Parental Reports on Late Effects and Follow-Up Needs: A Single-Center Assessment of Childhood Cancer Survivorship Care in Kenya
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design
2.3. Data Analysis
3. Results
3.1. Survivor and Parent Characteristics
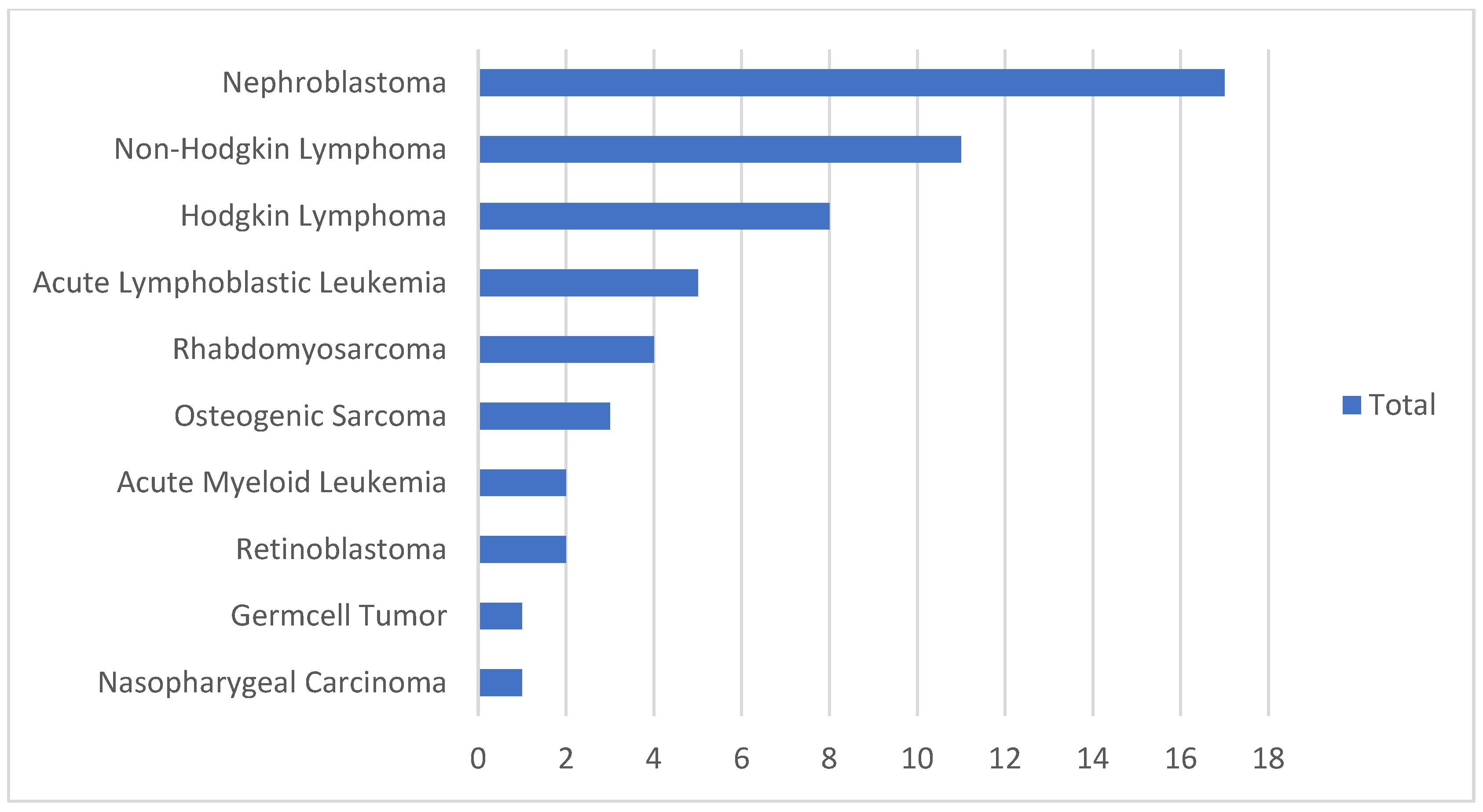
3.2. Childhood Cancer Treatment
3.3. Follow-Up
3.4. Transportation to MTRH
3.5. Medical History
3.6. Parent-Reported Symptoms
3.7. Information About Late Effects at MTRH
3.8. Preferred Follow-Up
3.9. Peer Support
3.10. Recommendations for Guidance of Survivors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LMICs | Low- and Middle-Income Countries |
| NHIF | National Health Insurance Fund |
| WHO | World Health Organization |
| HICs | High-Income Countries |
References
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71 Pt B, 101733. [Google Scholar] [CrossRef]
- Zabih, W.; Thota, A.B.; Mbah, G.; Freccero, P.; Gupta, S.; Denburg, A.E. Interventions to improve early detection of childhood cancer in low- and middle-income countries: A systematic review. Pediatr. Blood Cancer 2020, 67, e28761. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, J.; Ilbawi, A.; Sullivan, M.; Gray, A. The development and education of a workforce in childhood cancer services in low- and middle-income countries: A scoping review protocol. Syst. Rev. 2022, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer. Increasing Access, Advancing Quality, Saving Lives. Available online: https://iris.who.int/bitstream/handle/10665/347370/9789240025271-eng.pdf?sequence=1 (accessed on 9 March 2025).
