cancers 2022, 14, 1430. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Miyazaki, K.; Suzuki, K. CD56 for Multiple Myeloma: Lack of CD56 May Be Associated with Worse Prognosis. Acta Haematol. 2018, 140, 40–41. [Google Scholar] [CrossRef]
- McAvera, R.; Quinn, J.; Murphy, P.; Glavey, S. Genetic Abnormalities in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 11259. [Google Scholar] [CrossRef]
- Besse, L.; Sedlarikova, L.; Greslikova, H.; Kupska, R.; Almasi, M.; Penka, M.; Jelinek, T.; Pour, L.; Adam, Z.; Kuglik, P.; et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur. J. Haematol. 2016, 97, 93–100. [Google Scholar] [CrossRef]
- Shaughnessy, J.D., Jr.; Zhan, F.; Burington, B.E.; Huang, Y.; Colla, S.; Hanamura, I.; Stewart, J.P.; Kordsmeier, B.; Randolph, C.; Williams, D.R.; et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2006, 109, 2276–2284. [Google Scholar] [CrossRef]
- Sevcikova, S.; Paszekova, H.; Besse, L.; Sedlarikova, L.; Kubaczkova, V.; Almasi, M.; Pour, L.; Hajek, R. Extramedullary relapse of multiple myeloma defined as the highest risk group based on deregulated gene expression data. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 288–293. [Google Scholar] [CrossRef]
- Billecke, L.; Murga Penas, E.M.; May, A.M.; Engelhardt, M.; Nagler, A.; Leiba, M.; Schiby, G.; Kroger, N.; Zustin, J.; Marx, A.; et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br. J. Haematol. 2013, 161, 87–94. [Google Scholar] [CrossRef]
- Chen, T.; Sun, Z.; Cui, Y.; Ji, J.; Li, Y.; Qu, X. Identification of long noncoding RNA NEAT1 as a key gene involved in the extramedullary disease of multiple myeloma by bioinformatics analysis. Hematology 2023, 28, 2164449. [Google Scholar] [CrossRef]
- Egan, J.B.; Kortuem, K.M.; Kurdoglu, A.; Izatt, T.; Aldrich, J.; Reiman, R.; Phillips, L.; Baker, A.; Shi, C.X.; Schmidt, J.; et al. Extramedullary myeloma whole genome sequencing reveals novel mutations in Cereblon, proteasome subunit G2 and the glucocorticoid receptor in multi drug resistant disease. Br. J. Haematol. 2013, 161, 748–751. [Google Scholar] [CrossRef]
- Kriegova, E.; Fillerova, R.; Minarik, J.; Savara, J.; Manakova, J.; Petrackova, A.; Dihel, M.; Balcarkova, J.; Krhovska, P.; Pika, T.; et al. Whole-genome optical mapping of bone-marrow myeloma cells reveals association of extramedullary multiple myeloma with chromosome 1 abnormalities. Sci. Rep. 2021, 11, 14671. [Google Scholar] [CrossRef]
- Liu, Y.; Jelloul, F.; Zhang, Y.; Bhavsar, T.; Ho, C.; Rao, M.; Lewis, N.E.; Cimera, R.; Baik, J.; Sigler, A.; et al. Genetic Basis of Extramedullary Plasmablastic Transformation of Multiple Myeloma. Am. J. Surg. Pathol. 2020, 44, 838–848. [Google Scholar] [CrossRef]
- Long, X.; Xu, Q.; Lou, Y.; Li, C.; Gu, J.; Cai, H.; Wang, D.; Xu, J.; Li, T.; Zhou, X.; et al. The utility of non-invasive liquid biopsy for mutational analysis and minimal residual disease assessment in extramedullary multiple myeloma. Br. J. Haematol. 2020, 189, e45–e48. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Sirdesai, S.; Chen, M.; Khong, T.; Spencer, A. Circulating Tumour DNA Analysis for Tumour Genome Characterisation and Monitoring Disease Burden in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 1858. [Google Scholar] [CrossRef]
- Qu, X.; Chen, L.; Qiu, H.; Lu, H.; Wu, H.; Qiu, H.; Liu, P.; Guo, R.; Li, J. Extramedullary manifestation in multiple myeloma bears high incidence of poor cytogenetic aberration and novel agents resistance. Biomed. Res. Int. 2015, 2015, 787809. [Google Scholar] [CrossRef]
- Ryu, D.; Kim, S.J.; Hong, Y.; Jo, A.; Kim, N.; Kim, H.J.; Lee, H.O.; Kim, K.; Park, W.Y. Alterations in the Transcriptional Programs of Myeloma Cells and the Microenvironment during Extramedullary Progression Affect Proliferation and Immune Evasion. Clin. Cancer Res. 2020, 26, 935–944. [Google Scholar] [CrossRef]
