HPV and Cervical Cancer—Biology, Prevention, and Treatment Updates
Abstract
1. Introduction
2. Epidemiology
3. Classification of HPV Viruses
4. Risk Factors
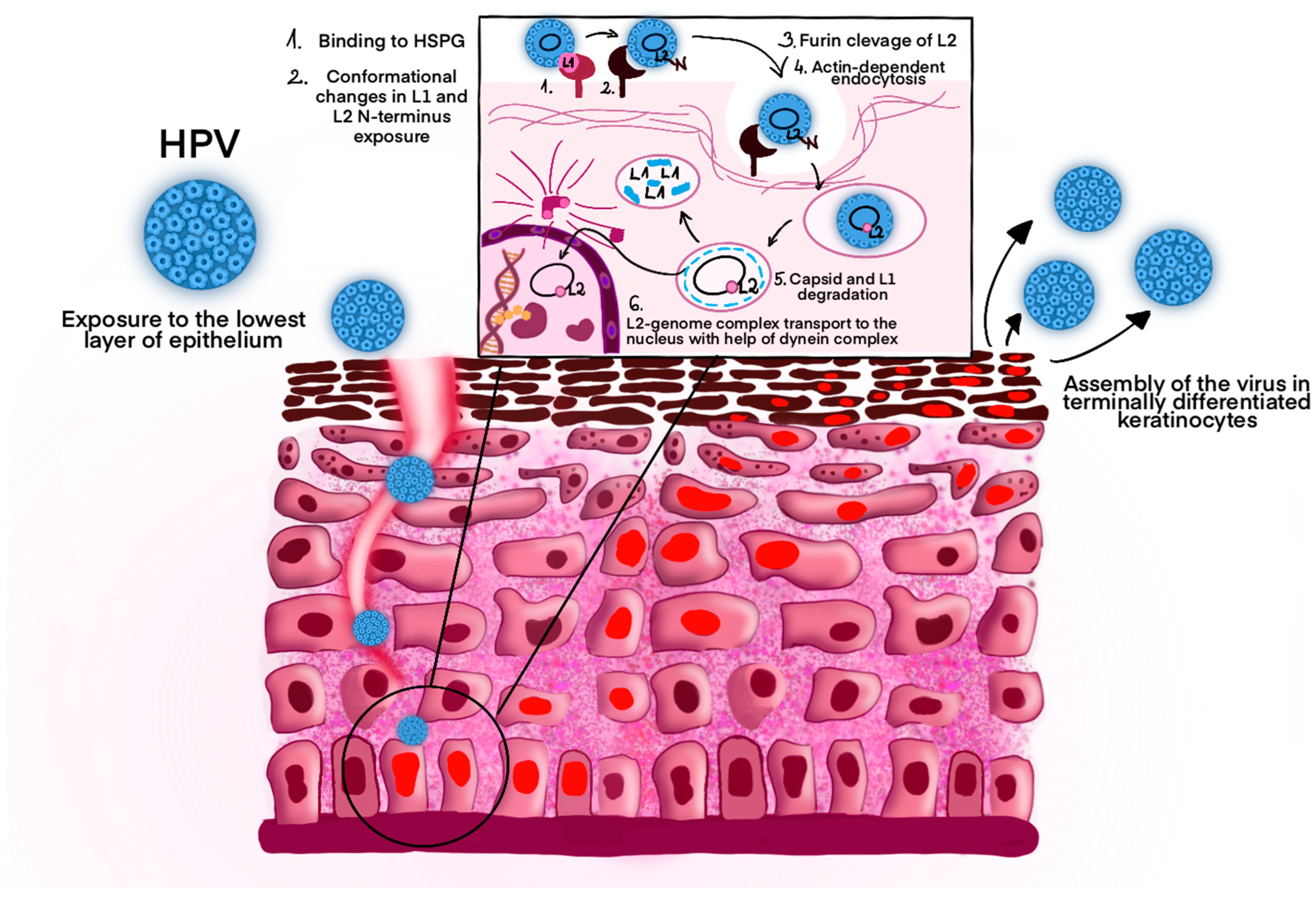
5. Pathogenesis and Symptoms of Infection
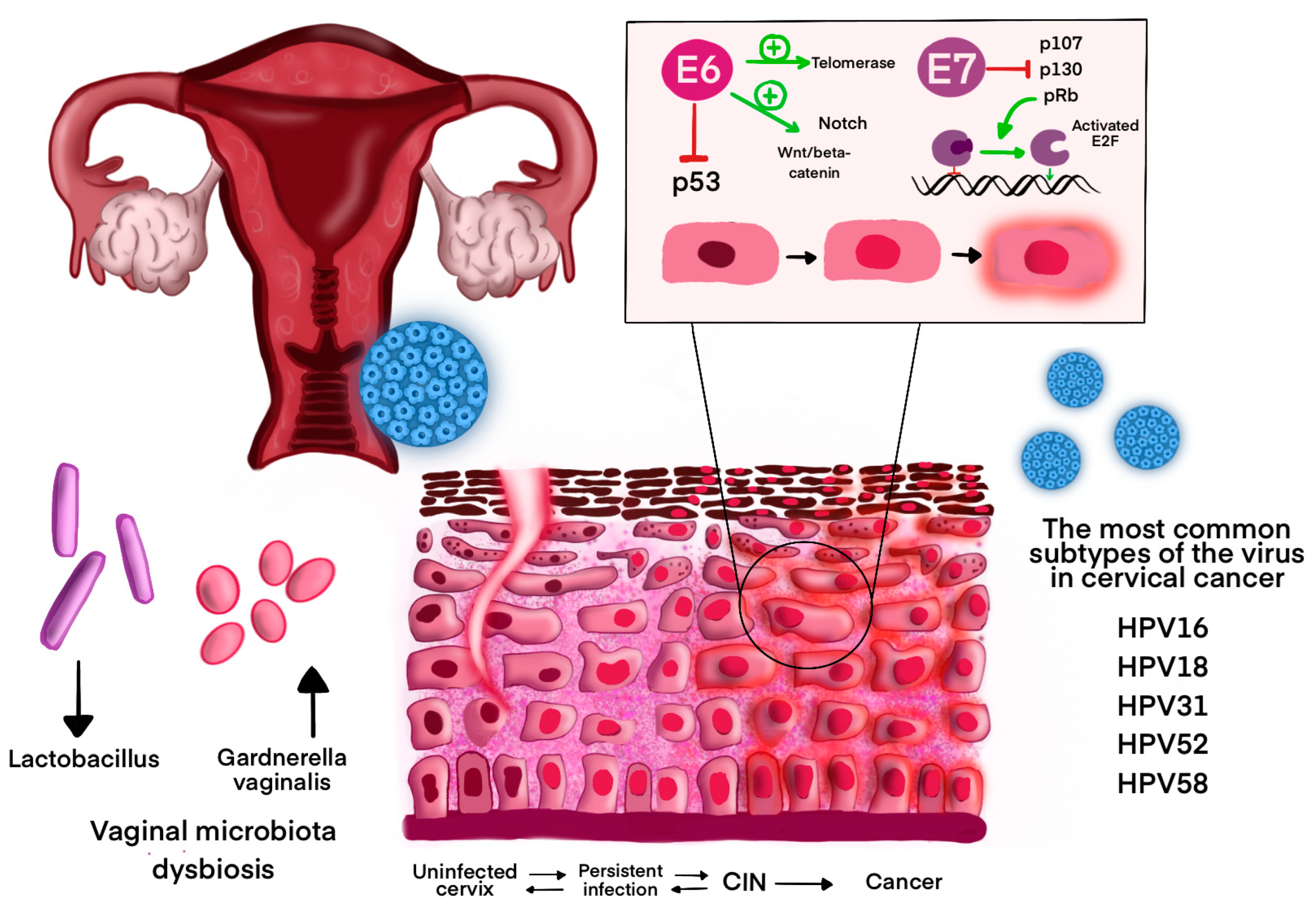
6. HPV-Related Cancers: Pathogenesis and Epidemiology
7. World Health Organization Guidelines—HPV Vaccine
- Girls aged 9–14 years: one or two doses.
- Young women aged 15–20 years: one or two doses.
- Women over 21 years: two doses spaced 6 months apart.
8. World Health Organization—HPV Vaccine News 2024
9. The HPV Vaccination Strategy: The Example of Poland
10. Self-Sampling for HPV Testing
11. Screening Tests in the Diagnosis of HPV Infections
12. The Role of Colposcopy in Cervical Cancer Screening—New Polish Recommendations
13. Treatment of HPV-Related Cervical Cancer
- Early-Stage Cervical Cancer (FIGO IA-IIA)
- Locally Advanced Cervical Cancer (LACC) (FIGO IIB–IVA, extending to the pelvic side wall or adjacent organs)
