Chest Wall Perforator Flaps in Breast Conservation: Versatile, Affordable, and Scalable: Insights from the Largest Single-Surgeon Audit from India
Abstract
:1. Introduction
2. Methodology
2.1. Patient Selection
2.2. Clinical Management
2.3. Surgical Procedures
2.3.1. Incision, Tumor Excision, and Oncological Clearance
2.3.2. Flap Selection
2.3.3. Pedicle Dissection
2.3.4. Pre-Operative Markings
2.3.5. Tumor Localization
2.3.6. Axillary Management
2.4. Post-Surgery Protocols
2.4.1. Assessment of Post-Surgery Complications
2.4.2. Post-Surgery Marking of Tumor Bed for Radiotherapy and Adjuvant Radiation Therapy Methodology
2.4.3. Patient-Reported Outcome Measures
2.5. Data Collection
2.6. Survival Analysis and Statistics
3. Results
3.1. Overview of the Study Cohort
3.2. Neoadjuvant Systemic Therapy (NAST—NACT/NAHT)
3.3. Surgical Outcomes
3.3.1. Surgical Margins and Nodal Clearance
3.3.2. Post Operative Complications
3.4. Adjuvant Radiotherapy
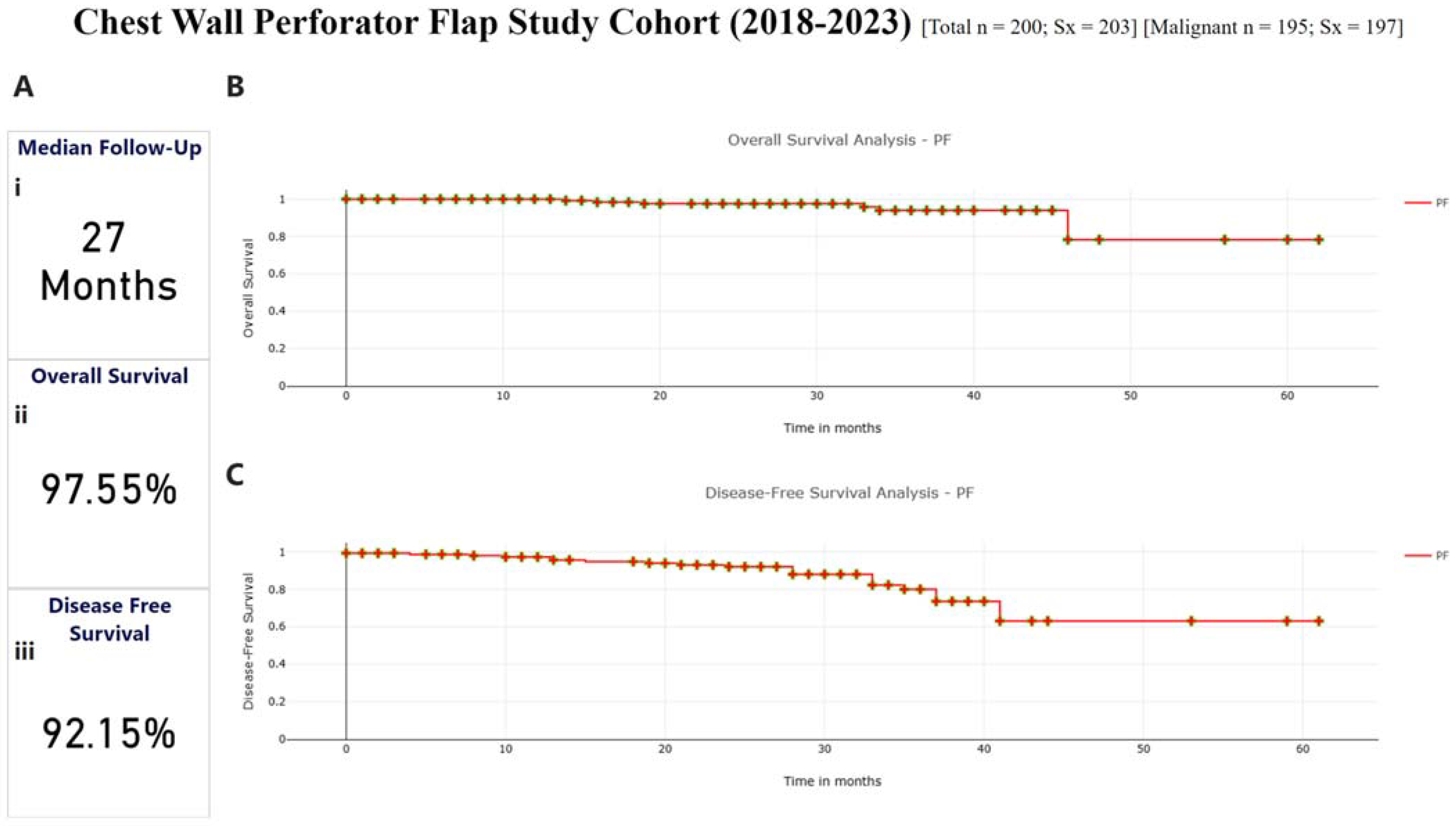
3.5. Survival Outcomes
3.6. Cosmetic Score Analysis
3.7. Patient-Reported Outcome Measures (PROMs)
4. Discussion
Surgeons’ Recommendations for Young Surgeons on CWPF Surgical Algorithm
- Preoperative Planning:
- Precisely identify the LTAP preoperatively to ensure its course to the lateral fold.