- Smetana, J.; Oppelt, J.; Stork, M.; Pour, L.; Kuglik, P. Chromothripsis 18 in multiple myeloma patient with rapid extramedullary relapse. Mol. Cytogenet. 2018, 11, 7. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, J.; Li, Y.; Cui, Y.; Fan, L.; Li, J.; Qu, X. Identification of evolutionary mechanisms of myelomatous effusion by single-cell RNA sequencing. Blood Adv. 2023, 7, 4148–4159. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, Y.; Chen, Z.; Zhang, J.; Zhu, Y.; Guo, R.; Zhang, R.; Shi, Q.; Li, J.; Chen, L. Characteristics and prognostic value of extramedullary chromosomal abnormalities in extramedullary myeloma. Chin. Med. J. 2022, 135, 2500–2502. [Google Scholar] [CrossRef]
- Yao, Q.; Morgan, G.J.; Chim, C.S. Distinct promoter methylation profile reveals spatial epigenetic heterogeneity in 2 myeloma patients with multifocal extramedullary relapses. Clin. Epigenetics 2018, 10, 158. [Google Scholar] [CrossRef]
- Jelinek, T.; Zihala, D.; Sevcikova, T.; Anilkumar Sithara, A.; Kapustova, V.; Sahinbegovic, H.; Venglar, O.; Muronova, L.; Broskevicova, L.; Nenarokov, S.; et al. Beyond the marrow: Insights from comprehensive next-generation sequencing of extramedullary multiple myeloma tumors. Leukemia 2024, 38, 1323–1333. [Google Scholar] [CrossRef]
- Zanwar, S.; Novak, J.; Howe, M.D.; Binder, M.; Gonsalves, W.I.; Braggio, E.; Rajkumar, V.; Jevremovic, D.; Dasari, S.; Kumar, S. The Mutational Landscape of Extramedullary Multiple Myeloma Reveals Novel Biologic Insights and Potential Therapeutic Targets for Exploration. Blood 2024, 144, 2744. [Google Scholar] [CrossRef]
- Schavgoulidze, A.; Corre, J.; Samur, M.K.; Mazzotti, C.; Pavageau, L.; Perrot, A.; Cazaubiel, T.; Leleu, X.; Macro, M.; Belhadj, K.; et al. RAS/RAF landscape in monoclonal plasma cell conditions. Blood 2024, 144, 201–205. [Google Scholar] [CrossRef]
- Paiva, B.; Calasanz, M.-J. RASping myeloma genomics. Blood 2024, 144, 129–131. [Google Scholar] [CrossRef]
- Ansari-Pour, N.; Samur, M.; Flynt, E.; Gooding, S.; Towfic, F.; Stong, N.; Estevez, M.O.; Mavrommatis, K.; Walker, B.; Morgan, G.; et al. Whole-genome analysis identifies novel drivers and high-risk double-hit events in relapsed/refractory myeloma. Blood 2023, 141, 620–633. [Google Scholar] [CrossRef]
- Maclachlan, K.H.; Garces, J.-J.; Shekarkhand, T.; Rajeeve, S.; Hashmi, H.; Hassoun, H.; Hultcrantz, M.; Korde, N.; Tan, C.R.; Mailankody, S.; et al. Genomic Complexity Correlates with the Degree of Marrow Independence of Malignant Plasma Cells in the Context of Extramedullary Disease. Blood 2024, 144, 248. [Google Scholar] [CrossRef]
- Bianchi, G.; Czarnecki, P.G.; Ho, M.; Roccaro, A.M.; Sacco, A.; Kawano, Y.; Gullà, A.; Samur, A.A.; Chen, T.; Wen, K.; et al. ROBO1 Promotes Homing, Dissemination, and Survival of Multiple Myeloma within the Bone Marrow Microenvironment. Blood Cancer Discov. 2021, 2, 338–353. [Google Scholar] [CrossRef]
- Pinho, A.V.; Van Bulck, M.; Chantrill, L.; Arshi, M.; Sklyarova, T.; Herrmann, D.; Vennin, C.; Gallego-Ortega, D.; Mawson, A.; Giry-Laterriere, M.; et al. ROBO2 is a stroma suppressor gene in the pancreas and acts via TGF-β signalling. Nat. Commun. 2018, 9, 5083. [Google Scholar] [CrossRef]
- He, H.; Di, Y.; Liang, M.; Yang, F.; Yao, L.; Hao, S.; Li, J.; Jiang, Y.; Jin, C.; Fu, D. The microRNA-218 and ROBO-1 signaling axis correlates with the lymphatic metastasis of pancreatic cancer. Oncol. Rep. 2013, 30, 651–658. [Google Scholar] [CrossRef]
- John, M.; Helal, M.; Duell, J.; Mattavelli, G.; Stanojkovska, E.; Afrin, N.; Leipold, A.M.; Steinhardt, M.J.; Zhou, X.; Žihala, D.; et al. Spatial transcriptomics reveals profound subclonal heterogeneity and T-cell dysfunction in extramedullary myeloma. Blood 2024, 144, 2121–2135. [Google Scholar] [CrossRef]
- Qi, Y.; Li, H.; Qi, K.; Zhu, F.; Cheng, H.; Chen, W.; Yan, Z.; Li, D.; Sang, W.; Fei, X.; et al. Clinical outcomes and microenvironment profiling in relapsed/refractory multiple myeloma patients with extramedullary disease receiving anti-BCMA CAR T-cell-based therapy. Am. J. Hematol. 2024, 99, 2286–2295. [Google Scholar] [CrossRef]