13.1. Early-Stage Cervical Cancer (FIGO IA–IIA)
13.2. Locally Advanced Cervical Cancer–LACC (FIGO IIB–IVA)
13.3. Recurrent or Metastatic Cervical Cancer (FIGO IVB)
- chemotherapy (paclitaxel, albumin-bound paclitaxel, docetaxel, fluorouracil, gemcitabine, pemetrexed, topotecan, vinorelbine, and irinotecan),
- bevacizumab,
- pembrolizumab,
- tisotumab vedotin-tftv,
- cemiplimab,
- Nivolumab for PD-L1–positive tumors,
- Selpercatinib for RET gene fusion–positive tumors,
- TRK inhibitors for NTRK gene fusion–positive tumors (larotrectinib, entrectinib),
- Trastuzumab deruxtecan for HER2-positive tumors,
- biomarkers with their associated targeted treatments as second-line/subsequent therapies [109].
14. Future Directions
14.1. Therapeutic Vaccine
- bacterial vector (L. monocytogenes, L. lactis, L. plantarum, L. casei, Salmonella, Shigella, and E. coli),
- viral vector (adenoviruses, adeno-associated viruses, alphaviruses, and vaccinia virus),
- peptide,
- protein,
- DNA,
- RNA replicon,
- dendritic cell,
- tumor cell [133].
