Comparative Analysis of Long-Term Renal Outcomes in Upper Tract Urothelial Carcinoma: Local Ablation Versus Radical Nephroureterectomy
Abstract
1. Introduction
2. Materials and Methods
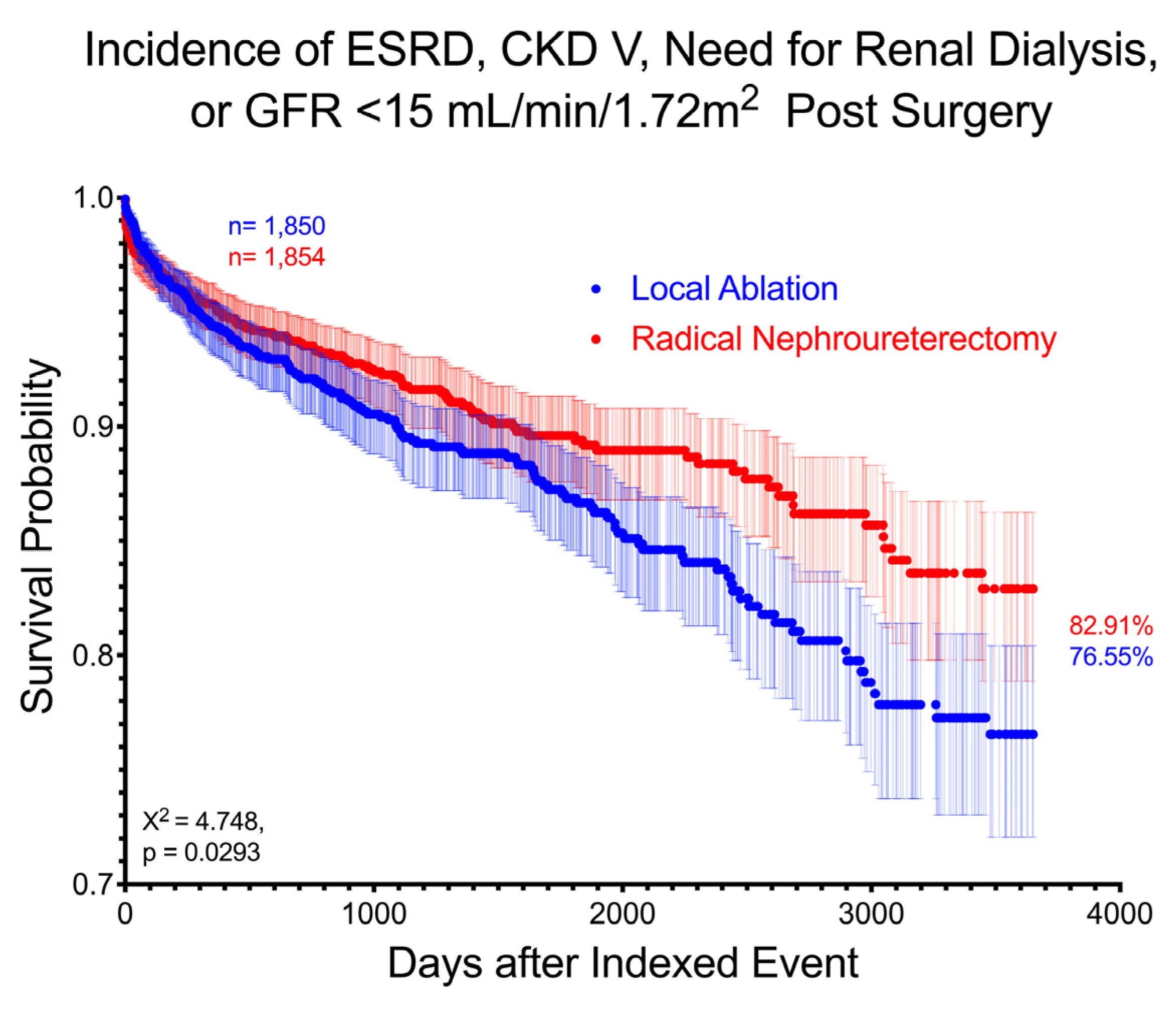
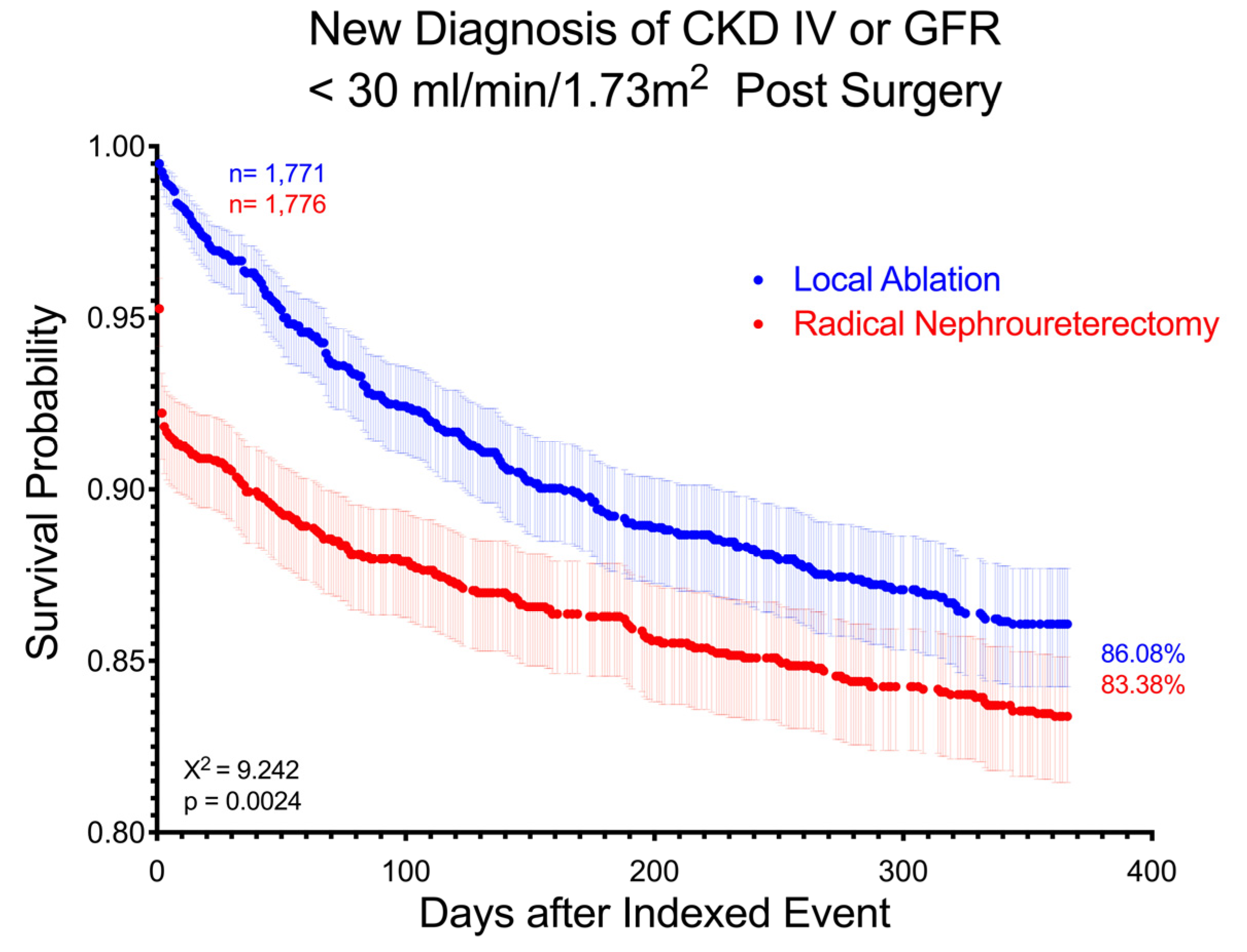
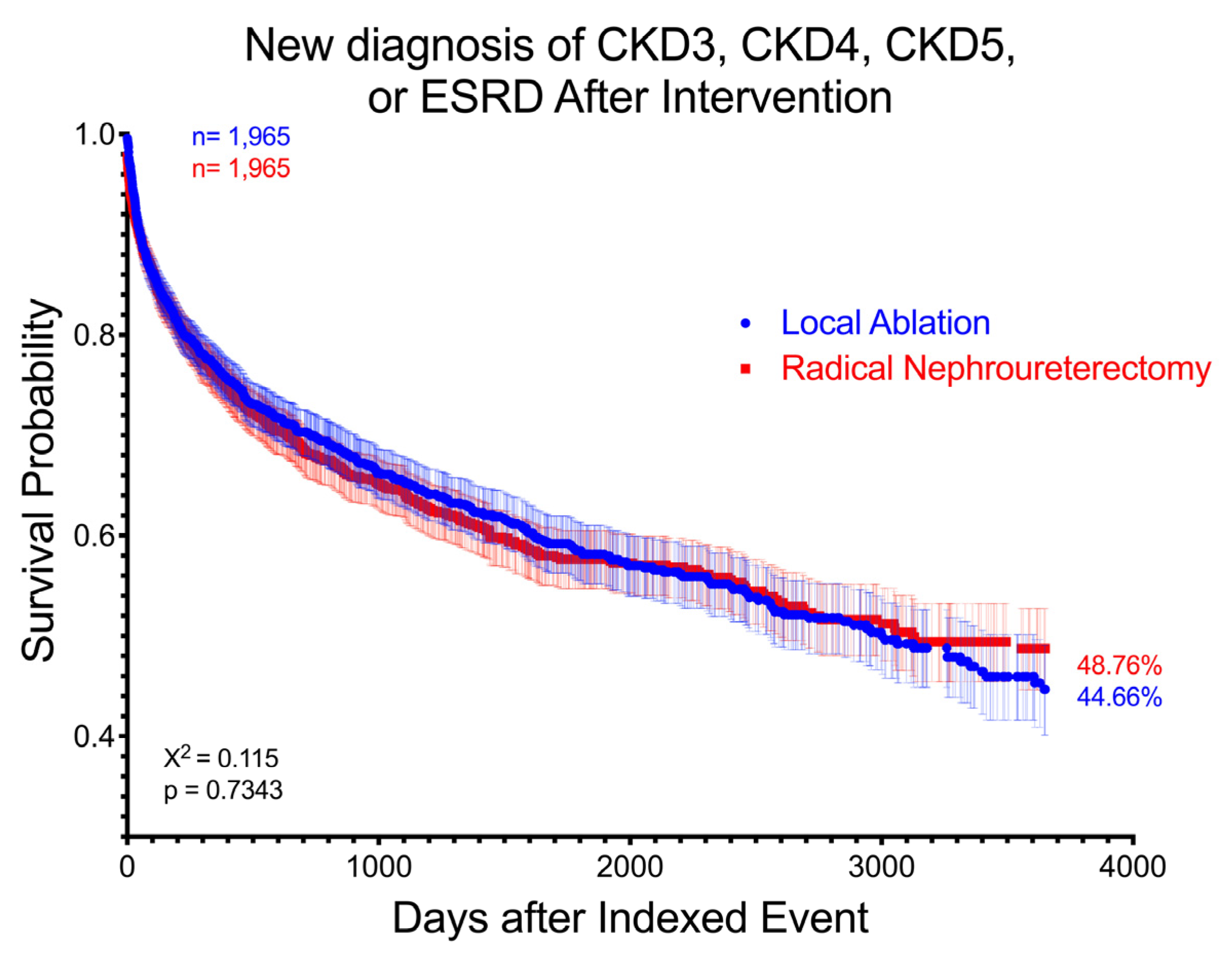
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| CT | Computed Tomography |
| eGFR | Estimated Glomerular Filtration Rate |
| ESRD | End-Stage Renal Disease |
| HR | Hazard Ratio |
| LA | Local Ablation |
| RNU | Radical Nephroureterectomy |
| RR | Risk Ratio |
| SU | Segmental Ureterectomy |
| UTI | Urinary Tract Infection |
| UTUC | Upper Tract Urothelial Carcinoma |
Appendix A
Appendix B
Appendix C
References
- McLaughlin, J.K.; Silverman, D.T.; Hsing, A.W.; Ross, R.K.; Schoenberg, J.B.; Yu, M.C.; Stemhagen, A.; Lynch, C.F.; Blot, W.J.; Fraumeni, J.F. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992, 52, 254–257. [Google Scholar] [PubMed]
- David, K.A.; Mallin, K.; Milowsky, M.I.; Ritchey, J.; Carroll, P.R.; Nanus, D.M. Surveillance of urothelial carcinoma. Cancer 2009, 115, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Zhang, J.; Chen, Y.; Wen, R.; Li, H. Microscopic hematuria predicts lower stage in patients with upper tract urothelial carcinoma. Cancer Manag. Res. 2018, 10, 4929–4933. [Google Scholar] [CrossRef] [PubMed]
- Potretzke, A.M.; Knight, B.A.; Vetter, J.M.; Anderson, B.G.; Hardi, A.C.; Bhayani, S.B.; Figenshau, R.S. Diagnostic Utility of Selective Upper Tract Urinary Cytology: A Systematic Review and Meta-analysis of the Literature. Urology 2016, 96, 35–43. [Google Scholar] [CrossRef]
- Coleman, J.A.; Clark, P.E.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Chou, R.; Hoffman-Censits, J.; Kulkarni, G.S.; Matin, S.F.; Pierorazio, P.M.; et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J. Urol. 2023, 209, 1071–1081. [Google Scholar] [CrossRef]