- Kastritis, E.; Zervas, K.; Symeonidis, A.; Terpos, E.; Delimbassi, S.; Anagnostopoulos, N.; Michali, E.; Zomas, A.; Katodritou, E.; Gika, D.; et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009, 23, 1152–1157. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Puertas, B.; González-Calle, V.; Sobejano-Fuertes, E.; Escalante, F.; Queizán, J.A.; Bárez, A.; Labrador, J.; Alonso-Alonso, J.M.; García de Coca, A.; Cantalapiedra, A.; et al. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers 2023, 15, 1558. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, X.; Li, X.; Yuan, C.; Chu, X.; Hao, L.; Wu, H.; Zhong, Y. Selinexor combined with bortezomib, lenalidomide, and dexamethasone for the treatment of newly diagnosed multiple myeloma with extramedullary disease. Sci. Rep. 2024, 14, 28557. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Garfall, A.L.; Nooka, A.K.; van de Donk, N.W.C.J.; Moreau, P.; Bhutani, M.; Oriol, A.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.; et al. MM-336 Long-Term Follow-Up From the Phase 1/2 MajesTEC-1 Trial of Teclistamab in Patients With Relapsed/Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2024, 24, S548. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Schinke, C.D.; Touzeau, C.; Minnema, M.C.; Donk, N.W.C.J.v.d.; Rodríguez-Otero, P.; Mateos, M.-V.; Rasche, L.; Ye, J.C.; Vishwamitra, D.; Ma, X.; et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41, 8036. [Google Scholar] [CrossRef]
- Pan, D.; Mouhieddine, T.H.; Fu, W.; Moshier, E.; Parekh, S.; Jagannath, S.; Rossi, A.C.; Richter, J.; Rodriguez, C.; Sanchez, L.J.; et al. Outcomes after CAR T Cells in Multiple Myeloma Patients with Extramedullary and Paramedullary Disease. Blood 2023, 142, 1006. [Google Scholar] [CrossRef]
- Dima, D.; Abdallah, A.O.; Davis, J.A.; Awada, H.; Goel, U.; Rashid, A.; DeJarnette, S.; Anwer, F.; Shune, L.; Raza, S.; et al. Impact of Extraosseous Extramedullary Disease on Outcomes of Patients with Relapsed-Refractory Multiple Myeloma receiving Standard-of-Care Chimeric Antigen Receptor T-Cell Therapy. Blood Cancer J. 2024, 14, 90. [Google Scholar] [CrossRef]
- Gagelmann, N.; Dima, D.; Merz, M.; Hashmi, H.; Ahmed, N.; Tovar, N.; Oliver-Caldés, A.; Stölzel, F.; Rathje, K.; Fischer, L.; et al. Development and Validation of a Prediction Model of Outcome After B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2024, 42, 1665–1675. [Google Scholar] [CrossRef]
- Nakashima, J.Y.; Khatri, V.; Cruz-Chamorro, R.J.; Zhou, J.; Patra, P.; Gonzalez, R.; De Avila, G.; Locke, F.L.; Liu, H.D.; Nishihori, T.; et al. Patterns of Failure in Multiple Myeloma with Extramedullary Disease Following Anti-BCMA Directed Chimeric Antigen Receptor (CAR) T-Cell Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S206–S207. [Google Scholar] [CrossRef]
- Vegivinti, C.T.R.; Lawrence Alexander Santhi, J.; Liu, L.; Keesari, P.R.; Thakur, R.; Hammami, M.B.; Kapu, V.; Pericherla, S.; Gopireddy, M.M.r.; Poojary, N.; et al. Efficacy of Bispecific Antibodies Vs CAR-T in the Treatment of Extramedullary Disease and High-Risk Cytogenetics in Relapsed Multiple Myeloma: A Systematic Review and Meta-Analysis. Blood 2023, 142, 1994. [Google Scholar] [CrossRef]
- Cohen, A.D.; Mateos, M.V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023, 141, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Ayuk, F.A.; Klyuchnikov, E.; Wolschke, C.; Berger, S.C.; Kroger, N. Impact of high-risk disease on the efficacy of chimeric antigen receptor T-cell therapy for multiple myeloma: A meta-analysis of 723 patients. Haematologica 2023, 108, 2799–2802. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Luo, W.; Liao, D.; Xie, W.; Wei, Q.; Zhang, Y.; Wang, X.; Wu, Z.; Kang, Y.; et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia 2024, 38, 149–159. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Gu, W.; Shi, M.; Lan, J.; Yan, Z.; Jin, L.; Xia, J.; Ma, S.; Liu, Y.; et al. Long-Term Follow-Up of Combination of B-Cell Maturation Antigen and CD19 Chimeric Antigen Receptor T Cells in Multiple Myeloma. J. Clin. Oncol. 2022, 40, 2246–2256. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, L.; Zhao, H.; Zhang, Y.; Wei, G.; Hong, R.; Wu, W.; Xu, H.; Wang, L.; Ni, F.; et al. Risk Factors Associated with Durable Progression-Free Survival in Patients with Relapsed or Refractory Multiple Myeloma Treated with Anti-BCMA CAR T-cell Therapy. Clin. Cancer Res. 2021, 27, 6384–6392. [Google Scholar] [CrossRef]