14.2. Oncoproteins E6 and E7 Antibodies as Serological Markers
14.3. Circulating Cell-Free DNA (cfDNA)
15. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta-Analysis of 1 Million Women with Normal Cytological Findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Kombe, A.J.K.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Chan, P.K.; et al. Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology 2018, 516, 86–101. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Castle, P.E.; Lorincz, A.T.; Wacholder, S.; Sherman, M.; Scott, D.R.; Rush, B.B.; Glass, A.G.; Schiffman, M. The Elevated 10-Year Risk of Cervical Precancer and Cancer in Women With Human Papillomavirus (HPV) Type 16 or 18 and the Possible Utility of Type-Specific HPV Testing in Clinical Practice. JNCI J. Natl. Cancer Inst. 2005, 97, 1072–1079. [Google Scholar] [CrossRef]
- Winer, R.L.; Lee, S.-K.; Hughes, J.P.; Adam, D.E.; Kiviat, N.B.; Koutsky, L.A. Genital Human Papillomavirus Infection: Incidence and Risk Factors in a Cohort of Female University Students. Am. J. Epidemiol. 2003, 157, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Kannampuzha, S.; Murali, R.; Namachivayam, A.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; et al. Exploring the Molecular Pathogenesis, Pathogen Association, and Therapeutic Strategies against HPV Infection. Pathogens 2022, 12, 25. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Porpora, M.G.; Recine, N.; Latino, M.A.; Sessa, R. HPV/Chlamydia trachomatis co-infection: Metagenomic analysis of cervical microbiota in asymptomatic women. New Microbiol. 2018, 41, 34–41. [Google Scholar] [PubMed]
- Yamaguchi, M.; Sekine, M.; Hanley, S.J.B.; Kudo, R.; Hara, M.; Adachi, S.; Ueda, Y.; Miyagi, E.; Enomoto, T. Risk factors for HPV infection and high-grade cervical disease in sexually active Japanese women. Sci. Rep. 2021, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Drake, V.E.; Fakhry, C.; Windon, M.J.; Stewart, C.M.; Akst, L.; Hillel, A.; Chien, W.; Ha, P.; Miles, B.; Gourin, C.G.; et al. Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer. Cancer 2021, 127, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-C.; Liu, W.-D.; Liu, Y.-H.; Ye, X.-H.; Chen, S.-D. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: A Meta-analysis of Epidemiological Studies. Asian Pac. J. Cancer Prev. 2015, 16, 3893–3900. [Google Scholar] [CrossRef]
- the HPV Study Group HPV Study; Molano, M.; Posso, H.; Weiderpass, E.; Brule, A.J.C.v.D.; Ronderos, M.; Franceschi, S.; Meijer, C.J.L.M.; Arslan, A.; Munoz, N. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br. J. Cancer 2002, 87, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Petignat, P.; Faltin, D.; Goffin, F.; Billieux, M.-H.; Stucki, D.; Sporri, S.; Vassilakos, P. Age-related performance of human papillomavirus testing used as an adjunct to cytology for cervical carcinoma screening in a population with a low incidence of cervical carcinoma. Cancer 2005, 105, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2019, 40, 602–608. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 2006, 119, 1108–1124. [Google Scholar] [CrossRef]
- Sabeena, S.; Bhat, P.; Kamath, V.; Arunkumar, G. Possible non-sexual modes of transmission of human papilloma virus. J. Obstet. Gynaecol. Res. 2017, 43, 429–435. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papilomavirus DNA. Virol. J. 2009, 6, 83. [Google Scholar] [CrossRef]
- Cason, J.; Mant, C.A. High-risk mucosal human papillomavirus infections during infancy & childhood. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2005, 32 (Suppl. 1), S52–S58. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Carrillo-Beltrán, D.; Aedo-Aguilera, V.; Calaf, G.M.; León, O.; Maldonado, E.; Tapia, J.C.; Boccardo, E.; Ozbun, M.A.; Aguayo, F. Tobacco Exposure Enhances Human Papillomavirus 16 Oncogene Expression via EGFR/PI3K/Akt/c-Jun Signaling Pathway in Cervical Cancer Cells. Front. Microbiol. 2018, 9, 3022. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.; Beral, V.; de González, A.B.; Colin, D.; Franceschi, S.; Goodill, A.; Green, J.; Peto, J.; Plummer, M.; Sweetland, S. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int. J. Cancer 2006, 118, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Hewavisenti, R.V.; Arena, J.; Ahlenstiel, C.L.; Sasson, S.C. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front. Immunol. 2023, 14, 1112513. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.; Muñoz, J.P.; Perez-Dominguez, F.; Carrillo-Beltrán, D.; Oliva, C.; Calaf, G.M.; Blanco, R.; Nuñez-Acurio, D. High-Risk Human Papillomavirus and Tobacco Smoke Interactions in Epithelial Carcinogenesis. Cancers 2020, 12, 2201. [Google Scholar] [CrossRef]