- Niţă, G.; Georgescu, D.; Mulţescu, R.; Draguţescu, M.; Mihai, B.; Geavlete, B.; Persu, C.; Geavlete, P. Prognostic factors in laser treatment of upper urinary tract urothelial tumours. J. Med. Life 2012, 5, 33–38. [Google Scholar]
- Myers, A.A.; Pak, R.W. Novel laser therapies and new technologies in the endoscopic management of upper tract urothelial carcinoma: A narrative review. Transl. Androl. Urol. 2023, 12, 1723–1731. [Google Scholar] [CrossRef]
- Soloway, M.S.; Masters, S. Urothelial susceptibility to tumor cell implantation influence of cauterization. Cancer 1980, 46, 1158–1163. [Google Scholar] [CrossRef]
- Grasso, M.; Fishman, A.I.; Cohen, J.; Alexander, B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: A 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012, 110, 1618–1626. [Google Scholar] [CrossRef]
- Maruyama, Y.; Araki, M.; Wada, K.; Yoshinaga, K.; Mitsui, Y.; Sadahira, T.; Nishimura, S.; Edamura, K.; Kobayashi, Y.; Watanabe, M.; et al. Long-term ureteroscopic management of upper tract urothelial carcinoma: 28-year single-centre experience. Ultrasound Med. Biol. 2020, 51, 130–137. [Google Scholar] [CrossRef]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Shore, N.D. Management of Low-grade Upper Tract Urothelial Carcinoma: An Unmet Need. Rev. Urol. 2020, 22, 1–8. [Google Scholar] [PubMed]
- Ham, W.S.; Park, J.S.; Jang, W.S.; Kim, J. Nephron-Sparing Approaches in Upper Tract Urothelial Carcinoma: Current and Future Strategies. Biomedicines 2022, 10, 2223. [Google Scholar] [CrossRef] [PubMed]
- Kaag, M.; Trost, L.; Thompson, R.H.; Favaretto, R.; Elliott, V.; Shariat, S.F.; Maschino, A.; Vertosick, E.; Raman, J.D.; Dalbagni, G. Preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma. BJU Int. 2013, 114, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Mantica, G.; Carrion, D.M.; Pang, K.H.; Ucar, T.; Parodi, S.; Tappero, S.; Lazarou, L.; Glykas, I.; Zabaftis, C.; Lourenco, M.; et