Abstract
Lymphomas represent a heterogeneous group of blood tumors, generally divided into non-Hodgkin lymphoma (NHL) (90% of all lymphomas) and Hodgkin lymphoma (HL). High-grade NHL can rapidly progress so that new strategies and potentially therapeutical options are needed. Recently, it was shown that Vitamin D (VitD) inhibits the growth of cancer cells, controls their invasion and metastasis, and strengthens the antitumor activity of various types of chemotherapeutic anticancer agents. Therefore, we reviewed the recent literature about the influence of VitD and its analogues (VDAs) on the treatment and the prognosis of B-cell lymphomas. As to the in vitro studies in different cell lines, VitD3 and VDAs enhanced the anti-proliferative efficacy of various chemotherapeutics and increased the expression of VitD receptor. In in vivo studies, blood levels of VitD were considered: higher values of plasma bioavailable VitD were correlated with better progression-free survival (PFS) and overall survival (OS), while an unfavorable PFS and OS were observed in VitD deficient groups. No clinical trial was made on the analogs, thus confirming the absence of in vivo positive role of these synthetic drugs. In conclusion, higher levels of circulating VitD are related to improved OS, reduced cancer-specific mortality, and better disease-free survival. VitD and analogs showed also positive effects in in vitro studies, while only VitD was able to improve clinical parameters. Furthermore, a complex approach with plant-based diet, adequate levels for motor exercise, and/or eventual VitD supplementation could be a valuable strategy to challenge lymphomas.
1. Introduction
Lymphomas, together with leukemias and myelomas, represent a heterogeneous group of blood tumors, originating from hematopoietic stem cell mutations or alteration in progenitor cells already oriented in a lymphoid direction. Lymphomas are generally divided into non-Hodgkin lymphoma (NHL) (90% of all lymphomas) and Hodgkin lymphoma (HL). NHL is further divided into B-cell, T-cell, and natural killer (NK) cell types, while HL is divided into classical and non-classical types [1,2,3]. Moreover, according to a clinical approach, lymphomas are of high grade (aggressive) or low grade (indolent). From an epidemiological point of view, indolent lymphomas are much more frequent than aggressive ones [4,5]. Furthermore, the latest classification of the World Health Organization (WHO) identified more than 80 lymphoma types and grouped them into three main classes based on their morphology, immunophenotype, genetic and molecular lesions profiles, clinical characteristics, and cellular derivation [3,4]. About 95% of the lymphomas are of B-cell origin, being the other T/NK-cell malignancies.
The Global Cancer Statistics 2022 from the International Agency for Research on Cancer (IARC) reported 20 million new cancer cases in 2022; amongst these, 553.010 were new cases of NHL and 82,409 new cases of HL, each with a mortality rank of 250.475 and 22.701 [6].
In last decades, an increase in the incidence of some subtypes of type B lymphomas, such as diffuse large B-cell lymphoma (DLBCL), has been observed [6,7]. This could be due to a combination of factors, including better recognition and diagnosis of lymphomas, as well as changes in environment and lifestyle. Several non-modifiable or modifiable risk factors are involved in the pathogenesis of B-cell lymphomas, such as tobacco and alcohol consumption, obesity, family history, autoimmune diseases, infections (including Epstein-Barr virus, Helicobacter pylori, hepatitis C virus), and inflammatory diseases [5,7]. However, other molecular factors also contribute to the pathogenesis of these types of tumors, such as the expression of B-cell receptor, crucial for the survival or proliferation of most malignant B-cells, the interaction of these transformed cells with other cells in the tumor microenvironment, and the recognition of an antigen that contributes to the survival and proliferation of B lymphoma cells [8].
NHLs have a wide range of histological aspects and clinical features, and patients commonly present lymphadenopathy or splenomegaly. In general, these heterogeneous features could make difficult the diagnosis, and timely identification is important because different therapies are available. In fact, the treatment of B-cell lymphomas depends on the specific subtype and staging of the disease [5]. High-grade NHL can rapidly progress, and prompt treatment is required, usually with a combination of chemotherapy with rituximab, a monoclonal antibody against CD20 B-cell specific surface antigen. Indolent lymphomas are not considered curable with conventional therapy. However, patients presenting localized lymphadenopathy may be treated by radiotherapy [5].
Thanks to advances in cancer therapy, many patients with different types of B lymphomas respond well to therapies and have good long-term survival prospects [9]. However, at present, lymphoid cancer treatments are not very successful. Therefore, new strategies and potentially therapeutical options are needed to increase efficiency and tolerance, compared to standard treatments, such as chemotherapy, immunochemotherapy with an antibody–drug conjugate or bispecific antibodies, or gene and cell therapies [10,11,12].
Recently, preclinical and epidemiological data have suggested that the main circulation form of Vitamin D (VitD), 25(OH)2D3, plays a crucial role in the pathogenesis, progression, and therapy of hematological cancers, and it was shown that patients with hematological diseases have lower serum levels of VitD3 [13]. In general, people with a lower VitD3 serum level have shown a higher risk of developing different types of cancer [11,14,15]. Although VitD3 primary function is the regulation of calcium and phosphate metabolism, its compounds inhibit the growth of cancer cells and control their invasion and metastasis through the induction of cell-cycle arrest, the control of apoptosis, the differentiation of cells, the reduction in angiogenesis, and the modulation of pro-inflammatory cytokine production [14,16]. Interestingly, in vitro and in vivo preclinical analyses indicated that VitD3 strengthens the antitumor activity of various types of chemotherapeutic anticancer agents, such as platinum analogues [17,18] and taxanes [18]. Moreover, it also increases the antitumor effect of cytotoxic biologic agents, such as dovitinib, a multi-kinase inhibitor [19].
However, although the significant anticancer properties displayed by VitD, unfortunately the high doses of administration necessary for its adjuvant anticancer effect could lead to hypercalcemia, an unfavorable condition for patients. In this regard, numerous clinical trials have demonstrated a better tolerability and promising results of several synthetic VitD analogues (VDAs), supporting a further investigation of their clinical utility, especially in combination with standard treatments, such as chemotherapeutic drugs and immunotherapy [20,21]. Therefore, the purpose of our study was to review the recent literature about the influence of VitD and its analogues on the treatment and the prognosis of B-cell lymphomas.
2. Materials and Methods
We searched PubMed and Medline databases to find all relevant English-language scientific papers dealing with the effects of VitD and its analogs on B lymphomas. Inclusion criteria were full texts entirely in English language with an abstract and at least one of the following features: clinical and/or preclinical studies on the role of VitD or VDAs in patients with B lymphomas; in vitro studies on the role of VitD or VDAs in B lymphoma cells. The latest studies, both in vitro and in vivo, were selected. We excluded studies written in a language different from English and case reports. Boolean operators AND/OR were used to combine search terms. The following search strings were used: “Vitamin D” OR “Vit D analogues” AND “lymphomas” OR “25-hydroxyvitamin D” AND “lymphomas”. V.U.B., F.S., J.F., P.M., and B.G. searched articles published in English until February 2025 and selected them on the basis of inclusion and exclusion criteria. In order to synopsize the scoping review process, a flow diagram was created. Reports of the scoping review were carried out on the basis of a guidance for authors when choosing a scoping review approach [22,23], as shown in Figure 1. This review followed the PRISMA guidelines. We registered our scoping review on OSF Registries with the following registration link: https://doi.org/10.17605/OSF.IO/NZXYQ (accessed on 20 February 2025).