- Use high-resolution imaging techniques (ultrasound, contrast mammography) to map out the tumor and perforators.
- Marking and Incision:
- Mark the axillary crease carefully.
- For LTAP, make a small incision that includes the sentinel node biopsy site and allows dissection up to the perforator’s origin.
- Flap Dissection:
- Begin flap dissection from the lateral to medial side for clear visualization and controlled handling of the perforator.
- Dissect the LTAP perforator up to its origin in the axillary artery to increase flap mobility and reduce traction.
- Mobilize the perforator within the muscle if additional reach is needed.
- ICG Utilization:
- If the facility is available, use Indo cyanine dye for SLN mapping.
- Inject ICG dye subdermally to identify SLN and assess vascularity pre- and post-dissection.
- Confirm perfusion of the LTAP flap to avoid under-perfused areas.
- Flap Placement:
- Tunnel the flap through the subcutaneous space to the defect site, ensuring adequate reach and minimal traction on the breast.
- Avoid fixing the flap tip directly to breast parenchyma to prevent asymmetry. Secure the flap to the chest wall using retaining sutures on superior and inferior borders.
- Margins and Tumor Resection:
- Maintain wide margins guided by intraoperative imaging and frozen section analysis to ensure complete tumor excision.
- Axillary Dissection:
- When required, perform axillary dissection in two stages: lateral and medial to the LTAP, ensuring meticulous preservation of the perforator.
- Supercharging:
- For larger defects, consider supercharging the LTAP with an additional LICAP to enhance vascularity.
- Validation and Documentation:
- Validate margins using frozen sections, and follow up with paraffin section confirmation.
- Rely on specimen imaging and pathology to confirm adequacy of resection and flap coverage.
- Patient-Specific Adjustments:
- Adapt flap size based on tumor location (e.g., smaller flaps for medial quadrant tumors).
- Ensure clear communication about surgical plans and outcomes to manage patient expectations effectively.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Axillary Dissection |
| ADM | Acellular Dermal Matrices |
| AICAP | Anterior Intercostal Artery Perforator Flap |
| ALND | Axillary Lymph Node Dissection |
| BC | Breast Cancer |
| BCS | Breast Conservation Surgery |
| BCT | Breast Conservation Therapy |
| CESM | Contrast Enhanced Spectral Mammography |
| CWPF | Chest Wall Perforator Flaps |
| DCIS | Ductal Carcinoma In Situ |
| ICG | Indocyanine Green |
| IDC | Intraductal Carcinoma |
| IMRT | Intensity-Modulated Radiation Therapy |
| KM | Kaplan–Meier |
| LABC | Locally Advanced Breast Cancer |
| LD muscle | Latissimus Dorsi muscle |
| LICAP | Lateral Intercostal Artery Perforator Flap |
| LTAP | Lateral Thoracic Artery Perforator Flap |
| MDT | Multi-disciplinary Team |
| MICAP | Medial Intercostal Artery Perforator Flap |
| NAC | Nipple Areolar Complex |
| NACT | Neo-Adjuvant Chemotherapy |
| NAHT | Neo-adjuvant Hormone Therapy |
| NAST | Neo-adjuvant Systemic Therapy |
| OBS | Oncoplastic Breast Surgery |
| pCR | Pathological Complete Response |
| PET | Positron Emission Tomography |
| pRD | Pathological Residual Disease |
| PROMs | Patient Reported Outcome Measures |
