Mitotane-Induced Hypothyroidism and Dyslipidemia in Adrenocortical Carcinoma: Sex Differences and Novel Evidence from a Thyroid Cell Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
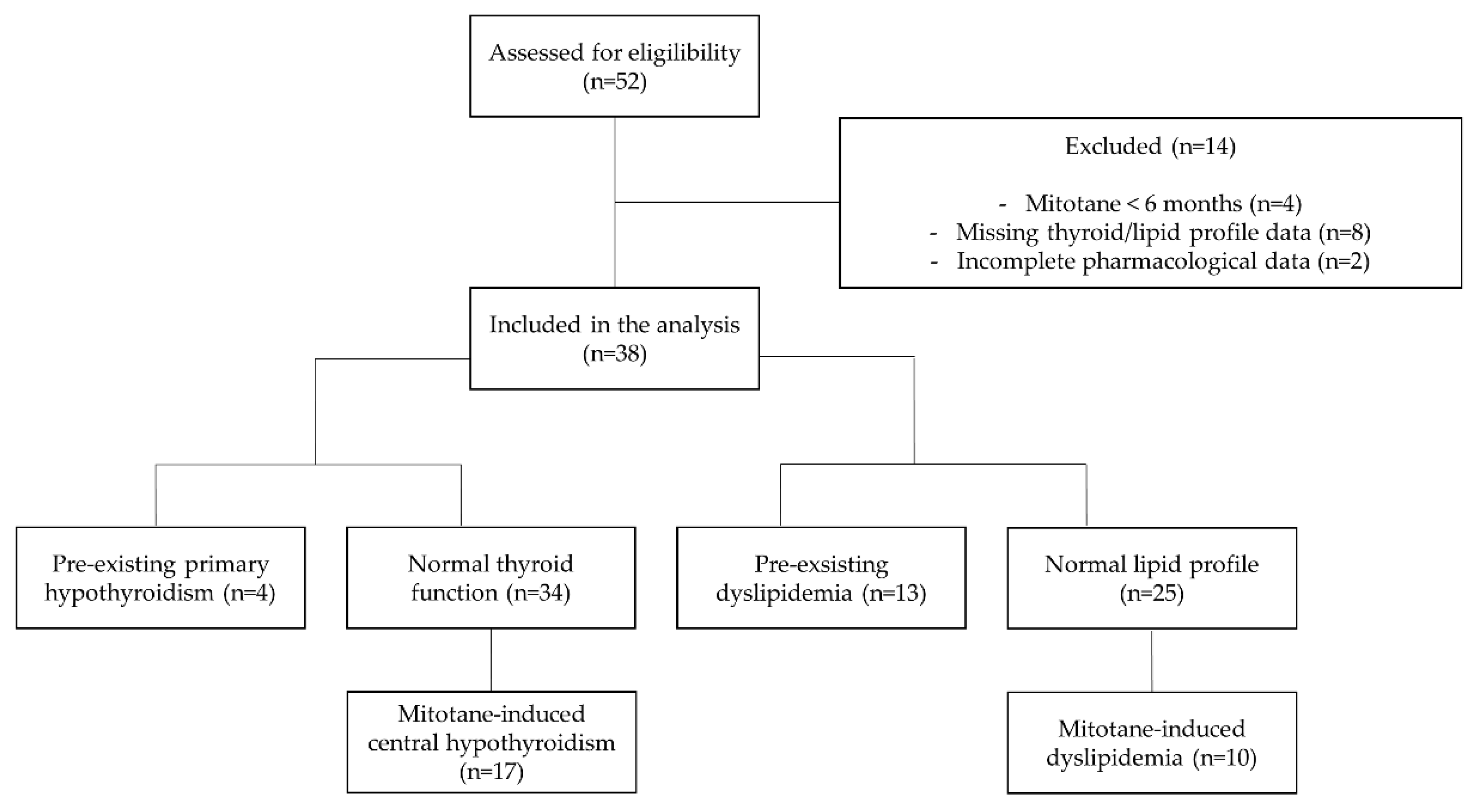