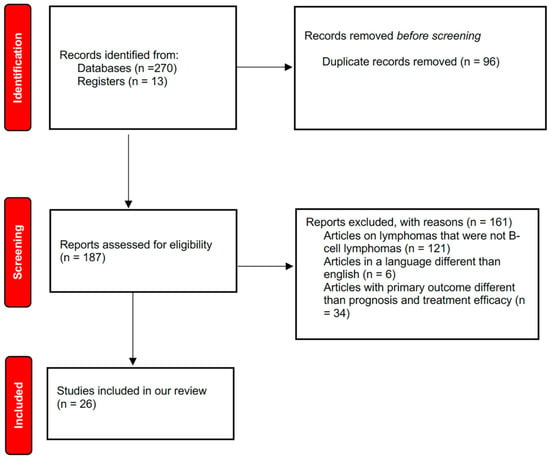
Figure 1.
Flow diagram of literature search.
3. Effects of Vitamin D and Its Analogues
3.1. Vitamin D and Its Analogues
VitD belongs to the group of fat-soluble vitamins. Its blood levels are the result of a combination between cutaneous synthesis through UV irradiation and intake with food or VitD supplements [11]. Its main circulating metabolite, 25-hydroxyvitamin D [25(OH)D], derives from the hydroxylation on position 25 of VitD in liver via 25-hydroxylase encoded by the gene CYP2R1. Then, 25(OH)D is bound to the VitD-binding protein and transported to the target tissues, mainly in kidneys, where a further hydroxylation on position 1, via 1α-hydroxylase coded by the CYP27B1 gene, occurs [24,25]. VitD can act both as a vitamin and a hormone, and its effects are mediated by the VitD receptor (VDR), an intracellular receptor member of the family of nuclear hormone receptors. VDR is formed by a DNA-binding domain that binds to a VitD response element (VDRE) by a C-terminal ligand-binding domain and by a third region that connects these two domains [26]. VitD interaction with VDR is responsible for its genomic effects. It can also exert non-genomic effects on different cell types via a membrane receptor. One of these effects is the increase in calcium and phosphate intestinal re-uptake, a process named transcalcification [24]. VitD also regulates the activity of chloride channel, stimulates protein kinase C and phospholipase C in different cellular types, such as osteoblasts, hepatocytes, and intestinal cells, and promotes the renal reabsorption of phosphate and the passage into the blood of calcium from the bones [27]. In the human body, blood levels of 25(OH)D are considered as a dependable estimation of the VitD status. Commonly, VitD levels between 50 and 75 nmol/L are considered as VitD insufficiency, while levels < 50 nmol/L are considered as VitD deficiency (VDD) [28]. VitD insufficiency status is a worldwide diffuse condition [29]; in particular, 20% of Afghanistan, Pakistan, Tunisia, and India people have lower levels (below 30 nmol/L). In this regard, epidemiological studies have suggested an increased risk of different types of cancers in patients with VDD [30,31] and, recently, an association between VitD levels and prognosis for cancer patients [32].
As above indicated, the main function of VitD is to maintain the proper levels of calcium and phosphorus in serum. Besides its major role of maintaining calcium–phosphate balance, it regulates most events, typical of cancer, being able to inhibit cellular proliferation, neoangiogenesis, and metastasis, to induce cellular differentiation and apoptosis, and to control the immune system [33].
It was observed that high doses of VitD provided anticancer activity and can cause hypercalcemia as a side effect. For this reason, various synthetic VDAs with a low calcemic effect versus a powerful antiproliferative, differentiating, and/or immune-modulatory role have been developed [34]. These drugs are created with structural modifications of the basic structure of VitD so that they can act more efficiently and more selectively, with reduced side effects.
However, conflicting data are present in the literature on the use of VDAs. In fact, some studies have described an elevated risk of complications (hypercalcemia, hypercalciuria, and hyperphosphatemia) when VDAs are used, compared to cholecalciferol and ergocalciferol, so that the use of VDAs should be cautiously evaluated and controlled, particularly in patients at a high risk of these complications [35]. On the contrary, other studies [36] indicated that some of the about 3000 synthetic VDAs produced by different pharmaceutical companies and research groups may interfere with the proliferation of the cancer cells in different cell lines, owing to their binding affinity to VDR. However, even if in human pancreatic tumors the VDA calcipotriol strikingly reduced the markers of inflammation and fibrosis [37], the positive activity of VDR ligands is still primarily referred to in vitro assays.
3.2. In Vitro Effects of Vitamin D and Its Analogues
As to the in vitro studies (Table 1), it was demonstrated that 1,25(OH)2D3 and certain VDAs displayed cytotoxic and pro-apoptotic actions upon DLBCL cells.

Table 1.
Summary of the in vitro studies.
With its high invasiveness, quick progression, and ease of spread, DLBCL is the most prevalent clinical subtype of aggressive non-Hodgkin’s lymphomas [37]. Based on statistical estimations, more than 40% of aggressive lymphomas are caused by DLBCL. According to reports, there are over 150,000 new DLBCL cases worldwide each year [43]. Sadly, relapse or refractory disease will occur in around 40% of DLBCL patients who are not responsive to conventional chemoimmunotherapy [44].
In vitro, these compounds caused the death of approximately 40% of DOHH2 cells, a cell line representative of DLBCL, after 24 h stimulation. Furthermore, 1,25(OH)2D3 and VDAs increased the expression of VDR protein in DOHH2 cells, which suggested a pronounced biological activity of these compounds upon malignant B-cell disease. Moreover, this study revealed that VitD3 and analogues can enhance the anti-proliferative efficacy of various chemotherapeutics, such as clomipramine [35].
In another study, Han et al. [38] demonstrated that, in the DLBCL Pfeiffer cell line, the subtype of non-Hodgkin lymphoma, calcitriol with the macrolide Rapamycin (RAPA) blocked proliferation, induced an increase in cells in G1 phase and amplified the cell-cycle arrest. It was also demonstrated that RAPA and calcitriol suppressed the expression of VDR and of 25-hydroxyvitamin d-24-hydroxylase (CYP24A1), the enzyme that causes 1,25(OH)2D3 degradation.
Several effects of VitD have been identified on immune cells such as T-, B-, NK-, monocytes and dendritic cells (DCs), as well as their involvement in tumors and autoimmune diseases [45,46,47,48].
Neumann et al. [39] showed VitD3 serum levels associated with maximum NK=cell-mediated antibody-dependent cellular cytotoxicity (ADCC). In fact, NK-cell-mediated ADCC is the major mechanism of action of both rituximab and obinutuzumab, whose efficacy improved significantly after VitD3 supplementation in VitD3 deficient and insufficient patients. In particular, obinutuzumab showed a stronger ADCC activity than rituximab. Therefore, the effect of VitD3 on NK-cell-mediated ADCC is crucial and can improve the clinical outcome of immunotherapies based on these antibodies.