| QoL | Quality of Life |
| RT | Radiation Therapy |
| SIB | Simultaneous Integrated Boost |
| SLNB | Sentinel Lymph Node Biopsy |
| TPS | Treatment Planning Software |
| TRM | Therapeutic Reduction Mammoplasty |
| UOQ | Upper Outer Quadrant |
| VMRT | Volumetric Modulated Arc Therapy |
References
- Mehrotra, R.; Yadav, K. Breast Cancer in India: Present Scenario and the Challenges Ahead. World J. Clin. Oncol. 2022, 13, 209–218. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Sankarapillai, J.; Mathew, A.; Nair, R.A.; Gangane, N.; Khuraijam, S.; Barmon, D.; Pandya, S.; Majumdar, G.; Deshmane, V.; et al. Breast Cancer Survival in India across 11 Geographic Areas under the National Cancer Registry Programme. Cancer 2024, 130, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Christiansen, P.; Carstensen, S.L.; Ejlertsen, B.; Kroman, N.; Offersen, B.; Bodilsen, A.; Jensen, M.-B. Breast Conserving Surgery versus Mastectomy: Overall and Relative Survival—A Population Based Study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2018, 57, 19–25. [Google Scholar] [CrossRef]
- van Maaren, M.C.; de Munck, L.; de Bock, G.H.; Jobsen, J.J.; van Dalen, T.; Linn, S.C.; Poortmans, P.; Strobbe, L.J.A.; Siesling, S. 10 Year Survival after Breast-Conserving Surgery plus Radiotherapy Compared with Mastectomy in Early Breast Cancer in the Netherlands: A Population-Based Study. Lancet Oncol. 2016, 17, 1158–1170. [Google Scholar] [CrossRef]
- Hassan, S.A.; Somashekhar, S.P.; Arun Kumar, N. Rate of Breast-Conserving Surgery vs Mastectomy in Breast Cancer: A Tertiary Care Centre Experience from South India. Indian J. Surg. Oncol. 2019, 10, 72–76. [Google Scholar] [CrossRef]
- Hamdi, M.; Van Landuyt, K.; Monstrey, S.; Blondeel, P. Pedicled Perforator Flaps in Breast Reconstruction: A New Concept. Br. J. Plast. Surg. 2004, 57, 531–539. [Google Scholar] [CrossRef]
- Hamdi, M.; Van Landuyt, K.; de Frene, B.; Roche, N.; Blondeel, P.; Monstrey, S. The Versatility of the Inter-Costal Artery Perforator (ICAP) Flaps. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Pujji, O.J.S.; Blackhall, V.; Romics, L.; Vidya, R. Systematic Review of Partial Breast Reconstruction with Pedicled Perforator Artery Flaps: Clinical, Oncological and Cosmetic Outcomes. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- McCulley, S.J.; Schaverien, M.V.; Tan, V.K.M.; Macmillan, R.D. Lateral Thoracic Artery Perforator (LTAP) Flap in Partial Breast Reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 686–691. [Google Scholar] [CrossRef]
- Pien, I.; Caccavale, S.; Cheung, M.C.; Butala, P.; Hughes, D.B.; Ligh, C.; Zenn, M.R.; Hollenbeck, S.T. Evolving Trends in Autologous Breast Reconstruction: Is the Deep Inferior Epigastric Artery Perforator Flap Taking Over? Ann. Plast. Surg. 2016, 76, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Soumian, S.; Parmeshwar, R.; Chandarana, M.; Marla, S.; Narayanan, S.; Shetty, G. Chest Wall Perforator Flaps for Partial Breast Reconstruction: Surgical Outcomes from a Multicenter Study. Arch. Plast. Surg. 2020, 47, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Romics, L.; Thekkinkattil, D.; Soliman, M.; Kaushik, M.; Barmpounakis, P.; Mortimer, C.; Courtney, C.A.; Goyal, A.; Garreffa, E.; et al. ‘PartBreCon’ Study. A UK Multicentre Retrospective Cohort Study to Assess Outcomes Following PARTial BREast reCONstruction with Chest Wall Perforator Flaps. Breast Scotl. 