- Anastasiou, E.; McCarthy, K.J.; Gollub, E.L.; Ralph, L.; van de Wijgert, J.H.; Jones, H.E. The relationship between hormonal contraception and cervical dysplasia/cancer controlling for human papillomavirus infection: A systematic review. Contraception 2021, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Zhang, C.; Ying, C.; Jiang, H. Diabetes associated with HPV infection in women aged over 50 years: A cross-sectional study from China’s largest academic woman’s hospital. Front. Endocrinol. 2022, 13, 972963. [Google Scholar] [CrossRef]
- Nunes, R.A.L.; Morale, M.G.; Silva, G.Á.F.; Villa, L.L.; Termini, L. Innate immunity and HPV: Friends or foes. Clinics 2018, 73, e549s. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Kaufman, R.H.; Adam, E.; Adler-Storthz, K. Human papillomavirus associated with vaginal intraepithelial neoplasia in women exposed to diethylstilbestrol in utero. Obstet. Gynecol. 1987, 70, 75–80. [Google Scholar]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat. Res. Mol. Mech. Mutagen. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- Schäfer, G.; Blumenthal, M.J.; Katz, A.A. Interaction of human tumor viruses with host cell surface receptors and cell entry. Viruses 2015, 7, 2592–2617. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Kines, R.C.; Roberts, J.N.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J. Virol. 2009, 83, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Sapp, M.; Bienkowska-Haba, M. Viral entry mechanisms: Human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 2009, 276, 7206–7216. [Google Scholar] [CrossRef]
- Bienkowska-Haba, M.; Patel, H.D.; Sapp, M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLOS Pathog. 2009, 5, e1000524. [Google Scholar] [CrossRef] [PubMed]
- Marušič, M.B.; Ozbun, M.A.; Campos, S.K.; Myers, M.P.; Banks, L. Human papillomavirus L2 facilitates viral escape from late endosomes via sorting nexin 17. Traffic 2011, 13, 455–467. [Google Scholar] [CrossRef]
- Florin, L.; Becker, K.A.; Lambert, C.; Nowak, T.; Sapp, C.; Strand, D.; Streeck, R.E.; Sapp, M. Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2. J. Virol. 2006, 80, 6691–6696. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, N.; Mitra, R.; McBride, A.A. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J. Virol. 2011, 85, 8981–8995. [Google Scholar] [CrossRef]
- Day, P.M.; Baker, C.C.; Lowy, D.R.; Schiller, J.T. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14252–14257. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. The Papillomavirus E2 proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Pang, Y.-Y.S.; Lowy, D.R.; Schiller, J.T. Maturation of papillomavirus capsids. J. Virol. 2005, 79, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Yajid, A.I.; Zakariah, M.A.; Zin, A.A.M.; Othman, N.H. Potential Role of E4 Protein in Human Papillomavirus Screening: A Review. Asian Pac. J. Cancer Prev. 2017, 18, 315–319. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Kyriazoglou, A.; Liontos, M.; Dimopoulos, M.A.; Gavriatopoulou, M. Current trends in the management and prevention of human papillomavirus (HPV) infection. J. Buon. 2020, 25, 1281–1285. [Google Scholar] [PubMed]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLOS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef]
- Tamarelle, J.; Thiébaut, A.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018, 25, 35–47. [Google Scholar] [CrossRef]
- Mortaki, D.; Gkegkes, I.D.; Psomiadou, V.; Blontzos, N.; Prodromidou, A.; Lefkopoulos, F.; Nicolaidou, E. Vaginal microbiota and human papillomavirus: A systematic review. J. Turk. Gynecol. Assoc. 2020, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Z.; Sun, T.; Zhu, L. Cervicovaginal microbiome, high-risk HPV infection and cervical cancer: Mechanisms and therapeutic potential. Microbiol. Res. 2024, 287, 127857. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Belleti, R.; Pinto, G.V.d.S.; Bolpetti, A.D.N.; da Silva, M.G.; Marconi, C. Cervicovaginal Gardnerella sialidase-encoding gene in persistent human papillomavirus infection. Sci. Rep. 2023, 13, 14266. [Google Scholar] [CrossRef]
- Milano, G.; Guarducci, G.; Nante, N.; Montomoli, E.; Manini, I. Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines 2023, 11, 1060. [Google Scholar] [CrossRef]
- Maglennon, G.A.; McIntosh, P.B.; Doorbar, J. Immunosuppression Facilitates the Reactivation of Latent Papillomavirus Infections. J. Virol. 2014, 88, 710–716. [Google Scholar] [CrossRef]
- Jamshidi, M.; Shekari, M.; Nejatizadeh, A.A.; Malekzadeh, K.; Baghershiroodi, M.; Davudian, P.; Dehghan, F.; Jamshidi, F. The impact of human papillomavirus (HPV) types 6, 11 in women with genital warts. Arch. Gynecol. Obstet. 2012, 286, 1261–1267. [Google Scholar] [CrossRef]
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Monsonego, J.; Magdelenat, H.; Catalan, F.; Coscas, Y.; Zerat, L.; Sastre, X. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int. J. Cancer 1991, 48, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Hashimoto, K.; Kitano, S.; Yamashita, S.; Toda, A.; Nakamura, K.; Kinose, Y.; Kodama, M.; Sawada, K.; Kimura, T. Estrogen induces genomic instability in high-risk HPV-infected cervix and promotes the carcinogenesis of cervical adenocarcinoma. Biochem. Biophys. Res. Commun. 2023, 659, 80–90. [Google Scholar] [CrossRef]