- Mostert, S.; Njuguna, F.; Langat, S.C.; Slot, A.J.; Skiles, J.; Sitaresmi, M.N.; van de Ven, P.M.; Musimbi, J.; Vreeman, R.C.; Kaspers, G.J. Two overlooked contributors to abandonment of childhood cancer treatment in Kenya: Parents’ social network and experiences with hospital retention policies. Psychooncology 2014, 23, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Israels, T.; Afungchwi, G.M.; Klootwijk, L.; Njuguna, F.; Hesseling, P.; Kouya, F.; Paintsil, V.; Landman, L.; Chitsike, I.; Chagaluka, G.; et al. Fever and neutropenia outcomes and areas for intervention: A report from SUCCOUR—Supportive Care for Children with Cancer in Africa. Pediatr. Blood Cancer 2021, 68, e29224. [Google Scholar] [CrossRef]
- Langat, S.; Njuguna, F.; Olbara, G.; Martijn, H.; Sieben, C.; Haverkort, M.; Njenga, D.; Vik, T.A.; Kaspers, G.; Mostert, S. Influence of health-insurance on treatment outcome of childhood cancer in Western Kenya. Support. Care Cancer 2023, 31, 467. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- The World Bank. Total Population of Kenya 2024. Available online: https://data.worldbank.org/indicator/SP.POP.0014.TO?end=2019&locations=KE&start=1960&view=chart (accessed on 9 March 2025).
- Severance, T.S.; Njuguna, F.; Olbara, G.; Kugo, M.; Langat, S.; Mostert, S.; Klootwijk, L.; Skiles, J.; Coven, S.L.; Overholt, K.M.; et al. An evaluation of the disparities affecting the underdiagnosis of pediatric cancer in Western Kenya. Pediatr. Blood Cancer 2022, 69, e29768. [Google Scholar] [CrossRef]
- Mbau, R.; Kabia, E.; Honda, A.; Hanson, K.; Barasa, E. Examining purchasing reforms towards universal health coverage by the National Hospital Insurance Fund in Kenya. Int. J. Equity Health 2020, 19, 19. [Google Scholar] [CrossRef]
- Ministry of Health. Social Health Insurance Fund (SHIF) Registration Set to Launch on March 1, 2024. Available online: https://www.health.go.ke/social-health-insurance-fund-shif-registration-set-launch-march-1-2024 (accessed on 24 June 2024).