2.1. Study Design
2.2. Patient Selection and Cell-Based Analysis
2.3. Statistical Analysis
3. Results
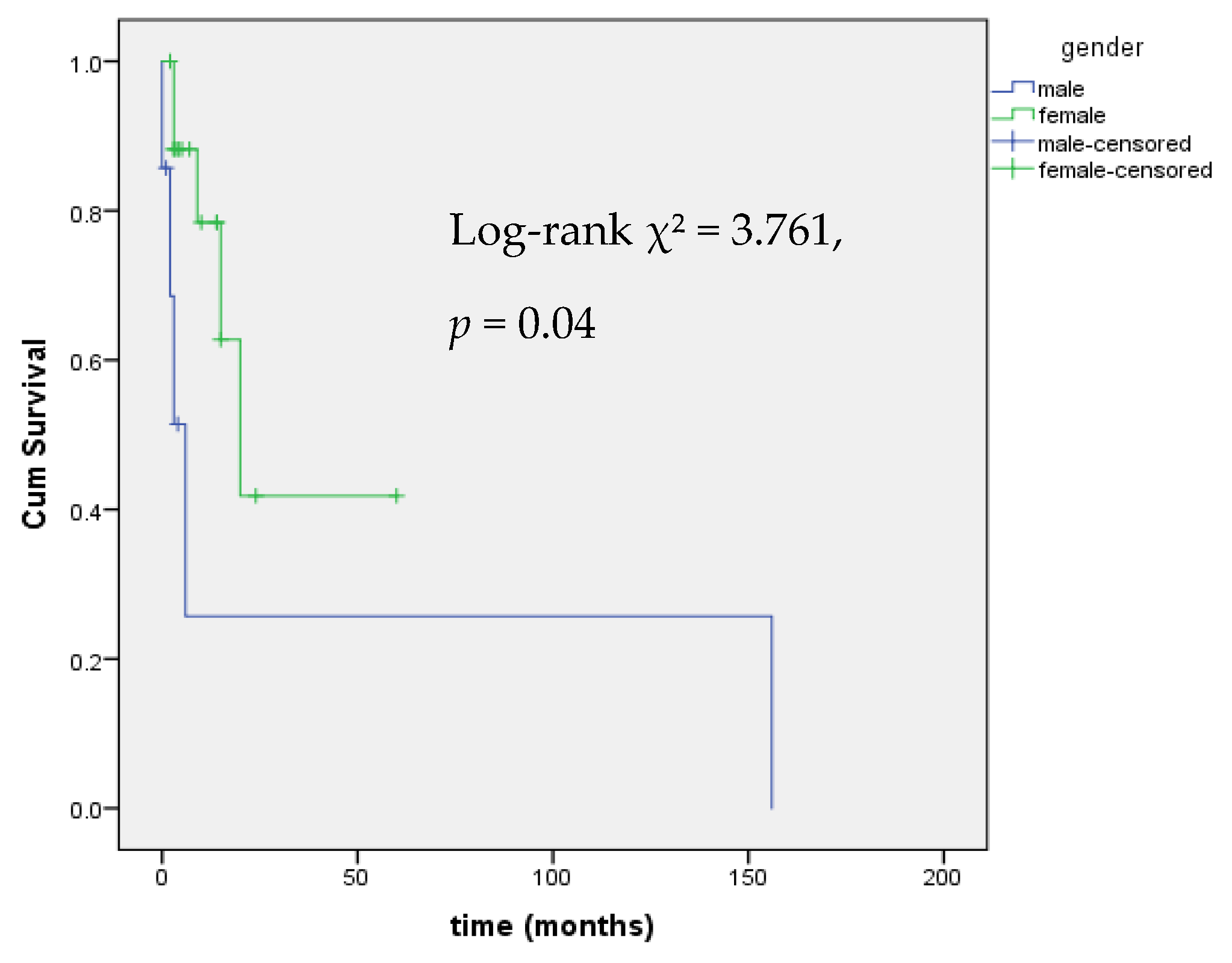
3.1. Central Hypothyroidism
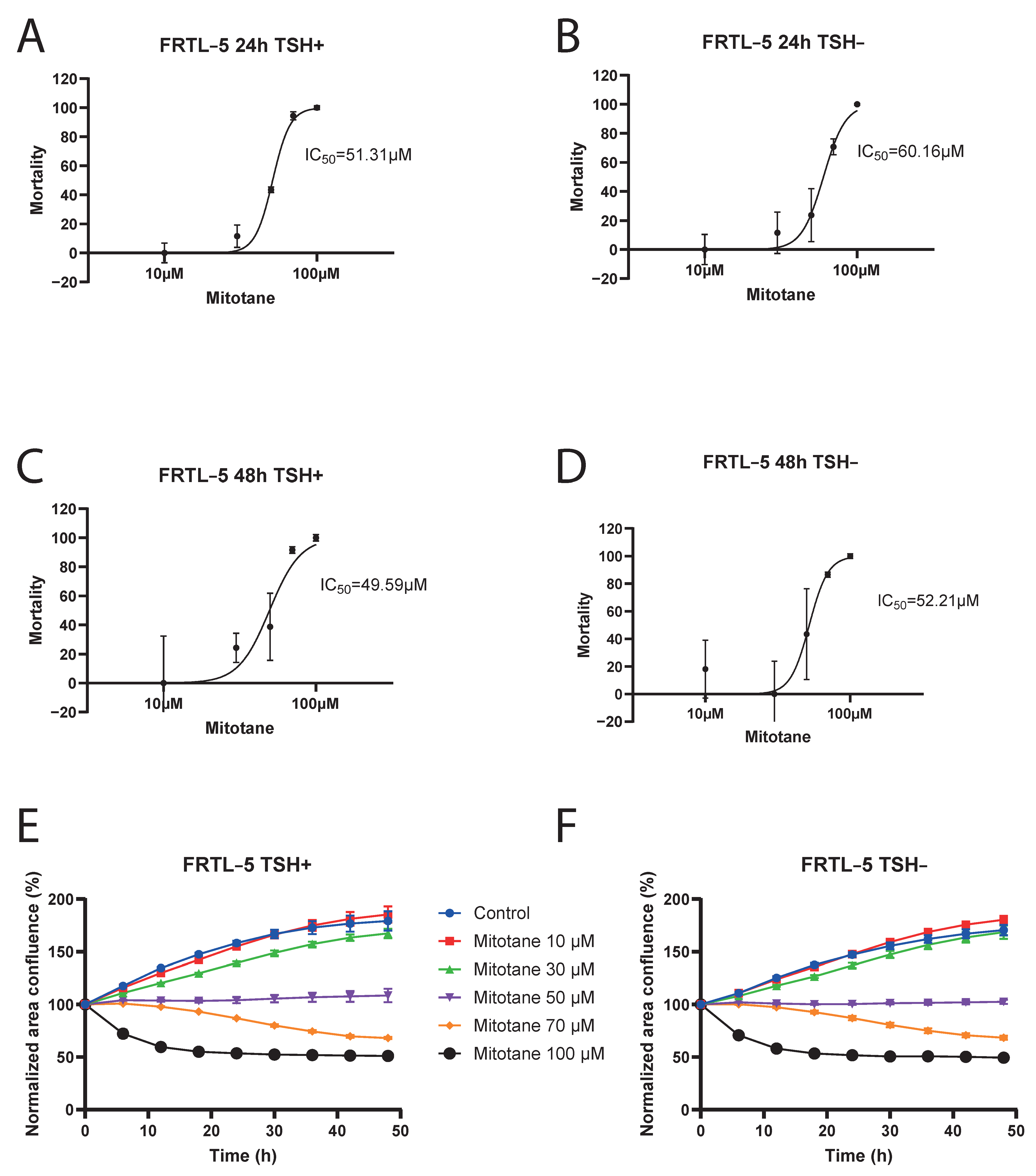