In line with these results, Bold et al. [40] showed immune modulatory effects of calcitriol on stimulated NK-cells in vitro. More in detail, NK-cells isolated from healthy volunteers, co-incubated with B-cell lymphoma DAUDI and U2932, stimulated with Interleukine-2, and treated with calcitriol by long-term stimulation presented an increased ADCC against these tumor cells.
Similar results were found in a different lymphoproliferative disorder such as HL. This disease accounts for approximately 30% of all malignant lymphomas in Europe and the United States [49]. Gharbaran et al. [41] demonstrated that HL-cell lines and primary Hodgkin and Reed/Sternberg cell lines showed low levels of VDR expression. In addition, VitD3 and VDAs (calcipotriol and EB1089) reduced the growth of HL-cell lines, associated with an increased accumulation of VDR, which induced and controlled the expression of genes.
However, the active forms of VitD3 and its analogues are not efficacious in all cancer cells, even if they show high levels of VDR expression [42]. The response to calcitriol and to the analog tacalcitol (PRI-2191) in leukemia and lymphoma cell lines could be influenced by the VDR polymorphism, especially Fok1 polymorphism and “bat” haplotype.
3.3. In Vivo Effects of Vitamin D
Several in vivo studies focused their attention mainly on DLBCL (Table 2).

Table 2.
Summary of the in vivo studies.
The influence of VDD on the therapeutic outcomes in elderly patients with DLBCL was investigated [50]. Patients with VitD levels ≤ 8 ng/mL had an event-free survival (EFS) of 59% and an overall survival (OS) of 70%, while patients with VitD levels > 8 ng/mL showed an EFS and OS of 79% and 82%, respectively.
In another study [51], plasma total 25(OH)D and bioavailable 25(OH)D levels at diagnosis in 332 newly diagnosed DLBCL patients were examined; the results were correlated with clinical characteristics, prognosis, and response to treatment. VDD (25-(OH)D < 30 ng/mL) was found in 92.8% of patients. Higher values of plasma bioavailable 25(OH)D were correlated with better progression-free survival (PFS) and OS, while higher levels of plasma total 25(OH)D significantly influenced PFS but not OS.
A lower PFS and OS were reported by Wang et al. [52], who investigated the prognostic value of VDD (25-(OH)D < 52.5 nmol/L) in 208 newly diagnosed DLBCL patients. It was shown that 25-(OH)D deficiency was an independent prognostic predictor for worse PFS and OS.
Nath et al. [53] evaluated the association between pre-therapy VitD levels and PFS, OS, and CAR-T-related toxicity and response in 111 relapsed/refractory DLBCL patients. In this study, 73 patients presented VDD (≤30 ng/mL), and they had worst 100-day complete response and 2-year OS.
Drake et al. [54] evaluated if circulating 25(OH)D levels influenced EFS and OS in a prospective cohort of 983 newly diagnosed patients with NHL. They reported lower EFS and OS in DLBCL patients with VDD, while they did not observe association between EFS and other NHL subtypes. Moreover, among patients with DLBCL, higher VitD levels were correlated with better EFS and OS.
Another type of B lymphoma is follicular lymphoma (FL), an indolent lymphoid tumor generated from germinal center B-cells. Recent developments in treatment for FL have significantly increased patient life. However, FL is still an incurable illness, nonetheless, with some patient groups exhibiting early disease development, histologic transformation, or a significant risk of damage from treatment. Furthermore, response rates and disease-control intervals decline with each new line of treatment for FL, which is a recurrent disease [61].
Two studies observed a worse prognosis in FL patients with VDD. In the first one [55], the impact of pretreatment with 25(OH)D on PFS between two independent cohorts of similarly treated prospective patients with newly diagnosed FL was evaluated. Amongst the patients with VDD < 20 ng/mL, the median follow-up was 5.4 years, while those with VDD < 10 ng/mL had a median follow-up of 6.6 years.
In the second study [56], the association between VDI (25(OH)D < 20 ng/mL) and adverse outcomes in FL patients was assessed. They observed a lower OS and EFS at 12 months in patients with VDI for the full cohort.
Another study by Eicher et al. [57] investigated the effect of VitD serum levels on PFS and OS in patients with different types of lymphomas undergoing high-dose chemotherapy (HDCT)/autologous stem cell transplantation (ASCT). It was shown a better PFS and OS in patients with VitD levels > 52 nmol/L.
Xu et al. [58] studied the prognostic value of VDD in patients with mantle cell lymphoma (MCL), another subtype of B-cell NHL. MCL is a rare type of B-cell neoplasm characterized by the growth of mature B-cells, typically expressing CD5. It predominantly affects older males, with a median age of around 65 years. Although it is generally classified as aggressive, it exhibits a wide range of clinical behaviors [62].
Qin et al. [59] studied the influence of serum 25-(OH)D deficiency on PFS and OS in 77 newly diagnosed HL patients. They observed that patients with VDD had lower PFS and OS compared with patients with normal VitD levels.
Moreover, Borchmann et al. [60] measured VitD levels before treatment in 351 prospectively treated patients with HL, correlating these results with their clinical outcomes. They found VDD in 50% of patients, being lower levels more common in relapsed/refractory patients. Moreover, patients with VDD had consistently impaired PFS.
3.4. Diet and Vitamin D
A limited number of studies have addressed associations between diet and risk for lymphoma, as well as the impact of VitD supplementation in this context.
Overall, in light of the scarcity of clinical evidence and contrasting results, “promotion of healthy lifestyles and empowering of lymphoma survivors should be implemented”, as suggested by Fondazione Italiana Linfomi researchers [63]. Indeed, in their systematic review, they concluded that the areas of development of clinical research are numerous, and it is wanted to implement plans encouraging and instructing patients on healthier lifestyles, such as consuming a healthy diet. Intriguingly, to date, VitD and other nutraceutical compounds might positively impact lymphoma prognosis even if safety issues should be carefully considered especially for the concomitant use of other dietary supplements and lymphoma-directed therapies [64].
4. Discussion and Conclusions
Lymphoma is the most common hematological malignancy in developed countries, whose evolution is induced by alterations in the hematopoietic cells. At present, although many strategies have been proposed for the treatment of these pathologies, myeloid and lymphoid cancer therapies are still not very successful. Therefore, researchers have focused their attention on new strategies and therapy options to increase the efficiency and tolerance of standard treatments, such as chemotherapy, radiotherapy, and immunotherapy. Amongst these strategies, calcitriol has shown an important role in the pathogenesis, progression, and therapy of hematological cancers. We reviewed both in vitro and in vivo studies that evaluated the influence of VitD and its analogs on prognosis and treatment efficacy in B lymphomas.
The in vitro studies revealed cytotoxic and pro-apoptotic effects of calcitriol and VDAs on NHL-cell lines, blocking proliferation and inducing the G1 phase or cycle cell arrest [38]. Besides controlling cancer cells’ viability directly, calcitriol and VDAs act also indirectly on cells of the innate and adaptive immune system, including macrophages as effector cells [65] with negative effects on tumor cells (Figure 2).
