CD8+CD28+PD1− T Cells as a Prognostic Biomarker in Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Sample Processing
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Demographics
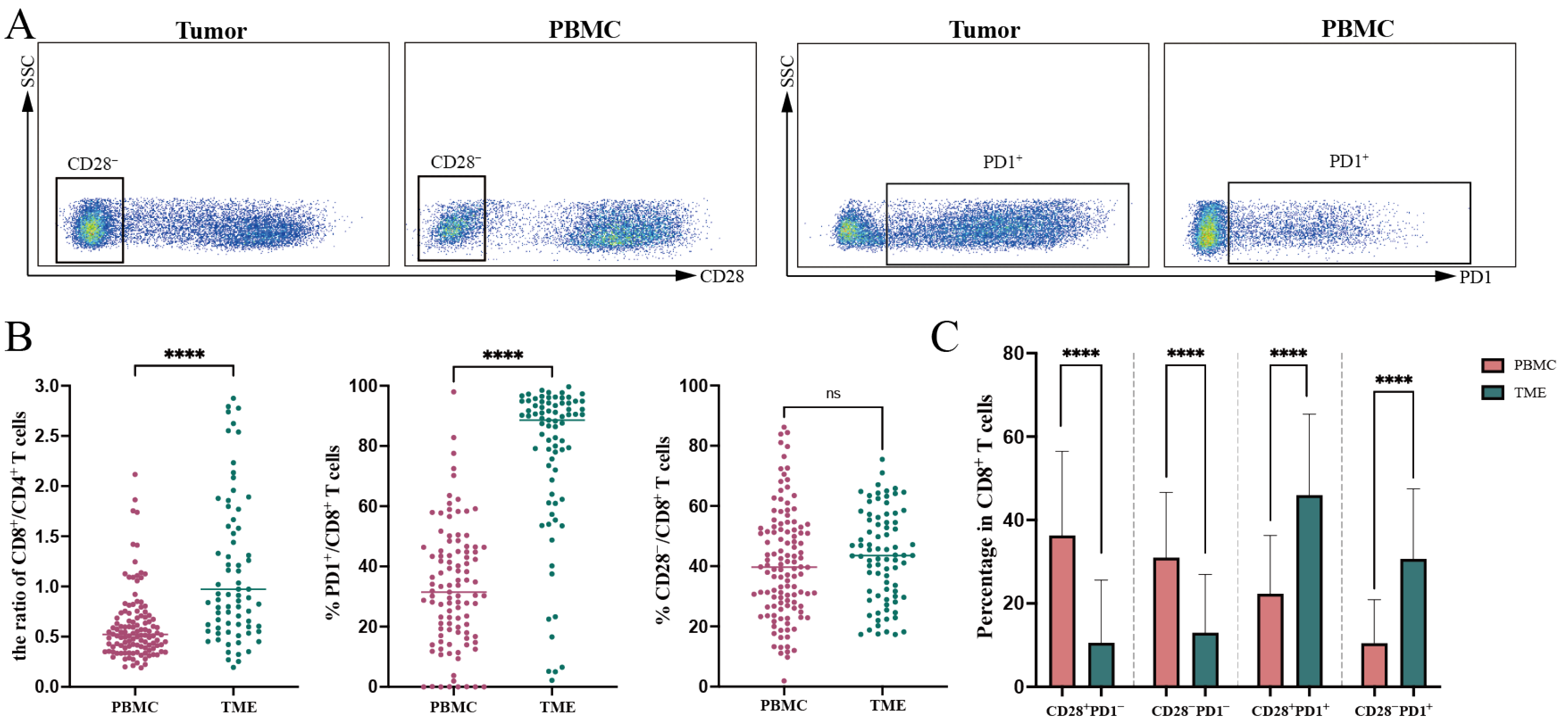
3.2. Expression of CD28 and PD1 on CD8+ T Cells in PBMC and TME
3.3. Correlation Between CD28 and PD1 Expression on CD8+ T Cells in PBMC and Clinical Characteristics
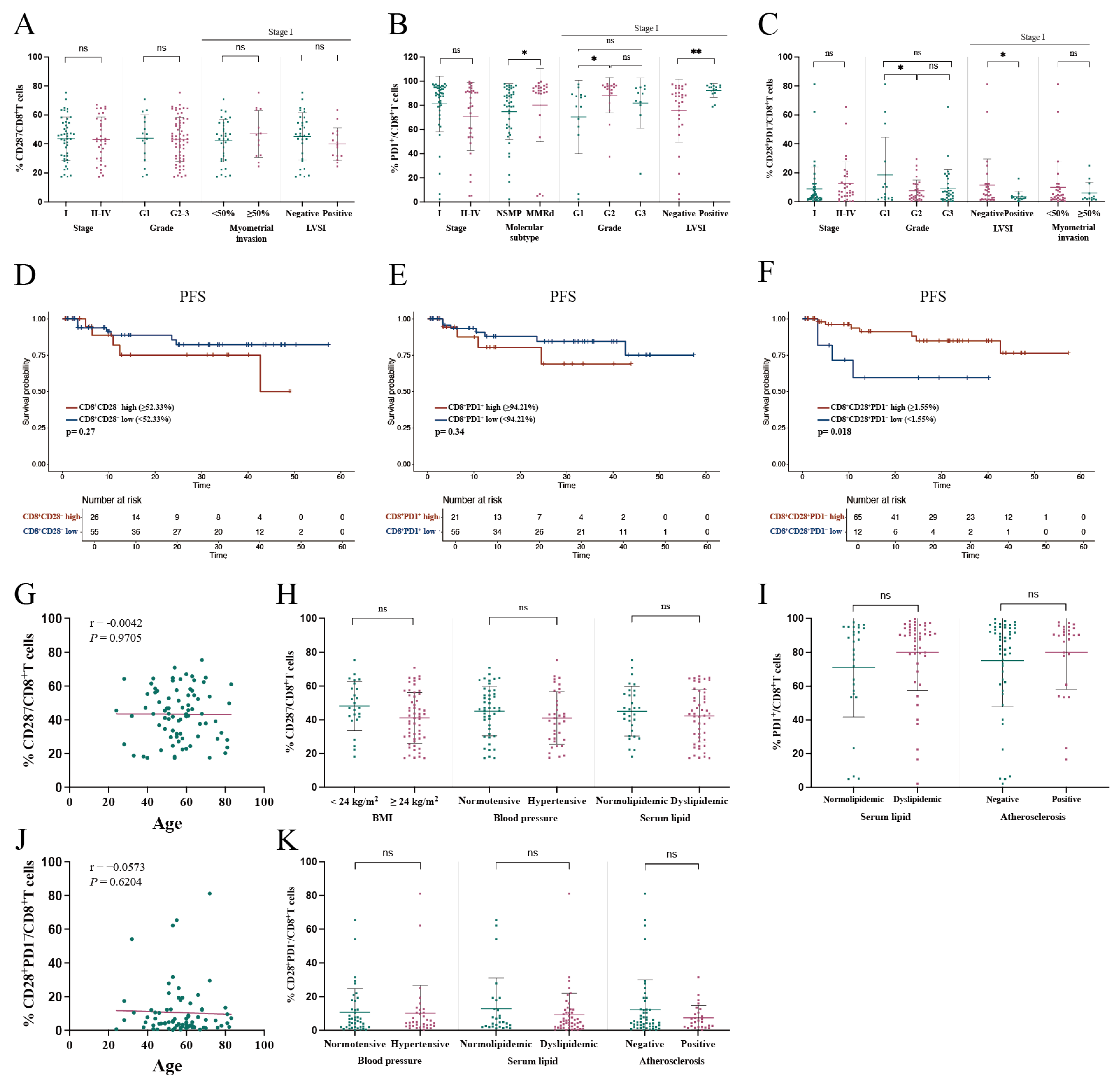
3.3.1. Association of CD28−/CD8+ T Cell Proportion in PBMC with Clinical Characteristics
3.3.2. Association of PD1+/CD8+ T Cell Proportion in PBMC with Clinical Characteristics
3.3.3. Association of CD28+PD1−/CD8+ T Cell Proportion in PBMC with Clinical Characteristics
3.4. Correlation Between CD28 and PD1 Expression on Tumor-Infiltrating CD8+ T Cells and Clinical Characteristics
3.4.1. Lack of Significant Correlation Between Tumor-Infiltrating CD28−/CD8+ T Cell Proportion and Clinical Characteristics
3.4.2. Association of PD1+/CD8+ T Cell Proportion in TME with Poor Clinical Characteristics
3.4.3. Association of CD28+PD1−/CD8+ T Cell Proportion in TME with Clinical Characteristics
3.5. Functional Activity of CD8+ T Cells in TME
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| EC | Endometrial cancer |
| PD1 | Programmed cell death protein 1 |
| LVSI | Lymphovascular space invasion |
| MSI | Microsatellite Instability |
| SASP | Senescence-associated secretory phenotype |
| MELF | Microcystic, elongated, and fragmented pattern |
| BMI | Body mass index |
| PFS | Progression-free survival |
| OS | Overall survival |
| PBMC | Peripheral blood mononuclear cells |
| PMA | Phorbol-12-myristate-13-acetate |
| TME | Tumor microenvironment |
| NSMP | No specific molecular profile |
| Tcm | Central memory T cells |
| Tem | Effector memory T cells |
| Temra | Effector memory T cells re-expressing CD45RA |
References