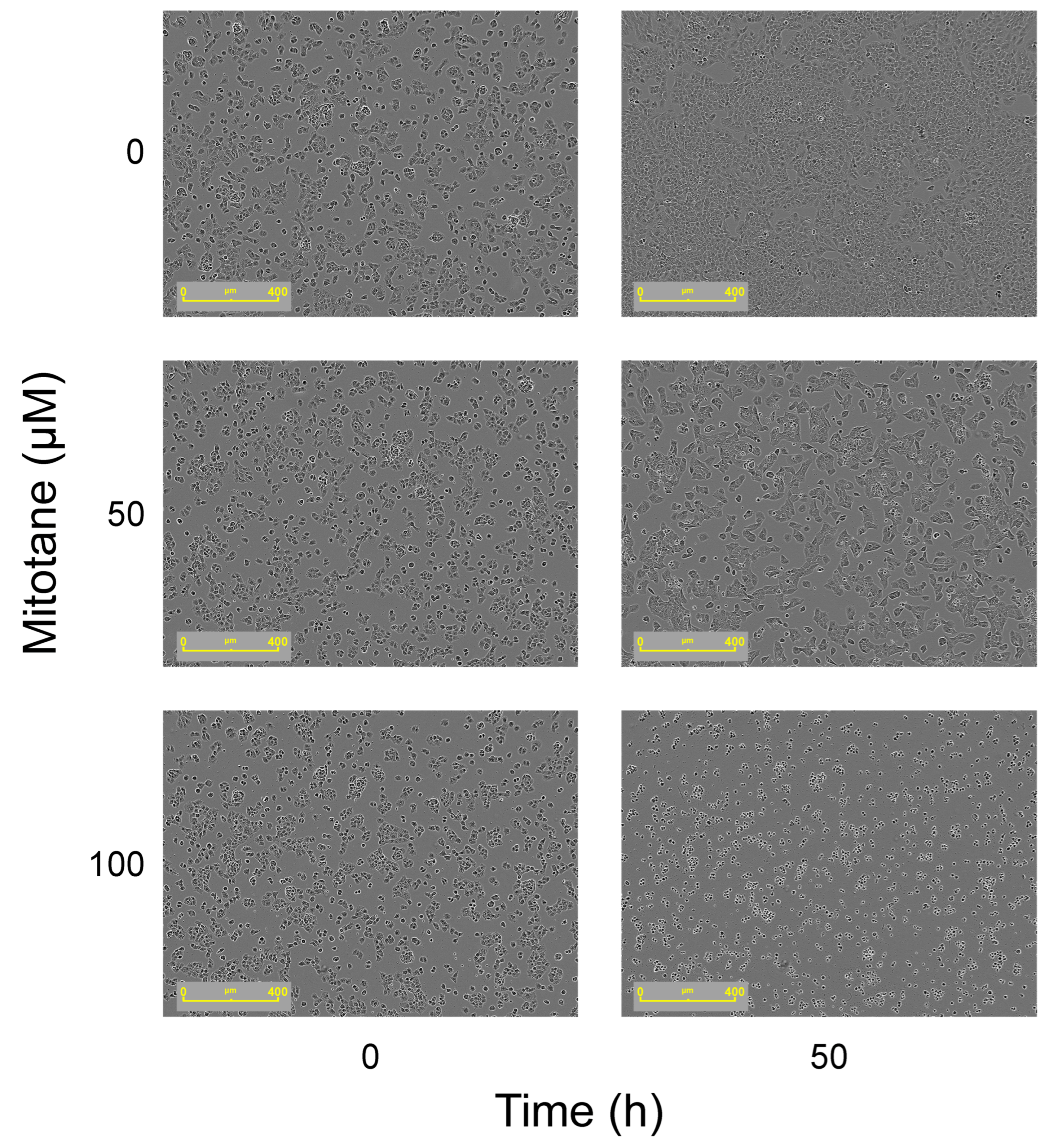
3.2. Effect of Mitotane on FRTL-5 Cells Viability
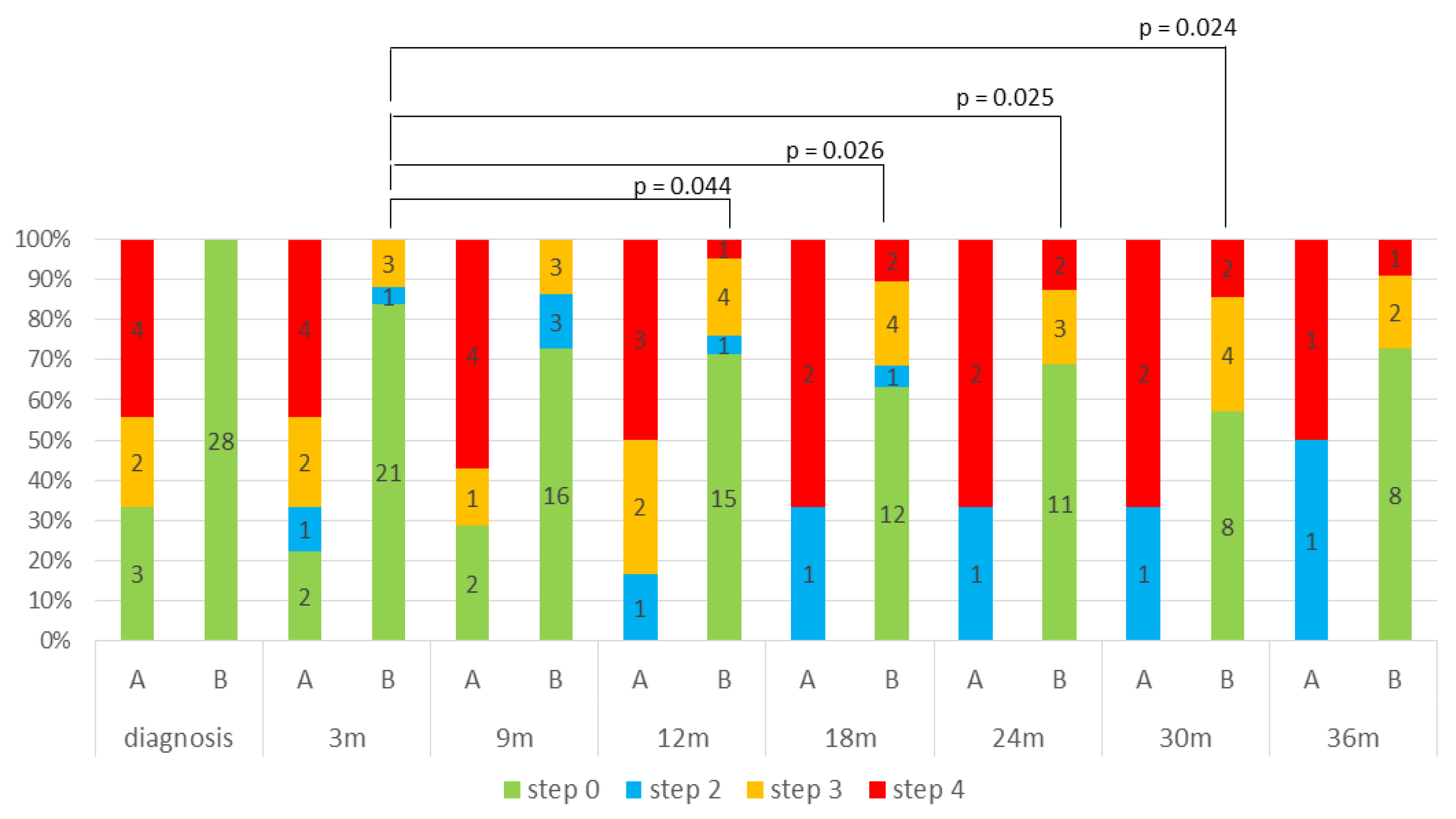
3.3. Dyslipidemia
3.4. Relationship Between Mitotane-Induced Central Hypothyroidism and Dyslipidemia