- Dunne, E.F.; Park, I.U. HPV and HPV-Associated Diseases. Infect. Dis. Clin. North Am. 2013, 27, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Neto, C.A.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ouda, A.M.; Elsabagh, A.A.; Elmakaty, I.M.; Gupta, I.; Vranic, S.; Al-Thawadi, H.; Al Moustafa, A.-E. HPV and Recurrent Respiratory Papillomatosis: A Brief Review. Life 2021, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Rudnicka, L. HPV Infections—Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024, 25, 7616. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Hatterschide, J.; Bohidar, A.E.; Grace, M.; Nulton, T.J.; Kim, H.W.; Windle, B.; Morgan, I.M.; Munger, K.; White, E.A. PTPN14 degradation by high-risk human papillomavirus E7 limits keratinocyte differentiation and contributes to HPV-mediated oncogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 7033–7042. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, L.; He, Y.; Qi, X. Human papillomavirus associated cervical lesion: Pathogenesis and therapeutic interventions. Medcomm 2023, 4, e368. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Vallejo-Ruiz, V.; Gutiérrez-Xicotencatl, L.; Medina-Contreras, O.; Lizano, M. Ver Molecular aspects of cervical cancer: A pathogenesis update. Front. Oncol. 2024, 14, 1356581. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- The European Cancer Organisation. Eliminating Cancers & Diseases in Europe; The European Cancer Organisation: Brussels, Belgium, 2019. [Google Scholar]
- Del Mistro, A.; Frayle, H.; Menegaldo, A.; Favaretto, N.; Gori, S.; Nicolai, P.; Spinato, G.; Romeo, S.; Tirelli, G.; da Mosto, M.C.; et al. Age-independent increasing prevalence of Human Papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep. 2020, 10, 9320. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeian, H.; Bai, Y.; Huang, R.; Chaurasia, A.; Darido, C. Navigating therapeutic strategies: HPV classification in head and neck cancer. Br. J. Cancer 2024, 131, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Libera, L.S.D.; de Carvalho, K.P.A.; Ramos, J.E.P.; Cabral, L.A.O.; Alencar, R.d.C.G.d.; Villa, L.L.; Alves, R.R.F.; Santos, S.H.R.; Carneiro, M.A.d.S.; Saddi, V.A. Human Papillomavirus and Anal Cancer: Prevalence, Genotype Distribution, and Prognosis Aspects from Midwestern Region of Brazil. J. Oncol. 2019, 2019, 6018269. [Google Scholar] [CrossRef]
- Hernandez, A.L.; Hilton, J.F.D.; Weatherly, C.S.D.; Berry-Lawhorn, J.M.; Jay, N.N.; Brickman, C.; Wang, C.-C.J.; Kauffman, J.; Calderon, J.; Farhat, S.; et al. Prevalence of Anal Human Papillomavirus Infection and Anal High-Grade Squamous Intraepithelial Lesions Among Men Who Have Sex With Men 50 Years and Older Living With or Without HIV. Am. J. Ther. 2024, 96, 439–446. [Google Scholar] [CrossRef]
- Machalek, D.A.; Poynten, M.; Jin, F.; Fairley, C.K.; Farnsworth, A.; Garland, S.M.; Hillman, R.J.; Petoumenos, K.; Roberts, J.; Tabrizi, S.N.; et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta-analysis. Lancet Oncol. 2012, 13, 487–500. [Google Scholar] [CrossRef]
- Glenn, W.K.; Ngan, C.C.; Amos, T.G.; Edwards, R.J.; Swift, J.; Lutze-Mann, L.; Shang, F.; Whitaker, N.J.; Lawson, J.S. High risk human papilloma viruses (HPVs) are present in benign prostate tissues before development of HPV associated prostate cancer. Infect. Agents Cancer 2017, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K. Evidence for a causal role by human papillomaviruses in prostate cancer—A systematic review. Infect. Agents Cancer 2020, 15, 41. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news/item/20-12-2022-WHO-updates-recommendations-on-HPV-vaccination-schedule (accessed on 26 November 2024).
- Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/human-papillomavirus-(hpv)/hpv-background-document--report-march-2022.pdf?sfvrsn=b600e252_1 (accessed on 26 November 2024).
- Available online: https://iris.who.int/bitstream/handle/10665/365350/WER9750-eng-fre.pdf?sequence=1 (accessed on 26 November 2024).
- WHO Position Paper (2022 Update). 2022. Available online: https://www.hpvcenter.se/human_reference_clones/ (accessed on 5 January 2025).
- Available online: https://cdn.who.int/media/docs/default-source/reproductive-health/sage_april2022meetinghighlights_11apr2022_final.pdf?sfvrsn=21bcfb4f_3 (accessed on 26 November 2024).
- Staadegaard, L.; Rönn, M.M.; Soni, N.; Bellerose, M.E.; Bloem, P.; Brisson, M.; Maheu-Giroux, M.; Barnabas, R.V.; Drolet, M.; Mayaud, P.; et al. Immunogenicity, safety, and efficacy of the HPV vaccines among people living with HIV: A systematic review and meta-analysis. EClinicalMedicine 2022, 52, 101585. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news/item/04-10-2024-who-adds-an-hpv-vaccine-for-single-dose-use (accessed on 26 November 2024).