- Que, Y.; Xu, M.; Xu, Y.; Almeida, V.D.F.; Zhu, L.; Wang, Z.; Wang, Y.; Liu, X.; Jiang, L.; Wang, D.; et al. Anti-BCMA CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With Extramedullary Disease: A Single Center Analysis of Two Clinical Trials. Front. Immunol. 2021, 12, 755866. [Google Scholar] [CrossRef]
- Li, C.; Cao, W.; Que, Y.; Wang, Q.; Xiao, Y.; Gu, C.; Wang, D.; Wang, J.; Jiang, L.; Xu, H.; et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin. Transl. Med. 2021, 11, e346. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Hu, G.; Wang, W.; Xiao, Y.; Cai, H.; Jiang, L.; Meng, L.; Yang, Y.; Zhou, X.; et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L.; et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.J.; Yang, S.S.; Sun, Y.; Wu, W.; Liu, Y.F.; Xu, J.; Zhuang, Y.; Zhang, W.; Weng, X.Q.; et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 9543–9551. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Riedhammer, C.; Bassermann, F.; Besemer, B.; Bewarder, M.; Brunner, F.; Carpinteiro, A.; Einsele, H.; Faltin, J.; Frenking, J.; Gezer, D.; et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia 2024, 38, 365–371. [Google Scholar] [CrossRef]
- Mohan, M.; Monge, J.; Shah, N.; Luan, D.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in relapsed refractory multiple myeloma: Multi-institutional real-world study. Blood Cancer J. 2024, 14, 35. [Google Scholar] [CrossRef]
- Joiner, L.; Bal, S.; Godby, K.N.; Costa, L.J. Teclistamab in patients with multiple myeloma and impaired renal function. Am. J. Hematol. 2023, 98, E322–E324. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Touzeau, C.; Krishnan, A.Y.; Moreau, P.; Perrot, A.; Usmani, S.Z.; Manier, S.; Cavo, M.; Martinez Chamorro, C.; Nooka, A.K.; Martin, T.G.; et al. Efficacy and safety of teclistamab in patients with relapsed/refractory multiple myeloma after BCMA-targeting therapies. Blood 2024, 144, 2375–2388. [Google Scholar] [CrossRef]
- Sidana, S.; Patel, K.K.; Peres, L.C.; Bansal, R.; Kocoglu, M.H.; Shune, L.; Atrash, S.; Smith, K.; Midha, S.; Ferreri, C.; et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood 2025, 145, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Lancman, G.; Rossi, A.; Chari, A.; Parekh, S.; Sanchez, L.; Rodriguez, C.; Cho, H.J.; Richter, J.; Thibaud, S.; et al. Extramedullary Relapse Post CAR-T. Blood 2022, 140, 4301–4302. [Google Scholar] [CrossRef]
- Costa, L.J.; Bahlis, N.J.; Usmani, S.Z.; van de Donk, N.W.C.J.; Nooka, A.K.; Perrot, A.; Qi, K.; Hodin, C.; Uhlar, C.; Zuppa, A.; et al. MM-328 Efficacy and Safety of Teclistamab in Patients With Relapsed/Refractory Multiple Myeloma (RRMM) With High-Risk (HR) Features: A Subgroup Analysis From the Phase 1/2 MajesTEC-1 Study. Clin. Lymphoma Myeloma Leuk. 2024, 24, S546–S547. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.A.; Ahmed, N.; Jia, X.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; et al. Safety and Efficacy of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Real-World Experience. Transplant. Cell Ther. 2024, 30, 308.e301–308.e313. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Magen, H.; Gatt, M.; Sebag, M.; Kim, K.; Min, C.-K.; Ocio, E.M.; Yoon, S.-S.; Chu, M.P.; Rodríguez-Otero, P.; et al. Talquetamab plus Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2025, 392, 138–149. [Google Scholar] [CrossRef]
- Mejia Saldarriaga, M.; Jayabalan, D.S.; Sowa, A.; Monge, J.; Rosenbaum, C.A.; Pearse, R.N.; Niesvizky, R.; Patel, S.; Bustoros, M. Genomic landscape of multiple myeloma with extramedullary disease: Results from a large patient database. J. Clin. Oncol. 2023, 41, 8058. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Minnema, M.C.; van der Holt, B.; Schjesvold, F.; Wu, K.L.; Broijl, A.; Roeloffzen, W.W.H.; Gadisseur, A.; Pietrantuono, G.; Pour, L.; et al. Treatment of primary plasma cell leukaemia with carfilzomib and lenalidomide-based therapy (EMN12/HOVON-129): Final analysis of a non-randomised, multicentre, phase 2 study. Lancet Oncol. 2023, 24, 1119–1133. [Google Scholar] [CrossRef]