2023, 71, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.S.K.; Mahajan, S.; Shruti, N.; Sharam, A.; Ahmed, R. Chest Wall Perforator Flap Partial Breast Reconstruction: A Retrospective Analysis of Surgical, Cosmetic and Survival Outcome. Ecancermedicalscience 2024, 18, 1681. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Shakya, S.R.; Nigam, S.; Sharma, A.; Datta, S.S.; Ahmed, R. Chest Wall Perforator Flaps in Partial Breast Reconstruction after Breast Conservation Surgery: An Additional Oncoplastic Surgical Option. Ecancermedicalscience 2020, 14, 1073. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Meybodi, F.; Pantiora, E.; Elder, E.; Cabel, F.; Hsu, J.; French, J.; Aristokleous, I.; Sjökvist, O.; Önefäldt, D.; et al. Chest Wall Perforator Flaps Are Safe and Can Decrease Mastectomy Rates in Breast Cancer Surgery: Multicentre Cohort Study. Br. J. Surg. 2024, 111, znae266. [Google Scholar] [CrossRef]
- Zeeshan, S.; Vohra, L.M.; Shamsi, U.S.; Zahid, N.; Ali, D.; Khan, N.; Garusi, C. A Single Centre Experience of Local Perforator Flaps in Oncoplastic Breast Surgery; a Cross-Sectional Study. Ann. Med. Surg. 2022, 84, 104916. [Google Scholar] [CrossRef]
- Chartier, C.; Safran, T.; Alhalabi, B.; Murphy, A.; Davison, P. Locoregional Perforator Flaps in Breast Reconstruction: An Anatomic Review & Quadrant Algorithm. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1328–1341. [Google Scholar] [CrossRef]
- de Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48,986 Women. JAMA Surg. 2021, 156, 628–637. [Google Scholar] [CrossRef]
- Kabeer, K.K.; Gowda, M.S.; Jafferbhoy, S.; Marla, S.; Narayanan, S.; Soumian, S. Impact of Chest Wall Perforator Flaps on Rates of Total Mastectomy in Breast Cancer. Indian J. Surg. Oncol. 2022, 13, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Mishra, R.; Kamthe, A.; Moosa, K.; Nare, S.; Alhat, R.; Navgire, R.; Vartak, A.; Busheri, L.; Pereira, J.; et al. Bridging the Gap in Oncoplastic Surgery Training and Assessing Its Impact on Breast Cancer Management in India. Glob. Surg. Educ. J. Assoc. Surg. Educ. 2024, 3, 100. [Google Scholar] [CrossRef]
- Panhofer, P.; Ferenc, V.; Schütz, M.; Gleiss, A.; Dubsky, P.; Jakesz, R.; Gnant, M.; Fitzal, F. Standardization of Morbidity Assessment in Breast Cancer Surgery Using the Clavien Dindo Classification. Int. J. Surg. 2014, 12, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Kim, M.C.; Lee, J.W.; Cho, Y.K.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Park, H.Y. Usefulness of Oncoplastic Volume Replacement Techniques after Breast Conserving Surgery in Small to Moderate-Sized Breasts. Arch. Plast. Surg. 2012, 39, 489–496. [Google Scholar] [CrossRef]
- Macmillan, R.D.; McCulley, S.J. Oncoplastic Breast Surgery: What, When and for Whom? Curr. Breast Cancer Rep. 2016, 8, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Kaufman, G.J.; Nos, C.; Buccimazza, I.; Sarfati, I.M. Improving Breast Cancer Surgery: A Classification and Quadrant per Quadrant Atlas for Oncoplastic Surgery. Ann. Surg. Oncol. 2010, 17, 1375–1391. [Google Scholar] [CrossRef]