- Wang, Q.; Zhang, W.; Cai, H.; Cao, Y. Understanding the perceptions of Chinese women of the commercially available domestic and imported HPV vaccine: A semantic network analysis. Vaccine 2020, 38, 8334–8342. [Google Scholar] [CrossRef]
- Zaman, K.; Schuind, A.E.; Adjei, S.; Antony, K.; Aponte, J.J.; Buabeng, P.B.; Qadri, F.; Kemp, T.J.; Hossain, L.; Pinto, L.A.; et al. Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9–14 years of age: Interim analysis from a phase 3 clinical trial. Vaccine 2024, 42, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-M.; Bi, Z.-F.; Zheng, Y.; Zhang, L.; Zheng, F.-Z.; Chu, K.; Li, Y.-F.; Chen, Q.; Quan, J.-L.; Hu, X.-W.; et al. Immunogenicity and safety of an Escherichia coli-produced human papillomavirus (types 6/11/16/18/31/33/45/52/58) L1 virus-like-particle vaccine: A phase 2 double-blind, randomized, controlled trial. Sci. Bull. 2023, 68, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Jin, H.; Fu, X. Comparative efficacy of human papillomavirus vaccines: Systematic review and network meta-analysis. Expert Rev. Vaccines 2023, 22, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, L.-W.; Li, K.; Huang, L.-R.; Li, J.-B.; Dong, Y.-L.; Li, W.; Ji, M.; Yang, Q.; Zhou, L.-Y.; et al. Comparison of the safety and persistence of immunogenicity of bivalent HPV16/18 vaccine in healthy 9–14-year-old and 18–26-year-old Chinese females: A randomized, double-blind, non-inferiority clinical trial. Vaccine 2023, 41, 7212–7219. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gov.pl/web/zdrowie/hpv (accessed on 26 November 2024).
- Available online: https://szczepienia.pzh.gov.pl/dla-lekarzy/szczepienia-hpv/schematy-szczepien-przeciw-hpv/ (accessed on 26 November 2024).
- Smolarczyk, K.; Duszewska, A.; Drozd, S.; Majewski, S. Parents’ Knowledge and Attitude towards HPV and HPV Vaccination in Poland. Vaccines 2022, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Drejza, M.; Rylewicz, K.; Lewandowska, M.; Gross-Tyrkin, K.; Łopiński, G.; Barwińska, J.; Majcherek, E.; Szymuś, K.; Klein, P.; Plagens-Rotman, K.; et al. HPV Vaccination among Polish Adolescents—Results from POLKA 18 Study. Healthcare 2022, 10, 2385. [Google Scholar] [CrossRef]
- Jankowski, M.; Grudziąż-Sękowska, J.; Wrześniewska-Wal, I.; Tyszko, P.; Sękowski, K.; Ostrowski, J.; Gujski, M.; Pinkas, J. National HPV Vaccination Program in Poland—Public Awareness, Sources of Knowledge, and Willingness to Vaccinate Children against HPV. Vaccines 2023, 11, 1371. [Google Scholar] [CrossRef]
- Lofters, A.; Vahabi, M. Self-sampling for HPV to enhance uptake of cervical cancer screening: Has the time come in Canada? Can. Med. Assoc. J. 2016, 188, 853–854. [Google Scholar] [CrossRef]
- Snijders, P.J.; Verhoef, V.M.; Arbyn, M.; Ogilvie, G.; Minozzi, S.; Banzi, R.; van Kemenade, F.J.; Heideman, D.A.; Meijer, C.J. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int. J. Cancer 2012, 132, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.; Snijders, P.; Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef] [PubMed]
- Agencja Oceny Technologii Medycznych i Taryfikacji Wydział Świadczeń Opieki Zdrowotnej, “Test HPV HR z genotypowaniem hrHPVobejmującym co najmniej typy 16 i 18”Ocena Zasadności Zakwalifikowania Świadczeniagwarantowanego z Zakresu Programów Zdrowotnychw Programie Profilaktyki Raka Szyjki Macicy. 2024. Available online: https://bip.aotm.gov.pl/zlecenia-mz-2024/1033-materialy-2024/8793-218-2024-zlc (accessed on 5 January 2025).
- Polskie Towarzystwo Ginekologiczne. Wytyczne dotyczące aplikacji testówmolekularnych identyfikujących DNA HPVHR w profilaktyce raka szyjki macicy. Stanow. Ekspertów PTG I KIDL Ginekol Pol. 2013, 84, 395–399. [Google Scholar]
- Trzeszcz, M.; Mazurec, M.; Jach, R.; Mazurec, K.; Jach, Z.; Kotkowska-Szeps, I.; Kania, M.; Wantuchowicz, M.; Prokopyk, A.; Barcikowski, P.; et al. Liquid-based screening tests results: Hpv, liquid-based cytology, and p16/ki67 dual-staining in private-based opportunistic cervical cancer screening. Diagnostics 2021, 11, 1420. [Google Scholar] [CrossRef]
- Michalek, I.M.; Manczuk, M.; dos Santos, F.L.C.; Macios, A.; Didkowska, J.; Nowakowski, A. Self-reported participation in cervical cancer screening among Polish women in 2004–2019. Ginekol. Polska 2023, 95, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nfz.gov.pl/aktualnosci/aktualnosci-centrali/mammografia-i-cytologia-wazne-zmiany-w-programach-profilaktycznych-na-nfz,8497.html (accessed on 26 November 2024).