al. The definition of ideal training of a urology resident from two different perspectives: Trainees vs. professors. Is there agreement in their idea of good training? Cent. Eur. J. Urol. 2023, 76, 162–166. [Google Scholar] [CrossRef]
- Thomas, C.Y.; Hemal, A.K. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013, 112, 425–426. [Google Scholar] [CrossRef]
- Labbate, C.V.; Hensley, P.J.; Miest, T.S.; Qiao, W.; Adibi, M.; Shah, A.Y.; Chery, L.; Papadopoulos, J.; Siefker-Radtke, A.O.; Gao, J.; et al. Longitudinal GFR trends after neoadjuvant chemotherapy prior to nephroureterectomy for upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 454.e17–454.e23. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yu, C.-C.; Yeh, H.-C.; Lee, H.-Y.; Jiang, Y.-H.; Lee, Y.-K.; Kuei, C.-H.; Wu, C.-C.; Huang, C.-Y.; Lin, W.-Y.; et al. Endoscopic management versus radical nephroureterectomy for localized upper tract urothelial carcinoma in a high endemic region. Sci. Rep. 2021, 11, 4040. [Google Scholar] [CrossRef]
- Tsujino, T.; Komura, K.; Inamoto, T.; Maenosono, R.; Hashimoto, T.; Adachi, T.; Hirasawa, Y.; Tokushige, S.; Ohno, T.; Yamazaki, S.; et al. Nephron-sparing ureteroscopic surgery vs. radical nephroureterectomy: Comparable survival-outcomes in upper tract urothelial carcinoma. World J. Urol. 2023, 41, 3585–3591. [Google Scholar] [CrossRef]
- Okuyama, Y.; Hatakeyama, S.; Tabata, R.; Fujimori, D.; Kawashima, Y.; Tanaka, T.; Fujita, N.; Okamoto, T.; Mori, K.; Yamamoto, H.; et al. Impact of nephroureterectomy on postoperative renal function in upper tract urothelial carcinoma: A multicenter retrospective study. Int. J. Urol. 2023, 30, 649–657. [Google Scholar] [CrossRef]