- Han, J.W.; Kwon, S.Y.; Won, S.C.; Shin, Y.J.; Ko, J.H.; Lyu, C.J. Comprehensive clinical follow-up of late effects in childhood cancer survivors shows the need for early and well-timed intervention. Ann. Oncol. 2009, 20, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.; Neufeld, P.; Harvey, J.; Downes, A.; Hayashi, R.J. Late Effects of Childhood Cancer, Participation, and Quality of Life of Adolescents. OTJR Occup. Particip. Health 2009, 29, 116–124. [Google Scholar] [CrossRef]
- Schulte, F.S.M.; Patton, M.; Alberts, N.M.; Kunin-Batson, A.; Olson-Bullis, B.A.; Forbes, C.; Russell, K.B.; Neville, A.; Heathcote, L.C.; Karlson, C.W.; et al. Pain in long-term survivors of childhood cancer: A systematic review of the current state of knowledge and a call to action from the Children’s Oncology Group. Cancer 2021, 127, 35–44. [Google Scholar] [CrossRef]
- Bowers, D.C.; Griffith, T.; Gargan, L.; Cochran, C.J.; Kleiber, B.; Foxwell, A.; Farrow-Gillespie, A.; Orlino, A.; Germann, J.N. Back pain among long-term survivors of childhood leukemia. J. Pediatr. Hematol. Oncol. 2012, 34, 624–629. [Google Scholar] [CrossRef]
- Lu, Q.; Krull, K.R.; Leisenring, W.; Owen, J.E.; Kawashima, T.; Tsao, J.C.I.; Zebrack, B.; Mertens, A.; Armstrong, G.T.; Stovall, M.; et al. Pain in long-term adult survivors of childhood cancers and their siblings: A report from the Childhood Cancer Survivor Study. Pain 2011, 152, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- van Deuren, S.; Penson, A.; van Dulmen-den Broeder, E.; Grootenhuis, M.A.; van der Heiden-van der Loo, M.; Bronkhorst, E.; Blijlevens, N.M.A.; Streefkerk, N.; Teepen, J.C.; Tissing, W.J.E.; et al. Prevalence and risk factors of cancer-related fatigue in childhood cancer survivors: A DCCSS LATER study. Cancer 2022, 128, 1110–1121. [Google Scholar] [CrossRef]
- Lackner, H.; Benesch, M.; Schagerl, S.; Kerbl, R.; Schwinger, W.; Urban, C. Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur. J. Pediatr. 2000, 159, 750–758. [Google Scholar] [CrossRef]
- Arpaci, T.; Kilicarslan Toruner, E. Assessment of problems and symptoms in survivors of childhood acute lymphoblastic leukaemia. Eur. J. Cancer Care 2016, 25, 1034–1043. [Google Scholar] [CrossRef]
- Seth, R.; Singh, A.; Seth, S.; Sapra, S. Late effects of treatment in survivors of childhood cancers: A single-centre experience. Indian J. Med. Res. 2017, 146, 216–223. [Google Scholar] [CrossRef]
- Signorelli, C.; Høeg, B.L.; Asuzu, C.; Centeno, I.; Estapé, T.; Fisher, P.; Lam, W.; Levkovich, I.; Manne, S.; Miles, A.; et al. International Survey of Psychosocial Care for Cancer Survivors in Low-/Middle- and High-Income Countries: Current Practices, Barriers, and Facilitators to Care. JCO Glob. Oncol. 2024, 10, e2300418. [Google Scholar] [CrossRef]
- Blaauwbroek, R.; Stant, A.D.; Groenier, K.H.; Kamps, W.A.; Meyboom, B.; Postma, A. Health-related quality of life and adverse late effects in adult (very) long-term childhood cancer survivors. Eur. J. Cancer 2007, 43, 122–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geenen, M.M.; Cardous-Ubbink, M.C.; Kremer, L.C.; van den Bos, C.; van der Pal, H.J.; Heinen, R.C.; Jaspers, M.W.; Koning, C.C.; Oldenburger, F.; Langeveld, N.E.; et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 2007, 297, 2705–2715. [Google Scholar] [CrossRef]
- Hudson, M.M.; Mertens, A.C.; Yasui, Y.; Hobbie, W.; Chen, H.; Gurney, J.G.; Yeazel, M.; Recklitis, C.J.; Marina, N.; Robison, L.R.; et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA 2003, 290, 1583–1592. [Google Scholar] [CrossRef]