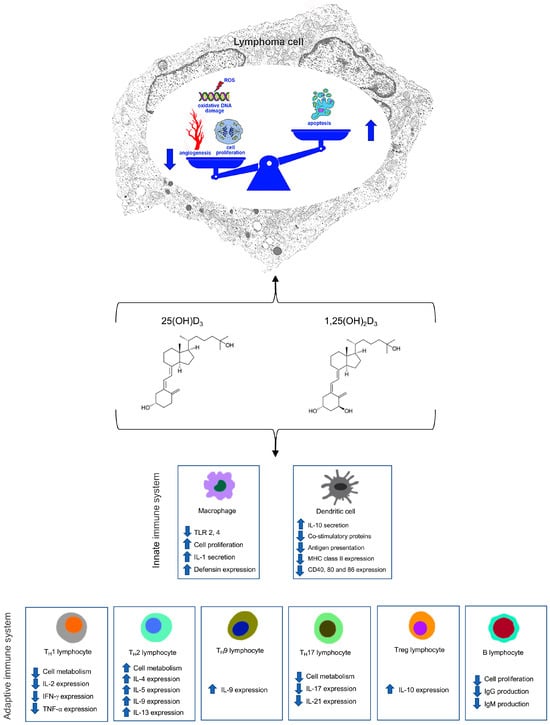
Figure 2.
Schematic representation of main actions of Vitamin D on lymphoma cell and on cells of the innate and adaptive immune system.  Increased;
Increased;  Decreased.
Decreased.
 Increased;
Increased;  Decreased.
Decreased.
The treatment with VitD and its analogues enhances the effect of different drugs such as antibiotics, chemotherapeutics, and biological drugs. In fact, these compounds, in co-administration with rapamycin, amplified cell-cycle arrest in Pfeiffer cell line [38]. The concomitant treatment with Obinutuzumab and calcitriol on NK-cells isolated from healthy volunteers stimulated an increased ADCC of these cells against B lymphoma cells [39].
Thus, the way by which lymphomas react to treatment may also be influenced by VitD. As reported above, it has been demonstrated that VitD actually increases the cellular cytotoxicity induced by rituximab [50]. In fact, deficiency in 25-hydroxyvitamin-D (25-OHD) has been found to be a poor prognostic indicator in patients receiving chemotherapy for follicular lymphoma and newly diagnosed DLBCL [54,55]. Moreover, better results in DLBCL have been linked to supplementation-achieved stabilization of VitD levels [13]. A phase III clinical trial (NCT03078855) is currently investigating the combination of VitD supplements and rituximab in non-Hodgkin lymphoma.
Finally, the management of relapsed/refractory large B-cell lymphoma has advanced dramatically as a result of immunotherapy combined with chimeric antigen receptor T-cell therapy (CAR-T), and three anti-CD19-CAR-T medicines are currently licensed for this indication [66]. According to a study, CAR-T recipients who are VitD insufficient had worse clinical outcomes [53].
Ultimately, consolidation for patients with recurrent lymphomas involves the use of autologous stem cell transplantation (ASCT) along with high-dose chemotherapy (HDCT). In a study, the impact of serum levels of VitD on progression-free survival (PFS) and overall survival (OS) was examined in patients with lymphomas undergoing ASCT. Higher VitD levels (>52 nmol/L) were linked to improved OS and PFS. Based on these findings, it appears that lower serum VitD levels are linked to worse outcomes for patients with lymphoma undergoing HDCT/ASCT. Prior to ASCT, VitD level optimization might be necessary [57].
Finally, another crucial anti-cancer activity of these substances is the interaction with VDR, a receptor generally largely expressed by cancer cells. In line with this knowledge, it has been shown that in the presence of a decreased expression of VDR, the treatment of high-grade B-cell lymphoma lines with higher concentrations of calcitriol induced a slightly appearance of this receptor [67].
Regarding the in vivo studies, they confirmed the positive influence of VitD on lymphomas prognosis and therapy efficacy [50]. In fact, patients with VDD always presented a worse PFS and OS when compared to patient with VitD levels within range.
These results confirm what several works already reported regarding VitD and its role in other types of solid tumors. In fact, a significant inverse association between VitD levels and breast, prostate, and colon cancer prognosis has already been found [68].
As to the possible explanation of the better outcomes in patients with high VitD levels, VitD and its analogues might modulate the tumor microenvironment reducing cancer prevention and progression [69,70]. In fact, cancer patients with higher VitD levels can have better general health conditions that might lead to improved overall survival [71]. By contrast, even if cancer tumors lead to extra-renal production of 1,25(OH)2D3 to fight the tumor [72,73], it has been found that 1,25D analogues are not useful in treating cancer [74].
So far, in the last years, it has been particularly focused on the role of additional factors as hypercalcemia in patients with lymphoma. It has been suggested that the major mechanism by which NHL patients develop hypercalcemia is not mediated by calcitriol or parathyroid-related protein. As a matter of fact, hypercalcemia is most prevalent in patients with diffuse large B-cell lymphoma of the nongerminal cell subtype. Overall, multiple evidence indicates that patients with calcitriol-mediated hypercalcemia showed a trend toward worse outcomes, suggesting that calcitriol might be a marker of high-grade lymphoma or a surrogate for more advanced disease [75,76,77,78]. Interestingly, in malignant lymphoma causing hypercalcemia, Ogawa and colleagues encountered a case of hypercalcemia induced by the overproduction of 1,25(OH)2VitD in DLBCL. They showed that immunostaining with the anti-CYP27B1 antibody was useful for identifying the ectopic expression of 1α-hydroxylase in lesions and facilitated the selection of corticosteroids as the treatment to control hypercalcemia in malignancy [79]. Indeed, evidence to support the excessive synthesis of 1,25(OH)2VitD by the ectopic expression of 25-hydroxyvitamin D3-1α-hydroxylase (1α-hydroxylase) in malignant lymphomas is limited [80,81]; however, the current research focused, in the management of the above conditions, on other pharmacological approaches such as hydration, elcatonin, bisphosphonates, and denosumab and, in some patients, corticosteroids and cinacalcet. Specifically, it is important to keep in mind that corticosteroids are effective not only in the treatment of lymphoma but also for 1,25(OH)2VitD-mediated hypercalcemia [79].
Indeed, VDD is associated with many pathological conditions, such as cancer, diabetes, autoimmune, cardiovascular, and infectious diseases [82].
Because VitD affects the immune system and the ability to fight off infections, it may be especially helpful for people with lymphoproliferative diseases. Even nonfatal infections can interfere with chemotherapy regimens and have crippling long-term effects [83], and patients with lymphoproliferative diseases are still at a high risk of serious and life-threatening infections [84,85] despite the widespread adoption of infection prevention practices.