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, D.; Pranjol, M.Z.I. Tumor-Infiltrated Lymphocytes, Macrophages, and Dendritic Cells in Endometrioid Adenocarcinoma of Corpus Uteri as Potential Prognostic Factors: An Immunohistochemical Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2016, 26, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, L.; Lei, Y.; Li, T.; Li, H.; Cheng, X. Integrated analysis of tumor mutation burden and immune infiltrates in endometrial cancer. Curr. Probl. Cancer 2021, 45, 100660. [Google Scholar] [CrossRef] [PubMed]
- Versluis, M.A.C.; Marchal, S.; Plat, A.; de Bock, G.H.; van Hall, T.; de Bruyn, M.; Hollema, H.; Nijman, H.W. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur. J. Cancer 2017, 86, 285–295. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Nga, H.T.; Nguyen, T.L.; Yi, H.S. T-Cell Senescence in Human Metabolic Diseases. Diabetes Metab. J. 2024, 48, 864–881. [Google Scholar] [CrossRef]
- Vallejo, A.N.; Weyand, C.M.; Goronzy, J.J. T-cell senescence: A culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol. Med. 2004, 10, 119–124. [Google Scholar] [CrossRef]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Zhang, Y.; Chen, Z.; Zhu, W.; Zhang, B.; Wang, Z.; Le, H. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int. Immunopharmacol. 2014, 18, 255–261. [Google Scholar] [CrossRef]
- Kinney, B.L.C.; Brammer, B.; Kansal, V.; Parrish, C.J.; Kissick, H.T.; Liu, Y.; Saba, N.F.; Buchwald, Z.S.; El-Deiry, M.W.; Patel, M.R.; et al. CD28-CD57+ T cells from head and neck cancer patients produce high levels of cytotoxic granules and type II interferon but are not senescent. Oncoimmunology 2024, 13, 2367777. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Li, C.H.; Huang, S.C.; Chang, D.Y.; Chou, L.Y.; Sheu, B.C. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer 2010, 116, 5777–5788. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Si, F.; Bagley, D.; Ma, F.; Zhang, Y.; Tao, Y.; Shaw, E.; Peng, G. Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J. Immunother. Cancer 2022, 10, e005020. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Song, G.; Wang, X.; Jia, J.; Yuan, Y.; Wan, F.; Zhou, X.; Yang, H.; Ren, J.; Gu, J.; Lyerly, H.K. Elevated level of peripheral CD8(+)CD28(-) T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapy. Cancer Immunol. Immunother. CII 2013, 62, 1123–1130. [Google Scholar] [CrossRef]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [CrossRef]
- Liu, J.N.; Kong, X.S.; Huang, T.; Wang, R.; Li, W.; Chen, Q.F. Clinical Implications of Aberrant PD-1 and CTLA4 Expression for Cancer Immunity and Prognosis: A Pan-Cancer Study. Front. Immunol. 2020, 11, 2048. [Google Scholar] [CrossRef]
- Burke, K.P.; Chaudhri, A.; Freeman, G.J.; Sharpe, A.H. The B7:CD28 family and friends: Unraveling coinhibitory interactions. Immunity 2024, 57, 223–244. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, Z.; Hu, Y.; Shang, S.; Wang, R.; Ma, J.; Zhang, Z.; Wu, M.; Wang, F.; Yu, J.; et al. High HPK1+PD-1+TIM-3+CD8+ T cells infiltration predicts poor prognosis to immunotherapy in NSCLC patients. Int. Immunopharmacol. 2024, 127, 111363. [Google Scholar] [CrossRef]
- Romagnoli, G.; D’Alessandris, Q.G.; Capone, I.; Tavilla, A.; Canini, I.; Lapenta, C.; Buccarelli, M.; Giordano, M.; Tirelli, V.; Sanchez, M.; et al. CD8+CD103+PD1+TIM3+ T cells in glioblastoma microenvironment correlate with prognosis. Immunology 2024, 171, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Palermo, B.; Franzese, O.; Frisullo, G.; D’Ambrosio, L.; Panetta, M.; Campo, G.; D’Andrea, D.; Sperduti, I.; De Nicola, F.; Goeman, F.; et al. CD28/PD1 co-expression: Dual impact on CD8+ T cells in peripheral blood and tumor tissue, and its significance in NSCLC patients’ survival and ICB response. J. Exp. Clin. Cancer Res. CR. 2023, 42, 287. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, J.; Turrini, R.; Minasyan, A.; Barras, D.; Crespo, I.; Grimm, A.J.; Casado, J.; Genolet, R.; Benedetti, F.; Wicky, A.; et al. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell 2021, 39, 1623–1642.e20. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Tang, H.; You, T.; Xu, X.; Li, S.; Li, Z.; Liu, Y.; Qiu, W.; Zhou, N.; Li, N.; et al. Peripheral CD8+CD28+ T lymphocytes predict the efficacy and safety of PD-1/PD-L1 inhibitors in cancer patients. Front. Immunol. 2023, 14, 1125876. [Google Scholar] [CrossRef]