- Gupta, S.; Howard, S.C.; Hunger, S.P.; Antillon, F.G.; Metzger, M.L.; Israels, T.; Harif, M.; Rodriguez-Galindo, C. Treating Childhood Cancer in Low- and Middle-Income Countries. In Cancer: Disease Control Priorities, 3rd ed.; International Bank for Reconstruction and Development: Washington, DC, USA, 2015; Chapter 7. [Google Scholar]
- Gleeson, H.K.; Shalet, S.M. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr. Relat. Cancer 2004, 11, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.; Francken, A.B.; Rouwé, C.; Kamps, W.A.; Postma, A. Reduction of adult height in childhood acute lymphoblastic leukemia survivors after prophylactic cranial irradiation. Pediatr. Blood Cancer 2005, 45, 139–143. [Google Scholar] [CrossRef]
- Fardell, J.E.; Wakefield, C.E.; De Abreu Lourenco, R.; Signorelli, C.; McCarthy, M.; McLoone, J.; Osborn, M.; Gabriel, M.; Anazodo, A.; Alvaro, F.; et al. Long-term health-related quality of life in young childhood cancer survivors and their parents. Pediatr. Blood Cancer 2021, 68, e29398. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Wakefield, C.E.; McLoone, J.K.; Johnston, K.A.; Mertens, A.C.; Osborn, M.; Cohn, R.J. Childhood Cancer Survivors’ Reported Late Effects, Motivations for Seeking Survivorship Care, and Patterns of Attendance. Oncologist 2023, 28, e276–e286. [Google Scholar] [CrossRef]
- Erickson, S.J.; Hile, S.; Kubinec, N.; Annett, R.D. Self-reported and parent proxy reported functional impairment among pediatric cancer survivors and controls. Health Qual. Life Outcomes 2020, 18, 142. [Google Scholar] [CrossRef]
- Ljungman, L.; Cernvall, M.; Grönqvist, H.; Ljótsson, B.; Ljungman, G.; von Essen, L. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: A systematic review. PLoS ONE 2014, 9, e103340. [Google Scholar] [CrossRef]
- Knijnenburg, S.L.; Kremer, L.C.; van den Bos, C.; Braam, K.I.; Jaspers, M.W. Health information needs of childhood cancer survivors and their family. Pediatr. Blood Cancer 2010, 54, 123–127. [Google Scholar] [CrossRef]
- Cherven, B.; Mertens, A.; Meacham, L.R.; Williamson, R.; Boring, C.; Wasilewski-Masker, K. Knowledge and risk perception of late effects among childhood cancer survivors and parents before and after visiting a childhood cancer survivor clinic. J. Pediatr. Oncol. Nurs. 2014, 31, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.M.; Koh, R.Y.V.; Lee, E.S. A scoping review on the factors associated with the lost to follow-up (LTFU) amongst patients with chronic disease in ambulatory care of high-income countries (HIC). BMC Health Serv. Res. 2023, 23, 883. [Google Scholar] [CrossRef] [PubMed]
- Wiangnon, S.; Veerakul, G.; Nuchprayoon, I.; Seksarn, P.; Hongeng, S.; Krutvecho, T.; Sripaiboonkij, N. Childhood cancer incidence and survival 2003–2005, Thailand: Study from the Thai Pediatric Oncology Group. Asian Pac. J. Cancer Prev. 2011, 12, 2215–2220. [Google Scholar]
- van Erp, L.M.E.; Maurice-Stam, H.; Beek, L.R.; Kremer, L.C.M.; den Hartogh, J.G.; van Gorp, M.; Huizinga, G.A.; Grootenhuis, M.A. Online cognitive-behavioral group intervention for young adult survivors of childhood cancer: A pilot study. J. Psychosoc. Oncol. 2023, 41, 518–538. [Google Scholar] [CrossRef] [PubMed]
- Bratteteig, M.; Rueegg, C.S.; Lie, H.C.; Thorsen, L.; Larsen, E.H.; Larsen, M.H.; Torsvik, I.K.; Götte, M.; Järvelä, L.S.; Kriemler, S.; et al. Physical activity behaviors and screen time in young childhood cancer survivors: The Physical Activity in Childhood Cancer Survivors Study. J. Cancer Surv. 2024. [Google Scholar] [CrossRef]