The finding that VitD deficiency is linked to an increased risk of infections is consistent with previous research showing that VitD supplementation may provide protection against infection [86]. Previous studies showed that nutritional deficiencies, particularly VitD deficiency, may contribute to the risk of infection by causing complex multisystem effects, including immunological defects. VitD actually affects how B-cells, T-cells, innate immune cells, and epithelial cells form, survive, and function [87]. Additionally, VitD forms endogenous AMPs called defensins through cytokine pathways, which kill invasion organisms inside cells [88].
Moreover, human B-cell development and function are impacted by VitD receptor activation. Through its nuclear receptors, 1,25-dihydroxyvitamin D3 influences B-cell migration and homing, immunoglobulin class switching to IgA at the expense of IgE, and B-cell differentiation. The effects of vitamin metabolites on B-cell survival factors, such as the control of BAFF and APRIL, the stimulation of TGF-β, or the inhibition of NF-κB, are well understood. Naïve B-cells differentiate into IgA+ plasmablasts when exposed to calcitriol [89].
Since CLL patients are said to have a decrease in antibody production [90,91], and because antibody therapy may benefit them [92], this factor is significant. IgG and IgA class-switching through abnormal CD40–CD40 ligand relations of and reduction in CD40 ligand; the suppression of CD95+ plasma cells in the bone marrow through interaction with CD95 ligand on CD5-B-cells; disproportionate inhibition by T-cells; iatrogenic myelosuppressive treatment; and the malfunctioning generation of polyclonal immunoglobulins and anomalous activity of non-neoplastic CD5-B-cells are among the causes of poor immunoglobulin concentrations [93,94].
Cesur et al. showed that, depending on the length, VitD use considerably raised serum IgG levels in comparison to individuals who did not take it and that the study stayed above the baseline over the long run [95]. It has been found that the 25(OH)VitD levels during VitD use have a positive and substantial connection with the most recent measurement of blood IgG. The study found that blood IgG levels remained above the initial level for a considerable amount of time and rose significantly based on the length between those who utilized VitD and those who did not.
As VitD administration continued, a positive and substantial correlation between the latest measured immunoglobulin G and 25(OH)D levels was seen [95].
The information presented above is especially pertinent to some patient subgroups, such as those infected with SARS-COVID-19. Relapsed immunocompromised COVID-19 patients who have had CD20 depleting therapy may benefit from combined therapy using selective intravenous immunoglobulin and antiviral medications, according to a recent study. The virus load dropped without reoccurring after the patient underwent intravenous immunoglobulin treatment [96]. VitD supplementation may enhance immunoglobulins’ protective function and enhance the prognosis of these individuals, as, while VitD supplementation may lessen the severity of a patient’s illness, low VitD levels are linked to increased infection rates, more severe disease, and higher fatality rates among COVID-19 patients [97].
In conclusion, the data in our possession suggest that higher levels of circulating VitD is associated with improved OS, reduced cancer-specific mortality, and better disease-free survival for various cancer types, with particular regard to lymphomas. Furthermore, VitD and analogs showed also positive effects in in vitro studies, while only VitD was able to improve clinical parameters. It is also the case that more studies are necessary to better understand the clinical benefits of VitD supplementation in lymphoma patients and, additionally, to develop model animals and clinical trials on the effects of VDAs in these diseases.
Given the aforementioned, we think that all lymphoma patients should be evaluated and given supplements, even though the exact role of VitD in these subjects is still unclear, particularly with regard to the best dosage to use. Regarding dosing, several different dosages have been suggested for various lymphoproliferative disorders. The authors of a study supplemented patients with VitD according to their age and 25-hydroxyvitamin D levels. Every three months, levels of the goal 25-hydroxyvitamin D level, which was set at ≥30 ng/mL [98], were measured. We believe it is fair to recommend 1000 IU of VitD per day for patients, with greater dosages required to address severe deficiencies. However, there are a lot of real-world issues to deal with, such as VitD poisoning.
It is very uncommon to become intoxicated from regularly prescribed dosages. The majority of intoxication instances are caused by self-initiated supplementation, formulation problems, or unintentional overdoses associated with prescription regimens. However, serious side effects are possible, such as renal failure necessitating hemodialysis [99]. Treatment options for cases of hypercalcemia brought on by excessive exogenous VitD3 supplementation have generally involved daily dosages above 50,000 IU for at least two to three months, along with intravenous fluids, loop diuretics, calcitonin, and glucocorticoids [100].
Lastly, a variety of factors, including age, gender, and ethnicity, must be taken into account. A study looked at VitD levels that were stratified by several factors at once. This approach determined which populations were more susceptible to negative health effects as a result of low VitD levels. For instance, non-Hispanic Black females of reproductive age had significant levels of deficit [101].
Moreover, it appears noteworthy to underline the role of a healthy diet in this context. Actually, limited evidence suggests that low-fat, plant-based diets may reduce the risk of this disease [102,103]. Data from the European Prospective Investigation into Cancer and Nutrition study revealed for the first time that adherence to a Mediterranean diet was modestly associated with a reduced risk of overall lymphoma [103]. However, it is not yet known whether other dietary factors, nutraceuticals, and/or dietary supplements can influence the course of lymphomas as studies aiming to evaluate the impact of the Mediterranean diet and VitD levels on late toxicities and secondary cancers were lacking. However, this aspect should further stimulate the scientific community to pay ever more attention to the promotion of lifestyles aimed at the prevention of late toxicities related to anti-cancer treatments [104].
However, due to its limited solubility in gastrointestinal tract aqueous fluids, VitD is a non-polar lipid with low bioavailability. Better absorption may be made possible by a process called micellization, which distributes fatty compounds into aqueous micellar spheres. Recent studies have demonstrated that VitD3 nanoemulsion formulations based on nanotechnology outperform traditional coarse emulsions in terms of homogeneity and bioavailability. These procedures might enhance biological effects of VitD in hematological patients [105,106].
On the basis of these observations, we trust that a multifaceted approach including a plant-based diet, adequate levels for physical activity [107], and/or eventually supplemented by VitD, could represent a valuable strategy to challenge lymphomas and, more in general, non-communicable diseases and their comorbidities.