- Giesen, N.; Chatterjee, M.; Scheid, C.; Poos, A.M.; Besemer, B.; Miah, K.; Benner, A.; Becker, N.; Moehler, T.; Metzler, I.; et al. A phase 2 clinical trial of combined BRAF/MEK inhibition for BRAFV600E-mutated multiple myeloma. Blood 2023, 141, 1685–1690. [Google Scholar] [CrossRef]
- Heuck, C.J.; Jethava, Y.; Khan, R.; van Rhee, F.; Zangari, M.; Chavan, S.; Robbins, K.; Miller, S.E.; Matin, A.; Mohan, M.; et al. Inhibiting MEK in MAPK pathway-activated myeloma. Leukemia 2016, 30, 976–980. [Google Scholar] [CrossRef]
- Costa, L.J.; Schjesvold, F.; Popat, R.; Siegel, D.; Usmani, S.Z.; Ali, S.A.; Chu, M.P.; Hartley-Brown, M.A.; Bahlis, N.J.; Oriol, A.; et al. Mezigdomide (MEZI) in Novel-Novel Combinations for Relapsed or Refractory Multiple Myeloma (RRMM): Preliminary Results from the CA057-003 Trial. Blood 2024, 144, 677. [Google Scholar] [CrossRef]
| Study Type | Year Published (Time Period) | Imaging Used to Assess for EMD | Population | Type of EM Involvement | Incidence of EMM | Treatments | PFS | OS | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Single-center retrospective analysis (USA) | 2022 (1970–2018) | Not reported | 1304 patients (256 EMD) | PSD (1º): 230 (17.6%) EMM (1º): 26 (2%) PSD (2º): 142 (14.6%) EMM (2º): 50 (5.1%) | 1º: 2% 2º: 5.1% | Chemotherapy (n = 873); PI (n = 180); IMiD (n = 70); PI + IMiD (n = 150); Monoclonal antibody (n = 16) ASCT (n = 413) | Not reported | mOS (1º): 20 (EMM) vs. 44 (PSD) vs. 45 (no EMM) months mOS (2º): 13 (EMM) vs. 14 (PSD) vs. 20 (no EMM) months | [18] |
| Meta-analysis of 8 trials: GIMEMA-MM-05-05, GIMEMA-MM-03-05, RV-MM-PI-209, RV-MM-EMN-441, EMN01, MMY2069, IST-CAR-506, IST-CAR-561 | 2020 (2010–2018) | Skeletal survey, MRI, or CT | 2322 NDMM (267 EMD) | PSD (1º): 243 (10.4%) EMM (1º): 12 (0.5%) Unclassified: 12 (0.5%) | 1º: 0.5% | IMiD backbone (n = 166), PI backbone (n = 66), IMiD + PI backbone (n = 35) followed by ASCT (n = 155) | mPFS: 24.3 (PSD) vs. 26.1 (EMM) vs. 25.2 (no EMD) months | mOS: 67.3 (PSD) vs. 70.1 (EMM) vs. 79.9 (no EMD) months | [19] |
| Multi-center retrospective analysis (11 European countries) | 2020 (2010–2017) | PET/CT (n = 50), MRI (n = 35), CT (n = 133) | 226 ND/RRMM with EMD | PSD (1º): 38 (16.8%) EMM (1º): 92 (40.7%) PSD (2º): 12 (5.3%) EMM (2º): 84 (37.2%) | Not reported | PI, IMiD PI + IMiD Monoclonal antibody | mPFS (1º): 51.7 (PSD) vs. 38.9 (EMM) months mPFS (2º): 20.9 (PSD) vs. 11.4 (EMM) months | mOS (1º): NR (PSD) vs. 46.5 (EMM) months; mOS (2º): 39.8 (PSD) vs. 13.6 (EMM) months | [20] |
| EBMT registry analysis | 2019 (2003–2015) | Not reported | 488 NDMM with EMD | PSD (1º): 76% EMM (1º): 18% | Not reported | Upfront ASCT (single or tandem or auto-allo) within 12 months of diagnosis | 4y PFS: 44% (PSD) vs. 39% (EMM) | 4y OS: 72% (PSD) vs. 60% (EMM) vs. 46% (both) | [21] |
| EBMT registry analysis | 2018 (2005–2014) | Not reported | 3744 NDMM (682 EMD) | PSD (1º): 543 (14.5%) EMM (1º): 139 (3.7%) | 1º: 3.7% | Upfront ASCT (single or tandem) within 12 months of diagnosis | 3y PFS: 50% (PSD) vs. 39.9% (EMM) vs. 47.9% (no EMD) | 3y OS: 77.7% (PSD) vs. 58% (EMM) vs. 80.1% (no EMM) | [17] |
| Single-center retrospective analysis (South Korea) | 2017 (2009–2016) | PET/CT | 64 NDMM with EMD | PSD (1º): 42 (65.6%) EMM (1º): 22 (34.4%) | Not reported | Vd (n = 7), Td (n = 23), VTd (n = 11), VMP (n = 16), MP (n = 6); followed by ASCT (n = 28) | mPFS: 16.1 (PSD) vs. 16 (EMM) months | 2y OS (1º): 52.6% (PSD) vs. 35.1% (EMM) | [22] |
| Single-center retrospective analysis (India) | 2017 (Not reported) | PET/CT, MRI | 271 NDMM (44 EMD) | PSD (1º): 30 (11.1%); EMM (1º): 8 (3%); PSD + EMM (1º): 6 (2.2%) | 1º: 5.2% | Novel agents (n = 27), Conventional chemo (n = 17); followed by ASCT (n = 44) | mPFS: 18 (all EM) vs. 44 (no EM) months | mOS: 32 (all EM) vs. 100 (no EM) months | [23] |