- Casillas, J.; Goyal, A.; Bryman, J.; Alquaddoomi, F.; Ganz, P.A.; Lidington, E.; Macadangdang, J.; Estrin, D. Development of a text messaging system to improve receipt of survivorship care in adolescent and young adult survivors of childhood cancer. J. Cancer Surviv. 2017, 11, 505–516. [Google Scholar] [CrossRef]
- Kakusa, B.W.; Xu, L.W.; Vaca, S.D.; Nalwanga, J.; Kiryabwire, J.; Ssenyonjo, H.; Mukasa, J.; Muhumuza, M.; Haglund, M.M.; Grant, G.A. Central Nervous System Tumors in Uganda: Outcomes of Surgical Treatment and Complications Assessed Through Telephone Survey. World Neurosurg. 2019, 129, e866–e880. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Barnea, D.; Cohn, R.J.; Cypriano, M.S.; Fresneau, B.C.; Haupt, R.; Hjorth, L.; Ishida, Y.; Kruseova, J.; Kuehni, C.E.; et al. Models of Care for Survivors of Childhood Cancer from Across the Globe: Advancing Survivorship Care in the Next Decade. J. Clin. Oncol. 2018, 36, 2223–2230. [Google Scholar] [CrossRef]
- Hill, R.E.; Wakefield, C.E.; Cohn, R.J.; Fardell, J.E.; Brierley, M.E.; Kothe, E.; Jacobsen, P.B.; Hetherington, K.; Mercieca-Bebber, R. Survivorship Care Plans in Cancer: A Meta-Analysis and Systematic Review of Care Plan Outcomes. Oncologist 2020, 25, e351–e372. [Google Scholar] [CrossRef]
- Odedina, F.T.; Rodrigues, B. Cancer Advocacy in Africa: Case studies of innovative practices. Infect. Agent Cancer 2013, 8 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Ryan, D.; Chafe, R.; Hodgkinson, K.; Chan, K.; Stringer, K.; Moorehead, P. Interventions to improve the aftercare of survivors of childhood cancer: A systematic review. Pediatr. Hematol. Oncol. J. 2018, 3, 90–98. [Google Scholar] [CrossRef]
- Embuldeniya, G.; Veinot, P.; Bell, E.; Bell, M.; Nyhof-Young, J.; Sale, J.E.; Britten, N. The experience and impact of chronic disease peer support interventions: A qualitative synthesis. Patient Educ. Couns. 2013, 92, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Williamson Lewis, R.; Effinger, K.E.; Wasilewski-Masker, K.; Mertens, A.; Xiao, C. Self-reported late effect symptom clusters among young pediatric cancer survivors. Support. Care Cancer 2021, 29, 8077–8087. [Google Scholar] [CrossRef] [PubMed]
- Zhukovsky, D.S.; Rozmus, C.L.; Robert, R.S.; Bruera, E.; Wells, R.J.; Chisholm, G.B.; Allo, J.A.; Cohen, M.Z. Symptom profiles in children with advanced cancer: Patient, family caregiver, and oncologist ratings. Cancer 2015, 121, 4080–4087. [Google Scholar] [CrossRef]
| N/Median | %/IQR | ||
|---|---|---|---|
| SURVIVORS | |||
| Age | At diagnosis | 5.0 | 3.0–8.0 |
| Sex | Male | 36 | 67% |
| Female | 18 | 33% | |
| Type of cancer | Solid tumor | 28 | 52% |
| Hematological tumor | 26 | 48% | |
| Health insurance during treatment | Present | 52 | 96% |
| Absent | 2 | 4% | |
| Health insurance during follow-up | Present | 48 | 89% |
| Absent | 6 | 11% | |
| Modality of treatment | Chemotherapy | 53 | 98% |
| Surgery | 25 | 46% | |
| Radiotherapy | 15 | 28% | |
| Duration of treatment (n = 53) | <6 months | 24 | 45% |
| ≥6 months | 29 | 55% | |
| Duration of follow-up * | <1 year | 11 | 20% |
| 1–<3 years | 29 | 54% | |
| 3–5 years | 9 | 17% | |
| >5 years | 5 | 9% | |
| Follow-up status ** | In follow-up | 28 | 52% |
| Lost to follow-up | 26 | 48% | |
| School attendance | Primary school | 44 | 81% |
| High school | 10 | 19% |
| N/Median | %/IQR | ||
|---|---|---|---|
| PARENTS | |||
| Age (in years) | Father (n = 42) | 45.0 | 39.0–48.3 |
| Mother (n = 53) | 39.0 | 32.0–43.0 | |
| Marital status (n = 53) | Married | 38 | (72%) |
| Divorced/separated | 7 | (13%) | |
| Single | 5 | (9%) | |
| Widowed | 3 | (6%) | |
| Number of children in family | Median | 4.0 | 3.0–5.0 |