Author Contributions
Conceptualization: V.B., A.A., H.R.M., M.B. and L.M.; Data Curation: B.G., J.F., F.S., P.M. and V.U.B.; Writing—Original Draft: A.A., D.P. and L.M.; Writing—Review and Editing: V.B., A.A., H.R.M., M.B., D.P. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gallamini, A.; Juweid, M. Lymphoma [Internet]; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care Clin. Off. Pract. 2016, 43, 661–675. [Google Scholar] [CrossRef]
- De Leval, L.; Jaffe, E.S. Lymphoma Classification. Cancer J. 2020, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar] [PubMed]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef]
- Seifert, M.; Scholtysik, R.; Küppers, R. Origin and Pathogenesis of B Cell Lymphomas. Methods Mol. Biol. 2019, 1956, 1–33. [Google Scholar] [PubMed]
- Hang, H.; Zhou, H.; Ma, L. Prognostic factors and clinical survival outcome in patients with primary mediastinal diffuse large B-cell lymphoma in rituximab era: A population-based study. Medicine 2024, 103, e37238. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, M.; Awan, F.T. Remission induction, consolidation, and novel agents in development for adults with acute myeloid leukaemia. Hematol. Oncol. 2010, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kulling, P.M.; Olson, K.C.; Olson, T.L.; Feith, D.J.; Loughran, T.P., Jr. Vitamin D in hematological disorders and malignancies. Eur. J. Haematol. 2017, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Hohaus, S.; Tisi, M.C.; Bellesi, S.; Maiolo, E.; Alma, E.; Tartaglia, G.; Corrente, F.; Cuccaro, A.; D’Alo’, F.; Basile, U.; et al. Vitamin D deficiency and supplementation in patients with aggressive B-cell lymphomas treated with immunochemotherapy. Cancer Med. 2018, 7, 270–281. [Google Scholar] [CrossRef]
- Trump, D.L.; Deeb, K.; Johnson, C.S. Vitamin D: Considerations in the Continued Development as an Agent for Cancer Prevention and Therapy. Cancer J. 2010, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. Review: The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Bandera Merchan, B.; Morcillo, S.; Martin-Nuñez, G.; Tinahones, F.J.; Macías-González, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, W.H.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.B. Calcitriol Combined With Platinum-based Chemotherapy Suppresses Growth and Expression of Vascular Endothelial Growth Factor of SKOV-3 Ovarian Cancer Cells. Anticancer Res. 2021, 41, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Wölfl, S. Effects of 1,25(OH)2D3 on Cancer Cells and Potential Applications in Combination with Established and Putative Anti-Cancer Agents. Nutrients 2017, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- García-Quiroz, J.; Cárdenas-Ochoa, N.; García-Becerra, R.; Morales-Guadarrama, G.; Méndez-Pérez, E.A.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Segovia-Mendoza, M.; Prado-García, H.; Avila, E.; et al. Antitumoral effects of dovitinib in triple-negative breast cancer are synergized by calcitriol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2021, 214, 105979. [Google Scholar] [CrossRef] [PubMed]
- Wakle, K.S.; Mokale, S.N.; Sakle, N.S. Emerging perspectives: Unraveling the anticancer potential of vitamin D3. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2877–2933. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Donini, V.; Cannalire, G. Rickets Types and Treatment with Vitamin D and Analogues. Nutrients 2024, 16, 416. [Google Scholar] [CrossRef]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B. The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-DihydroxyvitaminD(3). Endocrinol. Metab. Clin. N. Am. 2010, 39, 255–269. [Google Scholar]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kaushik, R.; Chawla, P.; Upadhyay, S.; Rawat, D.; Akhtar, A. Vitamin-D as a multifunctional molecule for overall well-being: An integrative review. Clin. Nutr. ESPEN 2024, 62, 10–21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin D and Cancer. IARC Working Group Reports; International Agency for Research on Cancer: Geneva, Switzerland, 2008; Volume 5. [Google Scholar]
- Bird, R.P. Vitamin D and cancer. Adv. Food Nutr. Res. 2024, 109, 92–159. [Google Scholar] [PubMed]
- Qin, B.; Xu, B.; Ji, N.; Yao, S.; Pawlish, K.; Llanos, A.A.M.; Lin, Y.; Demissie, K.; Ambrosone, C.B.; Hong, C.C.; et al. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among black women. Am. J. Clin. Nutr. 2020, 111, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Piatek, K.; Schepelmann, M.; Kallay, E. The Effect of Vitamin D and Its Analogs in Ovarian Cancer. Nutrients 2022, 14, 3867. [Google Scholar] [CrossRef]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Hermanns, C.; Lauer, A.A.; Reichrath, J.; Erhardt, T.; Hartmann, T.; Grimm, M.O.W.; Grimm, H.S. Vitamin D and Its Analogues: From Differences in Molecular Mechanisms to Potential Benefits of Adapted Use in the Treatment of Alzheimer’s Disease. Nutrients 2023, 15, 1684. [Google Scholar] [CrossRef]
- Kozielewicz, P.; Grafton, G.; Kutner, A.; Curnow, S.J.; Gordon, J.; Barnes, N.M. Novel vitamin D analogues; cytotoxic and anti-proliferative activity against a diffuse large B-cell lymphoma cell line and B-cells from healthy donors. J. Steroid Biochem. Mol. Biol. 2016, 164, 98–105. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Han, J.; Tang, Y.; Zhong, M.; Wu, W. Antitumor effects and mechanisms of 1,25(OH)2D3 in the Pfeiffer diffuse large B lymphoma cell line. Mol. Med. Rep. 2019, 20, 5064–5074. [Google Scholar] [CrossRef]
- Neumann, F.; Acker, F.; Schormann, C.; Pfreundschuh, M.; Bittenbring, T.J. Determination of optimum vitamin D3 levels for NK cell-mediated rituximab- and obinutuzumab-dependent cellular cytotoxicity. Cancer Immunol. Immunother. 2018, 67, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Bold, A.; Gross, H.; Holzmann, E.; Smetak, M.; Birkmann, J.; Bertsch, T.; Triebel, J.; Sauer, K.; Wilhelm, M.; Hoeres, T. Immune activating and inhibiting effects of calcitriol on γδ T cells and NK cells. Immunobiology 2022, 227, 152286. [Google Scholar] [CrossRef]
- Gharbaran, R.; Zhang, B.; Valerio, L.; Onwumere, O.; Wong, M.; Mighty, J.; Redenti, S. Effects of vitamin D3 and its chemical analogs on the growth of Hodgkin’s lymphoma, in vitro. BMC Res. Notes 2019, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Gleba, J.J.; Mielko, K.A.; Wietrzyk, J.; Kłopotowska, D.; Banach, J.; Turlej, E.; Gebura, K.; Bogunia-Kubik, K.; Kutner, A. Polymorphism of VDR Gene and the Sensitivity of Human Leukemia and Lymphoma Cells to Active Forms of Vitamin D. Cancers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Song, Y.; Wang, X.; Mi, L.; Cai, C.; Zhao, D.; Wang, L.; Ma, J.; Zhu, J. Burden of lymphoma in China, 1990–2019: An analysis of the global burden of diseases, injuries, and risk factors study 2019. Aging 2022, 14, 3175–3190. [Google Scholar] [CrossRef]