- Raman, J.D.; Lin, Y.-K.; Kaag, M.; Atkinson, T.; Crispen, P.; Wille, M.; Smith, N.; Hockenberry, M.; Guzzo, T.; Peyronnet, B.; et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol. Oncol. Semin. Orig. Investig. 2013, 32, 47.e9–47.e14. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Zabor, E.C.; Tennenbaum, D.; Furberg, H.; Benfante, N.; Coleman, J.A.; Jaimes, E.A.; Russo, P. Renal function recovery after radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2017, 36, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakagawa, T.; Miyakawa, J.; Kawai, T.; Tabata, M.; Kaneko, T.; Taguchi, S.; Naito, A.; Hikatsu, M.; Sato, Y.; et al. Smaller decline of renal function after nephroureterectomy predicts poorer prognosis of upper tract urothelial carcinoma: A multicentre retrospective study. Ultrasound Med. Biol. 2021, 51, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Hwang, J.E.; Chung, H.S.; Cho, Y.H.; Kim, M.S.; Hwang, E.C.; Oh, K.J.; Kim, S.-O.; Jung, S.I.; Kang, T.W.; et al. Is preoperative chronic kidney disease status associated with oncologic outcomes in upper urinary tract urothelial carcinoma? A multicenter propensity score-matched analysis. Oncotarget 2017, 8, 66540–66549. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, Q.; Li, X.; Qian, C.; Xiong, G.; Zhang, L.; Chen, X.; Zhang, X.; Yu, W.; He, Z.; et al. Nomogram Predicting Renal Insufficiency after Nephroureterectomy for Upper Tract Urothelial Carcinoma in the Chinese Population: Exclusion of Ineligible Candidates for Adjuvant Chemotherapy. BioMed Res. Int. 2014, 2014, 529186. [Google Scholar] [CrossRef]
- Abrate, A.; Sessa, F.; Campi, R.; Preto, M.; Olivero, A.; Varca, V.; Benelli, A.; Sessa, M.; Sebastianelli, A.; Pavone, C.; et al. Segmental ureterectomy vs. radical nephroureterectomy for ureteral carcinoma in patients with a preoperative glomerular filtration rate less than 90 ml/min/1.73 m2: A multicenter study. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 601.e11–601.e16. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in Renal Function Following Nephroureterectomy May Affect the Use of Perioperative Chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Dudinec, J.V.; Ortiz-Melo, D.I.; Lipkin, M.E.; Abern, M.R.; Shah, A.M.; Inman, B.A. Advanced chronic kidney disease; A comparison between nephroureterectomy and nephron-sparing surgery for upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2022, 41, 295.e19–295.e25. [Google Scholar] [CrossRef]
| Before Matching | ||||
|---|---|---|---|---|
| LA | RNU | |||
| N = 4172 | N = 2007 | Standard Mean Diff. | ||
| Gender | Male | 2670 (64.0%) | 1224 (61.0%) | 0.062 |
| Female | 1414 (33.9%) | 724 (36.1%) | 0.047 | |
| Unknown | 88 (2.1%) | 59 (2.9%) | 0.051 | |
| Age at Index | 71.0 ± 9.6 | 69.2 ± 10.0 | 0.182 | |
| Race | White | 3179 (76.2%) | 1487 (74.1%) | 0.049 |
| Black or African American | 213 (5.1%) | 82 (4.1%) | 0.049 | |
| Asian | 222 (5.3%) | 54 (2.7%) | 0.134 | |
| American Indian or Alaska Native | 10 (0.2%) | 10 (0.5%) | 0.043 | |
| Native Hawaiian or Other Pacific Islander | 12 (0.3%) | 0 (0.0%) | 0.076 | |
| Other | 139 (3.3%) | 59 (2.9%) | 0.022 | |
| Unknown | 397 (9.5%) | 315 (15.7%) | 0.221 | |
| BMI [kg/m2] | 28.2 ± 6.0 | 28.2 ± 6.2 | 0.001 | |
| Creatinine [Mass/volume] | 1.2 ± 0.7 | 1.3 ± 0.9 | 0.063 | |
| GFR [mL/min 1.73 m2] | 60.8 ± 22.8 | 59.5 ± 20.4 | 0.060 | |
| AKI and CKD N17–N19 | 924 (22.1%) | 421 (21.0%) | 0.028 | |
| AKI N17 | 481 (11.5%) | 204 (10.2%) | 0.044 | |
| CKD N18 | 710 (17.0%) | 339 (16.9%) | 0.003 | |
| CKD III N18.3 | 417 (10.0%) | 183 (9.1%) | 0.030 | |
| CKD IV N18.4 | 121 (2.9%) | 41 (2.0%) | 0.055 | |
| CKD V N18.5 | 14 (0.3%) | 10 (0.5%) | 0.025 | |
| ESRD N18.6 | 37 (0.9%) | 40 (2.0%) | 0.093 | |
| Dialysis CPT 1012740 | 10 (0.2%) | 14 (0.7%) | 0.067 | |
| Glomerular Diseases N00–N08 | 45 (1.1%) | 37 (1.9%) | 0.064 | |