| Median (range) | 4 | (1–11) | |
| Parental education level * (n = 53) | Low | 22 | 42% |
| High | 31 | 58% | |
| Religion (n = 53) | Christian | 50 | 93% |
| Muslim | 3 | 6% | |
| Distance to MTRH | <50 km | 7 | 13% |
| 50–100 km | 12 | 22% | |
| >100 km | 35 | 65% | |
| Travel time to MTRH | <1 h | 5 | 9% |
| 1–3 h | 19 | 35% | |
| >3 h | 30 | 56% |
| Late Effects | Severity | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Frequency | Mild | Moderate | Severe | |||||
| n | % | n | % | n | % | n | % | |
| Pain | 20 | 37% | 13 | 65% | 4 | 20% | 3 | 15% |
| Fatigue | 14 | 26% | 7 | 50% | 5 | 36% | 2 | 14% |
| Ophthalmological problems | 14 | 26% | 9 | 64% | 3 | 21% | 2 | 14% |
| Gastrointestinal | 12 | 22% | 8 | 67% | 3 | 25% | 1 | 8% |
| Shorter stature than siblings | 10 | 19% | - | - | - | - | - | - |
| Ear/nose/throat problems * | 8 | 15% | 0 | 0% | 5 | 71% | 2 | 29% |
| Orthopedic problems * | 8 | 15% | 5 | 71% | 2 | 29% | 0 | 0% |
| Dental problems | 6 | 11% | 3 | 50% | 3 | 50% | 0 | 0% |
| Hearing loss | 4 | 7% | - | - | - | - | - | - |
| Psychological problems * | 3 | 6% | 0 | 0% | 2 | 100% | 0 | 0% |
| Cognitive problems | 3 | 6% | 0 | 0% | 3 | 100% | 0 | 0% |
| Cardiac problems | 3 | 6% | 0 | 0% | 3 | 100% | 0 | 0% |
| Other problems | 2 | 4% | 1 | 50% | 1 | 50% | 0 | 0% |
| Neurological problems | 1 | 2% | 0 | 0% | 1 | 100% | 0 | 0% |
| Endocrine problems | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% |
| Respiratory problems | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal problems | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% |
| Secondary malignancy | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% |
| 1. Education on the late effects of cancer treatment should be provided to both parents and survivors. |
| 2. Healthcare providers should conduct home visits with survivors. |
| 3. Regular phone calls to check on survivors and families. |
| 4. Organize survivorship meetings. |
| 5. Offer psychological assistance to survivors and their families. |
| 6. Provide financial support to parents and survivors, especially for those who travel long distances. |
| 7. MTRH should improve file retrieval during follow-up clinics. |
| 8. MTRH should provide more frequent follow-up clinics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mageto, S.N.; Lemmen, J.P.M.; Njuguna, F.M.; Midiwo, N.; Langat, S.C.; Vik, T.A.; Kaspers, G.J.L. Parental Reports on Late Effects and Follow-Up Needs: A Single-Center Assessment of Childhood Cancer Survivorship Care in Kenya. Curr. Oncol. 2025, 32, 162. https://doi.org/10.3390/curroncol32030162
Mageto SN, Lemmen JPM, Njuguna FM, Midiwo N, Langat SC, Vik TA, Kaspers GJL. Parental Reports on Late Effects and Follow-Up Needs: A Single-Center Assessment of Childhood Cancer Survivorship Care in Kenya. Current Oncology. 2025; 32(3):162. https://doi.org/10.3390/curroncol32030162
Chicago/Turabian StyleMageto, Susan Nyabate, Jesse P. M. Lemmen, Festus Muigai Njuguna, Nancy Midiwo, Sandra Cheptoo Langat, Terry Allan Vik, and Gertjan J. L. Kaspers. 2025. "Parental Reports on Late Effects and Follow-Up Needs: A Single-Center Assessment of Childhood Cancer Survivorship Care in Kenya" Current Oncology 32, no. 3: 162. https://doi.org/10.3390/curroncol32030162
APA StyleMageto, S. N., Lemmen, J. P. M., Njuguna, F. M., Midiwo, N., Langat, S. C., Vik, T. A., & Kaspers, G. J. L. (2025). Parental Reports on Late Effects and Follow-Up Needs: A Single-Center Assessment of Childhood Cancer Survivorship Care in Kenya. Current Oncology, 32(3), 162. https://doi.org/10.3390/curroncol32030162