| Single-center retrospective analysis (Italy) | 2016 (2000–2010) | XR, CT, MRI | 329 patients (103 with secondary EMD) | PSD (2º): 34 (10.3%) EMM (2º): 43 (13.1%) PCL (2º): 26 (7.9%) | 2º: 13.1% | Not reported specifically for EMM (chemotherapy, bortezomib, lenalidomide, thalidomide) | Not reported | mOS: 2.4 (PSD) vs. 1.6 (EMM + PCL) vs. 11 (no EMM) years | [24] |
| Multi-center phase 2 clinical trial (Japan) | 2016 | Not reported | 36 RRMM (5 EMM) | EMM-B: 4 EMM: 1 (2.8%) | 2.8% | Pomalidomide + Dex | Not reported specifically for EMM but none of the EMM patients achieved ≥ PR | [25] | |
| Single-center retrospective analysis (China) | 2015 (1993–2013) | XR, US, CT | 834 patients (68 EMD) | EMM (1º): 40 (4.8%) EMM (2º): 28 (3.4%) | 1º: 4.8% 2º: 3.4% | IMiD backbone (n = 11), PI backbone (n = 17), Alkylating agent (n = 4), Dexamethasone-based (n = 8) followed by ASCT (n = 5) | mTTP: 11.5 (EMM) vs. 25 (no EMM) months | mOS: 16.5 (EMM) vs. 40 (no EMM) months | [26] |
| Single-center retrospective analysis (USA; DFCI) | 2015 (2005–2011) | Not reported | 663 patients treated with ASCT (55 EMD) | EMM (1º): 13 (2%) EMM (2º): 42 (6.3%) | 1º: 2% 2º: 6.3% | Td (n = 25), RVd (n = 23), Vd (n = 15); ASCT (n = 55); AlloSCT (n = 15) | Not reported | mOS: 49.2 months (from MM diagnosis); 15.6 months (from EMM diagnosis) | [27] |
| Single-center retrospective analysis (USA; DFCI/MGH) | 2015 (2003–2012) | CT, PET/CT, MRI | 117 patients (40 with EMD at diagnosis; 40 at relapse) | PSD (1º): 38 (32.5%) EMM (1º): 2 (1.7%); PSD (2º): 21 (17.9%) EMM (2º): 8 (6.8%) PSD + EMM (2º): 11 (9.4%) | 1º: 1.7% 2º: 16.2% | 8 clinical trials of upfront bortezomib-based regimens with/without lenalidomide; followed by ASCT (n = 57) or AlloSCT (n = 4) | Not reported | 2º: mOS: 2.47 (PSD) vs. 0.9 (EMM) years | [28] |
| Single-center retrospective analysis (Czech Republic) | 2013 (2005–2008) | US, CT, MRI | 226 RRMM (55 with EMD at relapse) | PSD (2º): 23 (10.2%) EMM (2º): 32 (14.2%) | 2º: 14.2% | Treatment at time of EM relapse not reported | mTTP: 5.4 months | mOS: 38 (EM) vs. 109 (no EM) months; 30 (EMM) vs. 45 (PSD) months | [29] |
| Single-center retrospective analysis (Germany) | 2012 (2007–2010) | Not reported | 357 RRMM (24 EMD) | EMM (2º): 24 (6.7%) | 2º: 6.7% | RT (n = 16), ASCT (n = 6), velcade (n = 16), lenalidomide (n = 12), thalidomide (n = 8) | mPFS: 2 months | mOS: 7 months | [30] |
| Single-center retrospective analysis (USA; UAMS) | 2012 (2000–2010) | PET/CT | 1965 patients (101 EMD) | EMM (1º): 66 (3.4%) EMM (2º): 35 (1.8%) | 1º: 3.4% 2º: 1.8% | ASCT as part of total therapy and non-total therapy protocols | 5y PFS (1º): 21% (EMM) vs. 50% (no EMM) | 5y OS (1º): 31% (EMM) vs. 59% (no EMM) | [10] |
| Single-center phase 2 clinical trial (USA; Mayo Clinic) | 2011 (2007–2010) | PET/CT, CT, MRI | 174 RRMM (16 EMM) | EMM (1º): 3 (1.7%) EMM (2º): 13 (7.5%) | 1º: 1.7% 2º: 7.5% | Pomalidomide + Dex | Not reported | mOS: 16 (EMM) vs. NR (no EMM) months | [31] |
| Single-center retrospective analysis (Italy) | 2009 (1971–2007) | XR, CT, MRI | 1003 MM patients (132 with EMM; 76 at diagnosis, 56 at relapse) | PSD (1º): ~65 (6.5%) EMM (1º): ~11 (1.1%); PSD (2º): ~40 (4%) EMM (2º): ~16 (1.6%); PCL (2º): 9 (0.9%) | 1º: ~1.1% 2º: ~1.6% | 1971–1993: conventional-dose chemotherapy; 1994–1999: ASCT < 65yo; 2000–2007: PI, IMiD-based | mPFS: 18 (all EMM) vs. 30 (no EMM) months | mOS: 36 (all EMM) vs. 43 (no EMM) months | [11] |
| Multi-center retrospective analysis (7 centers in the Netherlands) | 2008 | Not reported | 172 patients who received sequential auto-alloSCT | EMM (2º): 11 (6.4%) | 2º: 6.4% | Donor lymphocyte infusions (n = 3), thalidomide (n = 6), bortezomib (n = 2), dexamethasone (n = 2), RT (n = 4) | mPFS: 2.4 years (not significantly different between EMM and no EMM) | 1y OS: 61% (EMM) vs. 73% (no EM) | [32] |
| Multi-center retrospective analysis (Grupo Espanol de Mieloma) | 2006 (1999–2004) | Imaging only if symptomatic | 70 patients who received alloSCT | EMM (2º): 10 (14.3%) | 2º: 14.3% | Not reported | Not reported | Not reported | [33] |