- Świderska-Kiec, J.; Czajkowski, K.; Zaręba-Szczudlik, J.; Kacperczyk-Bartnik, J.; Bartnik, P.; Romejko-Wolniewicz, E. Comparison of HPV testing and colposcopy in detecting cervical dysplasia in patients with cytological abnormalities. Vivo 2020, 34, 1307–1315. [Google Scholar] [CrossRef]
- Jach, R.; Mazurec, M.; Nowakowski, A. 110 COLPOSCOPY 2020: POLISH COLPOSCOPIC TERMINOLOGY Based on IFCPC 2011 Nomenclature Summary of the Clinical Experts Consensus Recommendations of the Polish Society of Gynecologists and Obstetricians and the Polish Society of Colposcopy and Cervical Patho. n.d. Available online: https://journals.viamedica.pl/ginekologia_perinatologia_prakt/article/view/68855/55118 (accessed on 5 January 2025).
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines® Insights: Cervical Cancer, Version 1.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Sznurkowski, J.J.; Bodnar, L.; Szylberg, Ł.; Zołciak-Siwinska, A.; Dańska-Bidzińska, A.; Klasa-Mazurkiewicz, D.; Rychlik, A.; Kowalik, A.; Streb, J.; Bidziński, M.; et al. The Polish Society of Gynecological Oncology Guidelines for the Diagnosis and Treatment of Cervical Cancer (v2024.0). J. Clin. Med. 2024, 13, 4351. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. on behalf of the ESMO Guidelines Committee. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Restaino, S.; Pellecchia, G.; Arcieri, M.; Bogani, G.; Taliento, C.; Greco, P.; Driul, L.; Chiantera, V.; Ercoli, A.; Fanfani, F.; et al. Management for Cervical Cancer Patients: A Comparison of the Guidelines from the International Scientific Societies (ESGO-NCCN-ASCO-AIOM-FIGO-BGCS-SEOM-ESMO-JSGO). Cancers 2024, 16, 2541. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023*. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Van Driel, W.; Tidy, J.; Williamson, K.; et al. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N. Engl. J. Med. 2024, 390, 819–829. [Google Scholar] [CrossRef]
- Viveros-Carreño, D.; Pareja, R.; Plante, M. De-escalation of surgical radicality for non-fertility preserving management in patients with early-stage cervical cancer: A systematic review. Int. J. Gynecol. Cancer 2024, 34, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Taliento, C.; Scutiero, G.; Arcieri, M.; Pellecchia, G.; Tius, V.; Bogani, G.; Petrillo, M.; Pavone, M.; Bizzarri, N.; Driul, L.; et al. Simple hysterectomy versus radical hysterectomy in early-stage cervical cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2024, 50, 108252. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.-Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339. [Google Scholar] [CrossRef]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., II; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- McCormack, M.; Eminowicz, G.; Gallardo, D.; Diez, P.; Farrelly, L.; Kent, C.; Hudson, E.; Panades, M.; Mathew, T.; Anand, A.; et al. Induction chemotherapy followed by standard chemoradiotherapy versus standard chemoradiotherapy alone in patients with locally advanced cervical cancer (GCIG INTERLACE): An international, multicentre, randomised phase 3 trial. Lancet 2024, 404, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Gomes, A.J.P.d.S.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Toita, T.; Wu, X.; Limón, J.C.V.; Tarnawski, R.; Mandai, M.; Shapira-Frommer, R.; Mahantshetty, U.; Estevez-Diz, M.d.P.; Zhou, Q.; et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Ang, D.J.M.; Chan, J.J. Evolving standards and future directions for systemic therapies in cervical cancer. J. Gynecol. Oncol. 2024, 35, e65. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Tewari, K.S.; Dubot, C.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Salman, P.; Yañez, E.; Gümüş, M.; et al. First-Line Pembrolizumab + Chemotherapy Versus Placebo + Chemotherapy for Persistent, Recurrent, or Metastatic Cervical Cancer: Final Overall Survival Results of KEYNOTE-826. J. Clin. Oncol. 2023, 41, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Gladieff, L.; Martínez-García, J.; Villacampa, G.; Takekuma, M.; De Giorgi, U.; Lindemann, K.; Woelber, L.; Colombo, N.; Duska, L.; et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): A randomised, open-label, phase 3 trial. Lancet 2023, 403, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Seraj, E.; Zarkavelis, G.; Petrakis, D.; Kollas, A.; Kafantari, A.; Assi, A.; Tatsi, K.; Pavlidis, N.; Pentheroudakis, G. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit. Rev. Oncol. 2016, 108, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Colombo, A.; Milani, R.; Placa, F.; Zanagnolo, V.; Mangioni, C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J. Gynecol. Oncol. 2017, 28, e34. [Google Scholar] [CrossRef]