- Kang, M.J.; Hong, H.K.; Eo, P.S.; Lee, J.S.; Lee, J.W.; Lee, J.; Park, H.Y.; Yang, J.D. Surgical Strategies for Partial Breast Reconstruction in Medial-Located Breast Cancer: A 12-Year Experience. J. Breast Cancer 2023, 26, 35–45. [Google Scholar] [CrossRef]
- Holmström, H.; Lossing, C. The Lateral Thoracodorsal Flap in Breast Reconstruction. Plast. Reconstr. Surg. 1986, 77, 933–943. [Google Scholar] [CrossRef]
- Hamdi, M. Oncoplastic and Reconstructive Surgery of the Breast. Breast 2013, 22 (Suppl. S2), S100–S105. [Google Scholar] [CrossRef]
- Hamdi, M.; Rasheed, M.Z. Advances in Autologous Breast Reconstruction with Pedicled Perforator Flaps. Clin. Plast. Surg. 2012, 39, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.G.; Tenovici, A.A. Staged Approach to Partial Breast Reconstruction to Avoid Mastectomy in Women with Breast Cancer. Gland Surg. 2017, 6, 336–342. [Google Scholar] [CrossRef]
- Kim, J.B.; Kim, D.K.; Lee, J.W.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Park, H.Y.; Lee, J.Y.; Yang, J.D. The Usefulness of Pedicled Perforator Flap in Partial Breast Reconstruction after Breast Conserving Surgery in Korean Women. Arch. Plast. Surg. 2018, 45, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-López, C.; Julian Ibañez, J.F.; Vilà, J.; Luna Tomás, M.A.; Navinés López, J.; Pascual Miguel, I.; Fernandez-Llamazares-Rodriguez, J.; Higueras-Suñe, C. Anterior Intercostal Artery Perforator Flap in Immediate Breast Reconstruction: Anatomical Study and Clinical Application. Microsurgery 2017, 37, 603–610. [Google Scholar] [CrossRef]
- Roy, P.G.; Mustata, L.; Hu, J.; Phillips, B.; Parulekar, V.; Bhattacharyya, M.; Harris, A.; Oliveros, S. Partial Breast Reconstruction with Lateral Chest Wall Perforator Flap to Facilitate Breast Conservation in Breast Cancer: First 100 Cases with Cancer Outcomes at 8 Years Follow-Up and the Lessons Learned. Cancer Manag. Res. 2021, 13, 9453–9466. [Google Scholar] [CrossRef] [PubMed]
- Orabi, A.; Youssef, M.M.G.; Manie, T.M.; Shaalan, M.; Hashem, T. Lateral Chest Wall Perforator Flaps in Partial Breast Reconstruction. J. Egypt. Natl. Cancer Inst. 2022, 34, 2. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gass, J.; Patel, K.; Holmes, D.; Kopkash, K.; Peiris, L.; Peled, A.; Ryan, J.; El-Tamer, M.; Reiland, J. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3436–3444. [Google Scholar] [CrossRef]



| Feature | Class | N = (200) |
|---|---|---|
| Age (years) | Median (Range) | 52.5 (31–78) |
| <40 | 24 | |
| 41–60 | 127 | |
| >60 | 49 | |
| Comorbidities | Yes | 80 |
| No | 119 | |
| NA | 1 | |
| Size of Breast | S | 28 |
| M | 150 | |
| L | 18 | |
| NA | 4 | |
| Ptosis | No Ptosis | 34 |
| Grade I Mild | 68 | |
| Grade II Moderate | 46 | |
| Grade III Severe | 48 | |
| NA | 4 | |
| Malignant/Benign | Malignant | 195 |
| Benign | 5 |
| Feature | Class | Total N = 195 Sx = 197 Malignant | |
|---|---|---|---|
| Molecular Subtype | ER/PR | 119 | 61.03% |
| HER2 | 39 | 20% | |
| TNBC | 37 | 18.97% | |
| Focality | Unifocal | 155 | 81% |
| Multifocal/Multicentric | 37 | 19% | |
| In Unifocal Clinical Tumor Size (cT) | cT1 | 51 | 25% |
| cT2 | 86 | 43% | |
| cT3 | 11 | 5% | |
| NA | 5 | - | |
| In Multifocal Clinical Tumor Size (cT) | cT1 | 11 | 5% |
| cT2 | 22 | 10% | |
| cT3 | 3 | 1.5% | |
| DCIS | Tis | 8 | 4% |
| Tumor Grade | I | 8 | 4% |
| II | 114 | 58% | |
| III | 55 | 28% | |