| Study Type | Year Published (Time Period) | Population | Type of T-Cell-Redirecting Therapy | Lines of Therapy Prior to CART/BiTe | Efficacy in EMM | Reference |
|---|---|---|---|---|---|---|
| Multi-center retrospective analysis (USA) | 2024 (data cutoff: 2023) | 152 RRMM (47 EMM) | Cilta-cel Ide-cel | Median: 6; range: 4–15 | ORR: 58% (EMM) vs. 96% (no EMM); mPFS: 5.1 (EMM) vs. 12.4 (no EMM) months; mOS: 12.2 (EMM) vs. 27.5 (no EMM) months | [109] |
| Multi-center retrospective analysis (international) | 2024 (not reported) | 269 RRMM (112 EMM) | Cilta-cel Ide-cel Investigational | Median: 6; range: 6–7 | EMM predicts for early relapse/progression post-CART (HR: 1.92) | [110] |
| Single-center retrospective study (USA; Moffitt) | 2024 (2021–2023) | 116 RRMM (20 EMM; 10 PSD; 7 EMM + PSD) | Cilta-cel Ide-cel | Median: 6; range: 4–9 | mPFS: 15.7 months (all EMM); mOS: 3.1 months (all EMM) | [111] |
| Meta-analysis of BiTEs vs. CARTs | 2023 (not reported) | 172 RRMM with EMM (type of EMM not specified) | CART | Not reported | ORR: 77% (EMM) | [112] |
| Single-center retrospective study (USA; Mayo Clinic) | 2023 (2000–2021) | 299 RRMM patients with EMM; EMM (1º): 95; EMM (2º): 204 | Cilta-cel Ide-cel Investigational | Median: 4; range: 4–8 | ORR: 75%; mPFS: 4.9 months | [47] |
| Single-center retrospective study (USA) | 2023 (2017–2023) | 134 RRMM; EMM (1º): 34; PSD (1º): 25; EMM (2º): 13 | Cilta-cel Ide-cel Investigational | Not Reported | HR for PFS of 1º EMM vs. no EMM: 1.87; HR for OS of 1º EMM vs. no EMM: 3.78 | [108] |
| Multi-center phase 2 clinical trial (CARTITUDE-2C) | 2023 (data cutoff: 2021) | 20 RRMM (cohort C) (5 EMM; type of EMM not specified) | Cilta-cel | Median: 8; range: 4–13 | ORR: 60% (EMM) | [113] |
| Multi-center phase 1/2b clinical trial (CARTITUDE-1) | 2021 (2018–2019) | 113 RRMM (13 EMM; type of EMM not specified) | Cilta-cel | Median: 6; range (4–8) | mDOR: 12.9 months (EMM) vs. 23.3 months (all patients) | [104] |
| Multi-center retrospective analysis (USA) | 2024 (2021–2023) | 351 RRMM (84 EMM) | Ide-cel | Median: 6; range: 5–8 | ORR: 52% (EMM) vs. 82% (no EMM); mPFS: 5.3 (EMM) vs. 11.1 (no EMM) months; mOS: 14.8 (EMM) vs. 26.9 (no EMM) months | [16] |
| Multi-center phase 2 clinical trial (KarMMa) | 2021 (2017–2020) | 128 RRMM (50 EMM; type of EMM not reported) | Ide-cel | Median: 6; range: 3–16 | ORR of EMM worse than no EMM (no specifics) | [103] |
| Multi-center phase 1 clinical trial (CRB-401) | 2019 (2016–2018) | 33 RRMM (9 EMM; type of EMM not reported) | Ide-cel | Median: 7; range: 3–14 | ORR: 89% (EMD) | [114] |
| Meta-analysis of 17 trials | 2023 | 723 RRMM | Investigational | Median: 5 | RR for ORR of EMM vs. no EMD: 0.97; RR for PFS of EMM vs. no EMM: 1.44; RR for OS of EMM vs. no EMM: 1.96 | [115] |
| Single-center phase 1/2a clinical trial (China) | 2024 (2018–2022) | 16 RRMM (6 EMD; 3 had EMM without medullary disease; type of EMM not reported) | Investigational (bispecific anti-BCMA and anti-CS1 CART) | Median: 4; range: 2–8 | ORR: 0% (in 3 patients with EMM without medullary disease) | [116] |
| Single-center prospective cohort study (China) | 2024 (2017–2023) | 31 RRMM patients with EMM | Investigational (anti-BCMA and anti-CD19 CART) | Median: 4; range: 3–12 | ORR: 90% (medullary and serological); 64.5% (imaging); mPFS: 5 months; mOS: 9.7 months | [98] |
| Single-center phase 2 clinical trial (China) | 2022 (2017–2021) | 69 RRMM (15 EMM; type of EMM not reported) | Investigational (anti-BCMA and anti-CD19 CART) | Median: 4; range (2–17) | ORR: 80%; mPFS: 8.3 (EM M) vs. 21.4 (no EMM) months; mOS: 12.3 (EMM) vs. NR (no EMM) months | [117] |
| Single-center phase 1 clinical trial (China) | 2021 (2018–2021) | 61 RRMM (7 EMM, 10 PSD, 10 EMM + PSD) | Investigational (anti-BCMA CART) | Median: 3; range (3–9) | CR: 59% (EMM) vs. 81% (no EMM); 1 yr PFS: 34.4% (EMM) vs. 60.2% (no EMM) | [118] |