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.-C.; Hung, C.-F. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Vici, P.; Pizzuti, L.; Mariani, L.; Zampa, G.; Santini, D.; Di Lauro, L.; Gamucci, T.; Natoli, C.; Marchetti, P.; Barba, M.; et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: Hope or reality from clinical studies. Expert Rev. Vaccines 2016, 15, 1327–1336. [Google Scholar] [CrossRef]
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seon, Y.B.; Hong, S.R.; Lee, C.-W.; Kim, S.; Woo, J.-W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Trejo-Cerro, O.; Kaplan, E.; Li, Z.; Albertsboer, F.; El Hammiri, N.; Mariz, F.C.; Banks, L.; Ottonello, S.; et al. A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity. npj Vaccines 2024, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Singini, M.G.; Singh, E.; Bradshaw, D.; Ramaliba, T.; Chen, W.C.; Motlhale, M.; Kamiza, A.B.; de Villiers, C.B.; Muchengeti, M.; Mathew, C.G.; et al. Usefulness of high-risk HPV early oncoprotein (E6 and E7) serological markers in the detection of cervical cancer: A systematic review and meta-analysis. J. Med. Virol. 2022, 95, e27900. [Google Scholar] [CrossRef]
- Downham, L.; Jaafar, I.; Rol, M.L.; Nyaga, V.N.; Valls, J.; Baena, A.; Zhang, L.; Gunter, M.J.; Arbyn, M.; Almonte, M. Accuracy of HPV E6/E7 oncoprotein tests to detect high-grade cervical lesions: A systematic literature review and meta-analysis. Br. J. Cancer 2023, 130, 517–525. [Google Scholar] [CrossRef]
- Cheung, T.H.; Yim, S.F.; Yu, M.Y.; Worley, M.J.; Fiascone, S.J.; Chiu, R.W.; Lo, K.W.; Siu, N.S.; Wong, M.C.; Yeung, A.C.; et al. Liquid biopsy of HPV DNA in cervical cancer. J. Clin. Virol. 2019, 114, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Rungkamoltip, P.; Temisak, S.; Piboonprai, K.; Japrung, D.; Thangsunan, P.; Chanpanitkitchot, S.; Chaowawanit, W.; Chandeying, N.; Tangjitgamol, S.; Iempridee, T. Rapid and ultrasensitive detection of circulating human papillomavirus E7 cell-free DNA as a cervical cancer biomarker. Exp. Biol. Med. 2020, 246, 654–666. [Google Scholar] [CrossRef] [PubMed]
| Brand Name [83] | Valency | Target–HPV Types |
|---|---|---|
| Cervarix | bivalent | 16, 18 |
| Cecolin | bivalent | 16, 18 |
| Walrinvax | bivalent | 16, 18 |
| Gardasil | quadrivalent | 16, 18 |
| Cervavax | quadrivalent | 16, 18, 6, 11 |
| Gardasil9 | nonvalent | 16, 18, 31, 33, 45, 52, 58, 6, 11 |
| Age at the time of commencement of vaccination | 2-valent vaccine | 4-valent vaccine 9-valent vaccine |
| 9–14 years old | 2-dose regimen second dose 5 to 13 months after the first dose | 2-dose regimen second dose 5 to 13 months after the first dose |
| if the second dose of vaccine is administered earlier than 5 months after the first dose, administer the third dose | ||
| 15 years old and older | 3-dose regimen (0, 1, 6 months) | 3-dose regimen (0, 2, 6 months) |
| FIGO Stage | Guidelines | New Options | Additional Information | References |
|---|---|---|---|---|
| FIGO IA | Trachelectomy | [111] | ||
| FIGO IB-IIA | Radical/simple hysterectomy | -laparoscopy or robot-assisted surgery cannot be regarded as the preferred treatment in comparison with open surgery -radiotherapy could be safer than surgery for postmenopausal patients | [111,112,113,114,115,116,117,118] | |
| FIGO IIB-IVA | Platinum-derived chemotherapy and concomitant external radiation therapy followed by brachytherapy | -addition of induction chemotherapy -addition of pembrolizumab to the conventional chemoradiotherapy | -exploration of durvalumab use in patients with high tumoral PD-L1 expression | [109,112,119,120,121,122,123,124,125,126,127,128] |
| FIGO IVB | Palliative chemo/chemoradiotherapy (combination of platinum-based chemotherapy) Second-line therapy | -pembrolizumab combined with chemotherapy (+/−bevacizumab) -addition of atezolizumab to a standard bevacizumab plus platinum regimen | -second-line therapy choice depends on tumor characteristics | [109,112,115,128,129,130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włoszek, E.; Krupa, K.; Skrok, E.; Budzik, M.P.; Deptała, A.; Badowska-Kozakiewicz, A. HPV and Cervical Cancer—Biology, Prevention, and Treatment Updates. Curr. Oncol. 2025, 32, 122. https://doi.org/10.3390/curroncol32030122
Włoszek E, Krupa K, Skrok E, Budzik MP, Deptała A, Badowska-Kozakiewicz A. HPV and Cervical Cancer—Biology, Prevention, and Treatment Updates. Current Oncology. 2025; 32(3):122. https://doi.org/10.3390/curroncol32030122
Chicago/Turabian StyleWłoszek, Emilia, Kamila Krupa, Eliza Skrok, Michał Piotr Budzik, Andrzej Deptała, and Anna Badowska-Kozakiewicz. 2025. "HPV and Cervical Cancer—Biology, Prevention, and Treatment Updates" Current Oncology 32, no. 3: 122. https://doi.org/10.3390/curroncol32030122
APA StyleWłoszek, E., Krupa, K., Skrok, E., Budzik, M. P., Deptała, A., & Badowska-Kozakiewicz, A. (2025). HPV and Cervical Cancer—Biology, Prevention, and Treatment Updates. Current Oncology, 32(3), 122. https://doi.org/10.3390/curroncol32030122