- Carpio, C.; Bouabdallah, R.; Ysebaert, L.; Sancho, J.M.; Salles, G.; Cordoba, R.; Pinto, A.; Gharibo, M.; Rasco, D.; Panizo, C.; et al. Avadomide monotherapy in relapsed/refractory DLBCL: Safety, efficacy, and a predictive gene classifier. Blood 2020, 135, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Lv, X.B.; Wang, X.; Zhang, B.; Meng, W.; Yu, F.; Hu, H. Downregulation of miR-302c and miR-520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Br. J. Cancer 2013, 109, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Weeres, M.A.; Robien, K.; Ahn, Y.O.; Neulen, M.L.; Bergerson, R.; Miller, J.S.; Verneris, M.R. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 2014, 193, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bruce, D.; Cantorna, M.T. Vitamin D receptor expression controls proliferation of naive CD8+ T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunol. 2014, 15, 6. [Google Scholar] [CrossRef]
- De Martinis, M.; Allegra, A.; Sirufo, M.M.; Tonacci, A.; Pioggia, G.; Raggiunti, M.; Ginaldi, L.; Gangemi, S. Vitamin D Deficiency, Osteoporosis and Effect on Autoimmune Diseases and Hematopoiesis: A Review. Int. J. Mol. Sci. 2021, 22, 8855. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Y.; Duan, X.; Li, J.; Zhao, W.; Wang, H. Bioavailable 25(OH)D level is associated with clinical outcomes of patients with diffuse large B-cell lymphoma: An exploratory study. Clin. Nutr. 2021, 40, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Liang, J.H.; Wang, L.; Zhu, H.Y.; Xia, Y.; Fan, L.; Li, J.Y.; Xu, W. The prognostic value of 25-hydroxy vitamin D deficiency and its interaction with c-Myc expression in diffuse large B cell lymphoma. Ann. Hematol. 2020, 99, 2377–2384. [Google Scholar] [CrossRef]
- Nath, K.; Tomas, A.A.; Flynn, J.; Fein, J.A.; Alperovich, A.; Anagnostou, T.; Batlevi, C.L.; Dahi, P.B.; Fingrut, W.B.; Giralt, S.A.; et al. Vitamin D Insufficiency and Clinical Outcomes with Chimeric Antigen Receptor T-Cell Therapy in Large B-cell Lymphoma. Transplant. Cell. Ther. 2022, 28, 751.e1–751.e7. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; Inwards, D.J.; et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Salles, G.; Goldman, B.; Fisher, R.I.; Brice, P.; Press, O.; Casasnovas, O.; Maloney, D.G.; Soubeyran, P.; Rimsza, L.; et al. Low Serum Vitamin D Levels Are Associated with Inferior Survival in Follicular Lymphoma: A Prospective Evaluation in SWOG and LYSA Studies. J. Clin. Oncol. 2015, 33, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.I.; Maurer, M.J.; Witzig, T.E.; Drake, M.T.; Ansell, S.M.; Nowakowski, G.S.; Thompson, C.A.; Inwards, D.J.; Johnston, P.B.; Micallef, I.N.; et al. Vitamin D insufficiency is associated with an increased risk of early clinical failure in follicular lymphoma. Blood Cancer J. 2017, 7, e595. [Google Scholar] [CrossRef] [PubMed]
- Eicher, F.; Mansouri Taleghani, B.; Schild, C.; Bacher, U.; Pabst, T. Reduced survival after autologous stem cell transplantation in myeloma and lymphoma patients with low vitamin D serum levels. Hematol. Oncol. 2020, 38, 523–530. [Google Scholar] [CrossRef]
- Xu, D.M.; Liang, J.H.; Wang, L.; Zhu, H.Y.; Xia, Y.; Fan, L.; Li, J.Y.; Xu, W. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in mantle cell lymphoma. J. Cancer Res. Clin. Oncol. 2020, 146, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Q.; Yin, H.; Wu, J.Z.; Chen, R.Z.; Xia, Y.; Wang, L.; Zhu, H.Y.; Fan, L.; Li, J.Y.; Liang, J.H.; et al. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in Hodgkin lymphoma. Leuk. Res. 2021, 105, 106580. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, S.; Cirillo, M.; Goergen, H.; Meder, L.; Sasse, S.; Kreissl, S.; Bröckelmann, P.J.; von Tresckow, B.; Fuchs, M.; Ullrich, R.T.; et al. Pretreatment Vitamin D Deficiency Is Associated With Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 3528–3537. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Muñoz, J.; Trotman, J. Current and future therapies for follicular lymphoma. Exp. Hematol. Oncol. 2024, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Silkenstedt, E.; Dreyling, M.; Beà, S. Biological and clinical determinants shaping heterogeneity in mantle cell lymphoma. Blood Adv. 2024, 8, 3652–3664. [Google Scholar] [CrossRef] [PubMed]
- Minoia, C.; Gerardi, C.; Allocati, E.; Daniele, A.; De Sanctis, V.; Bari, A.; Guarini, A. The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 3135. [Google Scholar] [CrossRef]
- Levy Yurkovski, I.; Andreazzoli, F.; Ben-Arye, E.; Attias, S.; Tadmor, T. Integrative Approaches in the Treatment of Patients Affected by Lymphoma. Curr. Oncol. Rep. 2023, 25, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Gouni, S.; Marques-Piubelli, M.L.; Strati, P. Follicular lymphoma and macrophages: Impact of approved and novel therapies. Blood Adv. 2021, 5, 4303–4312. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Renne, C.; Benz, A.H.; Hansmann, M.L. Vitamin D3 receptor is highly expressed in Hodgkin’s lymphoma. BMC Cancer 2012, 12, 215. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D Intake and the Risk of Colorectal Cancer: An Updated Meta-Analysis and Systematic Review of Case-Control and Prospective Cohort Studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Lu, C.; Wang, Y.; Peng, L.; Jiang, M.; Tang, Y.; Zhao, Q. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget 2017, 8, 81040–81051. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Tretli, S.; Torjesen, P.A.; Babigumira, R.; Schwartz, G.G. Serum 25-hydroxyvitamin D levels predict cancer survival: A prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin. Epidemiol. 2019, 11, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Adams, J.S. Regulation of extra-renal synthesis of 1,25(OH)2D. In Feldman and Pike’s Vitamin D, 5th ed.; Volume One: Biochemistry, Physiology and Diagnostics; Academic Press: Cambridge, MA, USA, 2024; pp. 155–187. [Google Scholar]
- Marcinkowska, E.; Wallace, G.R.; Brown, G. The Use of 1α,25-Dihydroxyvitamin D3 as an Anticancer Agent. Int. J. Mol. Sci. 2016, 17, 729. [Google Scholar] [CrossRef]