| Single-center retrospective analysis of 2 clinical trials (China) | 2021 (2017–2021) | 60 RRMM (25 EMM/PCL; 35 without EMM) | Investigational: 25 (CT103A: 7, murine anti-BCMA CART: 18) | Median: 4; range: 3–11 | ORR: 84% (EMM) vs. 94.4% (no EMM); mPFS: 121 (EMM) vs. 361 (no EMM) days; mOS: 248 (EMM) vs. 1024 (no EMM) days | [119] |
| Single-center phase 1 clinical trial (China) | 2021 (2017–2019) | 30 RRMM (14 EMM/PCL) | Investigational (murine anti-BCMA CART) | Median 4; range: 3–11 | ORR: 92.8% (EMM/PCL) | [120] |
| Single-center phase 1 clinical trial (China) | 2021 (2018–2020) | 18 RRMM (4 EMM, 1 PCL) | Investigational (CT103A) | Median: 4; range: 3–6 | ORR: 100%; 1-yr PFS: 20% (EMM) vs. 79.1% (no EMM); 1-yr OS: 60% (EMM) vs. 79.1% (no EMM) | [121] |
| Single-center phase 1 clinical trial (China) | 2021 (2019–2021) | 23 RRMM (7 PSD, 1 EMM, 1 unspecified) | Investigational (bispecific anti-BCMA and anti-CD38 CART) | Median: 4; range (2–9) | ORR: 89% (EMM) | [122] |
| Single-center phase 1 clinical trial (USA, UPenn) | 2019 (2015–2018) | 25 RRMM (7 EMD; type of EMD not reported) | Investigational (anti-BCMA CART) | Median: 7; range: 3–13 | ORR: 57% (EMM) | [123] |
| Multi-center phase 1 clinical trial (China) | 2019 (2017–2018) | 17 RRMM (3 EMM, 2 PSD) | Investigational (LCAR-B38M) | Median: 4; range: 3–11 | ORR: 100% (EMM) | [124] |
| Single-center phase 1 clinical trial (USA; NIH) | 2018 (not reported) | 16 RRMM (1 EMM) | Investigational (anti-BCMA CART) | 16 (EMM) | ORR: 100% (EMM) | [125] |
| Multi-center retrospective analysis (Germany) | 2024 (2022–2024) | 123 RRMM (43 EMM; type of EMM not specified) | Teclistamab | Median: 6; range: 3–14 | ORR: 37.2% (EMM) vs. 72.6% (no EMM); mPFS: 2.1 months (EMM) vs. NR (no EMM) | [126] |
| Multi-center retrospective analysis (USA) | 2024 (2023–2024) | 110 RRMM (48 EMM) | Teclistamab | Median: 6; range: 3–13 | ORR: 43% (EMM) vs. 58% (no EMM) | [127] |
| Multi-center retrospective analysis (USA) | 2024 (2022–2023) | 106 RRMM (45 EMM) | Teclistamab | Median: 6; range: 4–18 | ORR: 47% (EMM) vs. 80.3% (no EMM); HR for PFS of EMM: 2.56 | [109] |
| Single-center retrospective study (USA; Mayo Clinic) | 2023 (2000–2021) | 299 RRMM patients with EMM; EMM (1º): 95 EMM (2º): 204 | BiTE: 12 (BCMAxCD3: 10, GPRC5DxCD3: 1, FCRH5xCD3: 1) | Median: 5; range: 4–8 | ORR: 59%; mPFS: 3.9 months | [47] |
| Meta-analysis of BiTE vs. CARTs | 2023 (not reported) | 106 RRMM with EMM (type of EMM not specified) | BiTE (teclistamab, talquetamab) | Not reported | ORR: 48% (EMM) | [112] |
| Single-center retrospective study (USA) | 2023 (not reported) | 7 RRMM (2 EMM; type of EMM not specified) | Teclistamab | Median: 4; range: 4–7 | ORR: 0% (both patients had PD prior to second cycle) | [128] |
| Multi-center phase 1/2 clinical trial (MajesTEC-1; Cohort A) | 2022 (2020–2022) | 165 RRMM (28 EMM) | Teclistamab | Median: 5; range: 2–14 | ORR: 35.7% (EMM) vs. 68.6% (no EMM) | [129] |
| Multi-center phase 1/2 clinical trial (MajesTEC-1; Cohort C) | 2022 (2020–2022) | 40 RRMM previously treated with PI, IMiD, anti-CD38, and BCMA-targeted therapies (12 EMM) | Teclistamab | Median: 6; range: 3–14 | ORR: 58.3% (EMM) vs. 52.5% (all patients) | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, M.; Paruzzo, L.; Minehart, J.; Nabar, N.; Noll, J.H.; Luo, T.; Garfall, A.; Zanwar, S. Extramedullary Multiple Myeloma: Challenges and Opportunities. Curr. Oncol. 2025, 32, 182. https://doi.org/10.3390/curroncol32030182
Ho M, Paruzzo L, Minehart J, Nabar N, Noll JH, Luo T, Garfall A, Zanwar S. Extramedullary Multiple Myeloma: Challenges and Opportunities. Current Oncology. 2025; 32(3):182. https://doi.org/10.3390/curroncol32030182
Chicago/Turabian StyleHo, Matthew, Luca Paruzzo, Janna Minehart, Neel Nabar, Julia Han Noll, Thomas Luo, Alfred Garfall, and Saurabh Zanwar. 2025. "Extramedullary Multiple Myeloma: Challenges and Opportunities" Current Oncology 32, no. 3: 182. https://doi.org/10.3390/curroncol32030182
APA StyleHo, M., Paruzzo, L., Minehart, J., Nabar, N., Noll, J. H., Luo, T., Garfall, A., & Zanwar, S. (2025). Extramedullary Multiple Myeloma: Challenges and Opportunities. Current Oncology, 32(3), 182. https://doi.org/10.3390/curroncol32030182