| NA | 20 | - | |
| Type of Tumor (Biopsy) | IDC | 156 | 79.2% |
| IDC + DCIS | 25 | 12.8% | |
| ILC | 4 | 2% | |
| ILC + LCIS | 1 | 0.5% | |
| DCIS | 9 | 4.5% | |
| Others | 2 | 1% | |
| Quadrant (unifocal) (n = 160) | UOQ | 95 | 59% |
| CQ | 34 | 21% | |
| LIQ | 16 | 10% | |
| LOQ | 12 | 7.5 | |
| LQ | 1 | 0.6% | |
| UIQ | 4 | 2% | |
| UQ | 0 | - | |
| Clinical Tumor Stage (Underwent Upfront Surgery) 121/195 (62%) patients underwent upfront surgery | Stage 0 | 0 | - |
| Stage IA | 25 | 20% | |
| Stage IB | 0 | - | |
| Stage IIA | 45 | 37% | |
| Stage IIB | 25 | 20% | |
| Stage IIIA | 12 | 10% | |
| Stage IIIB | 0 | - | |
| Stage IIIC | 2 | 1.6% | |
| NA | 6 | - | |
| Clinical Node Positivity (Upfront Surgery) | 42 of 121 (34.71%) were node positive | ||
| Clinical Tumor Stage (Given NAST) 74/195 (37%) patients received NAST | Stage 0 | 1 | 1.3% |
| Stage IA | 5 | 6% | |
| Stage IB | 0 | - | |
| Stage IIA | 18 | 24.3% | |
| Stage IIB | 19 | 25.6% | |
| Stage IIIA | 29 | 39.1% | |
| Stage IIIB | 0 | - | |
| Stage IIIC | 2 | 2.7% | |
| NA | 0 | - | |
| Clinical Node Positivity (NAST) | 55 of 74 (74.32%) were node positive | ||
| Pathological Tumor Stage (Upfront Surgery) | Stage 0 | 9 | |
| Stage IA | 20 | ||
| Stage IB | 0 | ||
| Stage IIA | 43 | ||
| Stage IIB | 29 | ||
| Stage IIIA | 8 | ||
| Stage IIIB | 1 | ||
| Stage IIIC | 4 | ||
| NA | 7 | ||
| Pathological Tumor Stage (Post-NAST Surgery) | Stage 0 (pCR) | 17 | |
| Stage IA | 14 | ||
| Stage IB | 0 | ||
| Stage IIA | 20 | ||
| Stage IIB | 10 | ||
| Stage IIIA | 6 | ||
| Stage IIIB | 0 | ||
| Stage IIIC | 5 | ||
| NA | 2 | ||
| Post-Op Complications | No Complications | 178 | |
| Grade I Complications | 17 | ||
| Grade II Complications | 5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppiker, C.B.; Mishra, R.; Jain, V.; Sivadasan, P.; Deshmukh, C.; Varghese, B.; Dhar, U.; Vartak, A.; Athavale, N.; Gupta, N.; et al. Chest Wall Perforator Flaps in Breast Conservation: Versatile, Affordable, and Scalable: Insights from the Largest Single-Surgeon Audit from India. Curr. Oncol. 2025, 32, 165. https://doi.org/10.3390/curroncol32030165
Koppiker CB, Mishra R, Jain V, Sivadasan P, Deshmukh C, Varghese B, Dhar U, Vartak A, Athavale N, Gupta N, et al. Chest Wall Perforator Flaps in Breast Conservation: Versatile, Affordable, and Scalable: Insights from the Largest Single-Surgeon Audit from India. Current Oncology. 2025; 32(3):165. https://doi.org/10.3390/curroncol32030165
Chicago/Turabian StyleKoppiker, C. B., Rupa Mishra, Vaibhav Jain, Priya Sivadasan, Chetan Deshmukh, Beenu Varghese, Upendra Dhar, Anushree Vartak, Namrata Athavale, Neerja Gupta, and et al. 2025. "Chest Wall Perforator Flaps in Breast Conservation: Versatile, Affordable, and Scalable: Insights from the Largest Single-Surgeon Audit from India" Current Oncology 32, no. 3: 165. https://doi.org/10.3390/curroncol32030165
APA StyleKoppiker, C. B., Mishra, R., Jain, V., Sivadasan, P., Deshmukh, C., Varghese, B., Dhar, U., Vartak, A., Athavale, N., Gupta, N., Busheri, L., Lulla, V., Bhandari, S., & Joshi, S. (2025). Chest Wall Perforator Flaps in Breast Conservation: Versatile, Affordable, and Scalable: Insights from the Largest Single-Surgeon Audit from India. Current Oncology, 32(3), 165. https://doi.org/10.3390/curroncol32030165