- Shallis, R.M.; Rome, R.S.; Reagan, J.L. Mechanisms of Hypercalcemia in Non-Hodgkin Lymphoma and Associated Outcomes: A Retrospective Review. Clin. Lymphoma Myeloma Leuk. 2018, 18, e123–e129. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Kantorovich, V.; Liker, H.R.; Van Herle, A.J.; Cohan, P.; Zehnder, D.; Adams, J.S. Vitamin D-mediated hypercalcemia in lymphoma: Evidence for hormone production by tumor-adjacent macrophages. J. Bone Miner. Res. 2003, 18, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Mudde, A.H.; van den Berg, H.; Boshuis, P.G.; Breedveld, F.C.; Markusse, H.M.; Kluin, P.M.; Bijvoet, O.L.; Papapoulos, S.E. Ectopic production of 1,25-dihydroxyvitamin D by B-cell lymphoma as a cause of hypercalcemia. Cancer 1987, 59, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Hayes, M.E.; Yin, J.A.; Berry, J.L.; Mawer, E.B. Abnormal synthesis of 1,25-dihydroxyvitamin D in patients with malignant lymphoma. J. Clin. Endocrinol. Metab. 1994, 78, 1202–1207. [Google Scholar] [PubMed]
- Ogawa, M.; Morikawa, M.; Kobatake, M.; Murakami, T.; Yamamoto, Y.; Watanabe, R.; Yamada, K.; Nishiyama, K.; Yasutomo, Y.; Hara, K. Hypercalcemia Associated with the Ectopic Expression of 25-hydroxyvitamin D3-1α-hydroxylase in Diffuse Large B-cell Lymphoma. Intern. Med. 2022, 61, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Matsuda, M. 25-Hydroxyvitamin D3-1α-hydroxylase- and multiple cytokine-producing diffuse large B-cell lymphoma. Blood 2018, 131, 2271. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [PubMed]
- Goggin, K.P.; Lu, L.; Lee, D.E.; Howell, C.R.; Srivastava, D.; Brinkman, T.M.; Armstrong, G.T.; Bhakta, N.; Robison, L.L.; Ehrhardt, M.J.; et al. Severe Sepsis During Treatment for Childhood Leukemia and Sequelae Among Adult Survivors. JAMA Netw. Open 2024, 7, e242727. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Musolino, C.; Pioggia, G.; Gangemi, S. Secondary Immunodeficiency in Hematological Malignancies: Focus on Multiple Myeloma and Chronic Lymphocytic Leukemia. Front. Immunol. 2021, 12, 738915. [Google Scholar] [CrossRef] [PubMed]
- Aljabari, S.; Balch, A.; Larsen, G.Y.; Fluchel, M.; Workman, J.K. Severe Sepsis-Associated Morbidity and Mortality among Critically Ill Children with Cancer. J. Pediatr. Intensive Care 2019, 8, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Carboo, J.A.; Dolman-Macleod, R.C.; Malan, L.; Lombard, M.J. High-dose oral vitamin D supplementation for prevention of infections in children aged 0 to 59 months: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S. Vitamin D as a defensin. J. Musculoskelet. Neuronal Interact. 2006, 6, 344–346. [Google Scholar] [PubMed]
- Ramanarayanan, P.; Heine, G.; Worm, M. Vitamin A and vitamin D induced nuclear hormone receptor activation and its impact on B cell differentiation and immunoglobulin production. Immunol. Lett. 2023, 263, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, A.; Marcus, H.; Canaan, A.; Ergas, D.; David, M.; Berrebi, A.; Reisner, Y. A Model for Human B-Chronic Lymphocytic Leukemia in Human/Mouse Radiation Chimera: Evidence for Tumor-Mediated Suppression of Antibody Production in Low-Stage Disease. Blood 1997, 89, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Forconi, F.; Moss, P. Perturbation of the Normal Immune System in Patients with CLL. Blood 2015, 126, 573–581. [Google Scholar] [CrossRef]
- Bussel, J.B.; Cunningham-Rundles, C. Intravenous Usage of Gammaglobulin: Humoral Immunodeficiency, Immune Thrombocytopenic Purpura, and Newer Indications. Cancer Investig. 1985, 3, 361–366. [Google Scholar] [CrossRef]
- Cantwell, M.; Hua, T.; Pappas, J.; Kipps, T.J. Acquired CD40-Ligand Deficiency in Chronic Lymphocytic Leukemia. Nat. Med. 1997, 3, 984–989. [Google Scholar] [CrossRef]
- Cerutti, A.; Kim, E.C.; Shah, S.; Schattner, E.J.; Zan, H.; Schaffer, A.; Casali, P. Dysregulation of CD30+ T Cells by Leukemia Impairs Isotype Switching in Normal B Cells. Nat. Immunol. 2001, 2, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cesur, F.; Atasever, Z.; Özoran, Y. Impact of vitamin D3 supplementation on COVID-19 vaccine response and immunoglobulin G antibodies in deficient women: A randomized controlled trial. Vaccine 2023, 41, 2860–2867. [Google Scholar] [CrossRef]
- Maruki, T.; Nomoto, H.; Iwamoto, N.; Yamamoto, K.; Kurokawa, M.; Iwatsuki-Horimoto, K.; Yamayoshi, S.; Suzuki, Y.; Kawaoka, Y.; Ohmagari, N. Successful management of persistent COVID-19 using combination antiviral therapy (nirmatrelvir/ritonavir and remdesivir) and intravenous immunoglobulin transfusion in an immunocompromised host who had received CD20 depleting therapy for follicular lymphoma. J. Infect. Chemother. 2024, 30, 793–795. [Google Scholar] [CrossRef]
- Yang, J.M.; Li, Z.Q.; Zhong, Y.B.; Xie, H.Y.; Luo, Y.; Xiao, L.; Liao, J.H.; Wang, M.Y. Association Between Vitamin D and COVID-19-Related Outcomes: An Umbrella Review of Meta-Analyses. Nutr. Rev. 2025, 17, nuae225. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Welin, E.; Braeutigam, C.; Gilger, E.; Lane, A.; Salloum, R. Impact of a Vitamin D Replacement Algorithm in Children and Young Adults with Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2018, 40, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Farooq, N. Vitamin D toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Burrowes, H.B.; Rumph, J.T.; Wilkerson, J.; Jackson, C.L.; Jukic, A.M.Z. Vitamin D Levels in the United States: Temporal Trends (2011–2018) and Contemporary Associations with Sociodemographic Characteristics (2017–2018). Nutrients 2024, 16, 3414. [Google Scholar] [CrossRef]
- Chen, G.C.; Lv, D.B.; Pang, Z.; Liu, Q.F. Fruits and vegetables consumption and risk of non-Hodgkin’s lymphoma: A meta-analysis of observational studies. Int. J. Cancer 2013, 133, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Benavente, Y.; Saez, M.; Agudo, A.; Naudin, S.; Hosnijeh, F.S.; Noh, H.; Freisling, H.; Ferrari, P.; Besson, C.; et al. Adherence to the mediterranean diet and lymphoma risk in the european prospective investigation into cancer and nutrition. Int. J. Cancer 2019, 145, 122–131. [Google Scholar] [CrossRef]
- Marini, H.R.; Facchini, B.A.; di Francia, R.; Freni, J.; Puzzolo, D.; Montella, L.; Facchini, G.; Ottaiano, A.; Berretta, M.; Minutoli, L. Glutathione: Lights and Shadows in Cancer Patients. Biomedicines 2023, 11, 2226. [Google Scholar] [CrossRef]
- Huang, J.R.; Song, J.R.; Cai, W.S.; Shao, Z.W.; Zhou, D.Y.; Song, L. Enhancing vitamin D3 bioaccessibility: Unveiling hydrophobic interactions in soybean protein isolate and vitamin D3 binding via an infant in vitro digestion model. Food Chem. 2024, 451, 139507. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.K.; Dev, T.; Mittal, A.; Mani, K.; Narang, A.; Arora, P.; Singh, A.; Chadha, A.; Dang, N.; Goel, M.; et al. A randomised controlled trial comparing the efficacy of micellised and fat-soluble vitamin D3 supplementation in healthy adults. Br. J. Nutr. 2019, 121, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).