- Waite, J.C.; Wang, B.; Haber, L.; Hermann, A.; Ullman, E.; Ye, X.; Dudgeon, D.; Slim, R.; Ajithdoss, D.K.; Godin, S.J.; et al. Tumor-targeted CD28 bispecific antibodies enhance the antitumor efficacy of PD-1 immunotherapy. Sci. Transl. Med. 2020, 12, eaba2325. [Google Scholar] [CrossRef]
- Jia, H.; Guo, J.; Liu, Z.; Chen, P.; Li, Y.; Li, R.; Yang, Y.; Li, X.; Wei, P.; Zhong, J.; et al. High expression of CD28 enhanced the anti-cancer effect of siRNA-PD-1 through prompting the immune response of melanoma-bearing mice. Int. Immunopharmacol. 2022, 105, 108572. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]


| Panel Name | Target Markers |
|---|---|
| Panel 1 | CD4, CD8, CD28, PD1 |
| Panel 2 | PD1, LAG-3, TIM-3, CD45, CD3, CD4, CD8, CCR7, CD45RA, CD28 |
| Panel 3 | CD4, CD8, CD28, PD1 |
| Panel 4 | IFN-γ, Granzyme B, IL-2, TNF-α, Perforin |
| Characteristic | PBMC (n = 120) | Tumor (n = 81) |
|---|---|---|
| Age(years) | 54.6 ± 12.2 | 56.4 ± 12.8 |
| FIGO stage | ||
| I | 89 (74.2%) | 47 (58.0%) |
| II | 6 (5.0%) | 6 (7.4%) |
| III | 19 (15.8%) | 20 (24.7%) |
| IV | 6 (5.0%) | 8 (9.9%) |
| Grade | ||
| G1 | 41 (34.2%) | 16 (19.8%) |
| G2 | 54 (45.0%) | 33 (40.7%) |
| G3 | 25 (20.8%) | 32 (39.5%) |
| Histotype | ||
| Endometrioid | 106 (88.3%) | 71 (87.7%) |
| Non-endometrioid | 14 (11.7%) | 10 (12.3%) |
| Molecular subtype | ||
| POLEmut | 2 (1.7%) | 1 (1.2%) |
| MMRd | 16 (13.3%) | 24 (29.6%) |
| NSMP | 91 (75.8%) | 48 (59.3%) |
| p53abn | 10 (8.3%) | 7 (8.6%) |
| Metabolic disorders | ||
| Hypertension | 45 (37.5%) | 36 (44.4%) |
| Diabetes mellitus | 22 (18.3%) | 21 (25.9%) |
| Atherosclerosis | 31 (25.8%) | 27 (33.3%) |
| Fatty liver | 59 (49.2%) | 33 (40.7%) |
| Hyperlipidemia | 62 (51.7%) | 51 (63.0%) |