| Renal Tubulo-Interstitial Diseases N10–N16 | 1539 (36.9%) | 645 (32.1%) | 0.100 | |
| Acute Pyelonephritis N10 | 42 (1.0%) | 22 (1.1%) | 0.009 | |
| Obstructive and Reflux Uropathy N13 | 1500 (36.0%) | 619 (30.8%) | 0.109 | |
| Urolithiasis N20–N23 | 616 (14.8%) | 239 (11.9%) | 0.084 | |
| Other Disorder of Kidney and Ureter N25–N29 | 1675 (40.1%) | 1053 (52.5%) | 0.249 | |
| Unspecified Contracted Kidney N26 | 60 (1.4%) | 71 (3.5%) | 0.135 | |
| Other Diseases of Urinary System N30–N39 | 1461 (35.0%) | 563 (28.1%) | 0.150 | |
| Cystitis N30 | 427 (10.2%) | 123 (6.1%) | 0.150 | |
| UTI, Site Unspecified N39.0 | 733 (17.6%) | 293 (14.6%) | 0.081 | |
| Essential Hypertension I10 | 1917 (45.9%) | 879 (43.8%) | 0.043 | |
| Ischemic Heart Disease I20–I25 | 855 (20.5%) | 369 (18.4%) | 0.053 | |
| Cerebrovascular Disease I60–I69 | 267 (6.4%) | 89 (4.4%) | 0.087 | |
| Diabetes E08–E13 | 843 (20.2%) | 336 (16.7%) | 0.089 | |
| Metabolic Disorders E70–E88 | 1801 (43.4%) | 786 (39.2%) | 0.086 | |
| Chronic Lower Respiratory Diseases J40–J4A | 718 (17.2%) | 306 (15.2%) | 0.053 | |
| Alcohol-related Disorders F10 | 71 (1.7%) | 28 (1.4%) | 0.025 | |
| Nicotine Dependence F17.2 | 295 (7.1%) | 141 (7.0%) | 0.002 | |
| Hematuria R31 | 1933 (46.3%) | 820 (40.9%) | 0.111 | |
| Retention of Urine R33 | 260 (6.2%) | 81 (4.0%) | 0.100 | |
| Proteinuria R80 | 96 (2.3%) | 35 (1.7%) | 0.040 | |
| After Matching | ||||
|---|---|---|---|---|
| LA | RNU | |||
| N = 1965 | N = 1965 | Standard Mean Diff. | ||
| Gender | Male | 1202 (61.2%) | 1202 (61.2%) | <0.001 |
| Female | 713 (36.3%) | 705 (35.9%) | 0.009 | |
| Unknown | 50 (2.5%) | 58 (2.9%) | 0.027 | |
| Age at Index | 69.2 ± 10.0 | 69.3 ± 9.9 | 0.012 | |
| Race | White | 1454 (74.0%) | 1464 (74.5%) | 0.012 |
| Black or African American | 93 (4.7%) | 81 (4.1%) | 0.030 | |
| Asian | 52 (2.6%) | 54 (2.7%) | 0.006 | |