| Obesity | 36 (30.0%) | 26 (32.1%) |
| Vital status at last follow-up | ||
| Tumor-Free Survival | 110 (91.7%) | 69 (85.2%) |
| Relapse | 10 (8.3%) | 12 (14.8%) |
| Deceased | 4 (3.3%) | 6 (7.4%) |
| Markers | PBMC (%) | Tumor (%) |
|---|---|---|
| CD8+ | 36.48 ± 12.25 | 51.81 ± 17.61 |
| CD28+/CD8+ | 59.18 ± 18.19 | 56.64 ± 15.18 |
| CD28−/CD8+ | 40.82 ± 18.19 | 43.36 ± 15.18 |
| PD1+/CD8+ | 31.48 (18.01, 45.16) | 88.54 (66.33, 94.25) |
| CD28+PD1+/CD8+ | 21.05 (11.24, 33.35) | 47.74 (33.36, 61.03) |
| CD28−PD1+/CD8+ | 6.78 (3.28, 15.03) | 28.28 (19.33, 42.24) |
| PD1−/CD8+ | 68.57 (54.88, 82.01) | 11.93 (5.76, 31.36) |
| CD28+PD1−/CD8+ | 37.51 (22.57, 49.06) | 4.90 (2.12, 12.26) |
| CD28−PD1−/CD8+ | 28.50 (18.47, 40.33) | 6.92 (3.20, 18.54) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Stage | 3.395 | 2.120–5.438 | <0.001 | 2.148 | 1.227–3.760 | 0.007 |
| Myometrium invasion ≥ 50% | 9.158 | 2.904–28.883 | <0.001 | 10.230 | 2.092–50.024 | 0.004 |
| %CD28+PD1−/CD8+ TILs | 0.245 | 0.069–0.871 | 0.030 | 0.099 | 0.022–0.446 | 0.003 |
| Grade | 4.425 | 1.901–10.304 | <0.001 | 0.576 | ||
| LVSI | 4.945 | 1.748–13.988 | 0.003 | 0.846 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Yang, L.; Zhang, Y.; Guo, H. CD8+CD28+PD1− T Cells as a Prognostic Biomarker in Endometrial Cancer. Curr. Oncol. 2025, 32, 121. https://doi.org/10.3390/curroncol32030121
Nie Y, Yang L, Zhang Y, Guo H. CD8+CD28+PD1− T Cells as a Prognostic Biomarker in Endometrial Cancer. Current Oncology. 2025; 32(3):121. https://doi.org/10.3390/curroncol32030121
Chicago/Turabian StyleNie, Yufei, Lin Yang, Yanan Zhang, and Hongyan Guo. 2025. "CD8+CD28+PD1− T Cells as a Prognostic Biomarker in Endometrial Cancer" Current Oncology 32, no. 3: 121. https://doi.org/10.3390/curroncol32030121
APA StyleNie, Y., Yang, L., Zhang, Y., & Guo, H. (2025). CD8+CD28+PD1− T Cells as a Prognostic Biomarker in Endometrial Cancer. Current Oncology, 32(3), 121. https://doi.org/10.3390/curroncol32030121