| American Indian or Alaska Native | 10 (0.5%) | 10 (0.5%) | <0.001 | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0%) | 0 (0.0%) | - | |
| Other | 61 (3.1%) | 58 (3.0%) | 0.009 | |
| Unknown | 295 (15.0%) | 298 (15.2%) | 0.013 | |
| BMI [kg/m2] | 28.6 ± 6.1 | 28.2 ± 6.2 | 0.065 | |
| Creatinine [Mass/volume] | 1.2 ± 0.7 | 1.3 ± 0.8 | 0.044 | |
| GFR [mL/min 1.73 m2] | 60.5 ± 21.2 | 59.8 ± 20.3 | 0.034 | |
| AKI and CKD N17-N19 | 424 (21.6%) | 407 (20.7%) | 0.021 | |
| AKI N17 | 195 (9.9%) | 203 (10.3%) | 0.013 | |
| CKD N18 | 332 (16.9%) | 325 (16.5%) | 0.010 | |
| CKD III N18.3 | 186 (9.5%) | 179 (9.1%) | 0.012 | |
| CKD IV N18.4 | 37 (1.9%) | 40 (2.0%) | 0.011 | |
| CKD V N18.5 | 10 (0.5%) | 10 (0.5%) | <0.001 | |
| ESRD N18.6 | 27 (1.4%) | 31 (1.6%) | 0.017 | |
| Dialysis CPT 1012740 | 10 (0.5%) | 10 (0.5%) | <0.001 | |
| Glomerular Diseases N00–N08 | 33 (1.7%) | 33 (1.7%) | 0.010 | |
| Renal Tubulo-Interstitial Diseases N10–N16 | 628 (32.0%) | 636 (32.4%) | 0.009 | |
| Acute Pyelonephritis N10 | 18 (0.9%) | 22 (1.1%) | 0.020 | |
| Obstructive and Reflux Uropathy N13 | 640 (31.0%) | 611 (31.1%) | 0.001 | |
| Urolithiasis N20–N23 | 233 (11.9%) | 237 (12.1%) | 0.006 | |
| Other Disorder of Kidney and Ureter N25–N29 | 1012 (51.5%) | 1016 (51.7%) | 0.004 | |
| Unspecified Contracted Kidney N26 | 39 (2.0%) | 69 (3.5%) | 0.093 | |
| Other Diseases of Urinary System N30–N39 | 594 (30.2%) | 554 (28.2%) | 0.036 | |
| Cystitis N30 | 122 (6.2%) | 123 (6.3%) | 0.004 | |
| UTI, Site Unspecified N39.0 | 286 (14.6%) | 289 (14.7%) | 0.003 | |
| Essential Hypertension I10 | 843 (42.9%) | 859 (43.7%) | 0.045 | |
| Ischemic Heart Disease I20–I25 | 365 (18.6%) | 358 (18.2%) | 0.009 | |
| Cerebrovascular Disease I60–I69 | 92 (4.7%) | 88 (4.5%) | 0.010 | |
| Diabetes E08–E13 | 331 (16.8%) | 329 (16.7%) | 0.003 | |
| Metabolic disorders E70–E88 | 752 (38.3%) | 767 (39.0%) | 0.016 | |
| Chronic Lower Respiratory Diseases J40–J4A | 302 (15.4%) | 300 (15.3%) | 0.003 | |
| Alcohol-related Disorders F10 | 32 (1.6%) | 28 (1.4%) | 0.017 | |
| Nicotine Dependence F17.2 | 142 (7.2%) | 136 (6.9%) | 0.012 | |
| Hematuria R31 | 799 (40.7%) | 806 (41.0%) | 0.007 | |
| Retention of Urine R33 | 73 (3.7%) | 81 (4.1%) | 0.021 | |
| Proteinuria R80 | 32 (1.6%) | 35 (1.8%) | 0.012 | |
| CKD (eGFR mL/min/1.73 m2) | LA | RNU | HR (CI) |
|---|---|---|---|
| Stage 3 or greater (0–59) | 537 days | 25 days | 0.58 (0.51–0.66) |
| Stage 3 (30–59) | 764 days | 110 days | 0.58 (0.51–0.65) |
| Stage 4 (15–29) | 828 days | 427 days | 0.95 (0.82–1.09) |
| Stage 5 (0–14) | 2021 days | 1085 days | 1.13 (0.91–1.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baer, B.R.; Matheny, M.V.; Mercedes, R.H.; Raman, J.D. Comparative Analysis of Long-Term Renal Outcomes in Upper Tract Urothelial Carcinoma: Local Ablation Versus Radical Nephroureterectomy. Curr. Oncol. 2025, 32, 125. https://doi.org/10.3390/curroncol32030125
Baer BR, Matheny MV, Mercedes RH, Raman JD. Comparative Analysis of Long-Term Renal Outcomes in Upper Tract Urothelial Carcinoma: Local Ablation Versus Radical Nephroureterectomy. Current Oncology. 2025; 32(3):125. https://doi.org/10.3390/curroncol32030125
Chicago/Turabian StyleBaer, Blake R., Meghan V. Matheny, Raidizon H. Mercedes, and Jay D. Raman. 2025. "Comparative Analysis of Long-Term Renal Outcomes in Upper Tract Urothelial Carcinoma: Local Ablation Versus Radical Nephroureterectomy" Current Oncology 32, no. 3: 125. https://doi.org/10.3390/curroncol32030125
APA StyleBaer, B. R., Matheny, M. V., Mercedes, R. H., & Raman, J. D. (2025). Comparative Analysis of Long-Term Renal Outcomes in Upper Tract Urothelial Carcinoma: Local Ablation Versus Radical Nephroureterectomy. Current Oncology, 32(3), 125. https://doi.org/10.3390/curroncol32030125