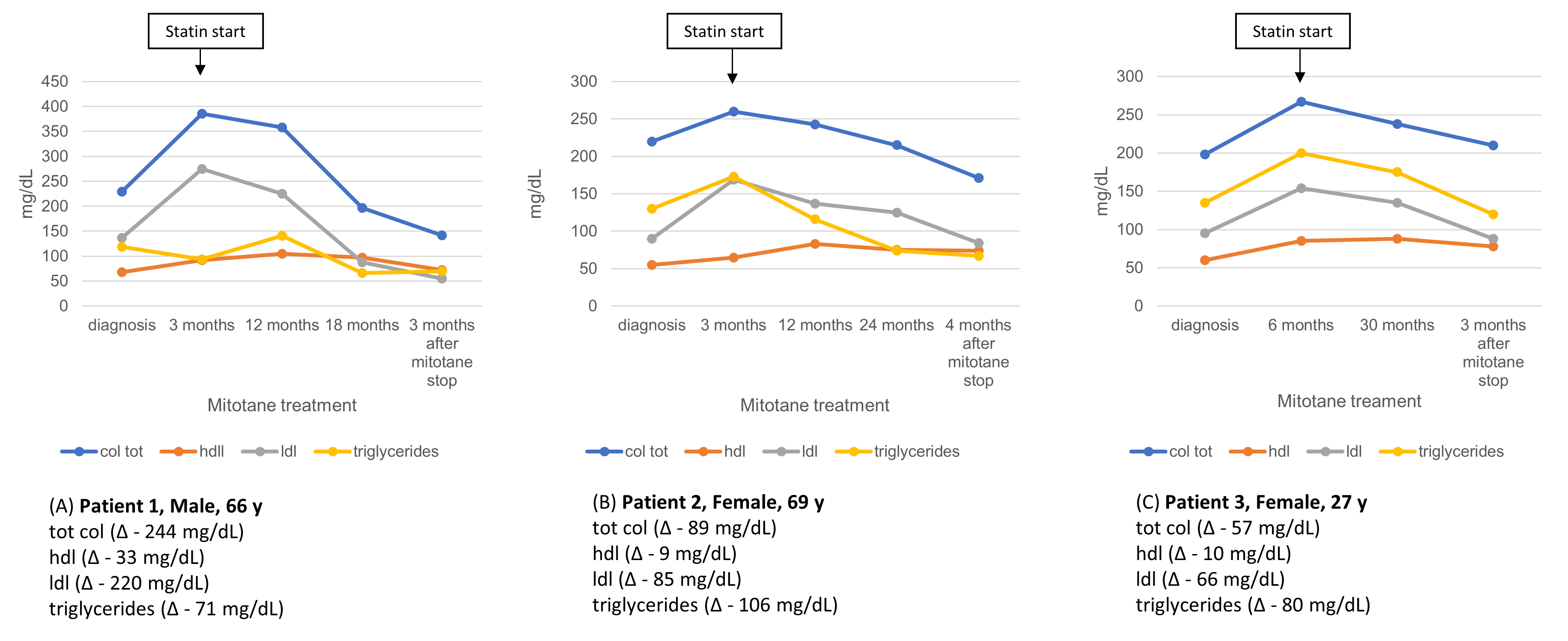
3.5. Lipid Profile Evolution After Mitotane Discontinuation
4. Discussion
4.1. Central Hypothyroidism
4.2. Dyslipidemia
4.3. Relationship Between Mitotane-Induced Central Hypothyroidism and Dyslipidemia
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Step | LDL-c Reduction | Therapy Options |
|---|---|---|
| 0 | - | No therapy |
| 1 | <30% | Bempedoic acid Ezetimibe |
| 2 | 31–40% | Rosuvastatin 5 mg Atorvastatin 10 mg Bempedoic acid + Ezetimibe |
| 3 | 41–50% | Rosuvastatin 10 mg Rosuvastatin 20 mg Atorvastatin 20 mg Atorvastatin 40 mg |
| 4 | >51% | Rosuvastatin 5 mg + Ezetimibe Rosuvastatin 10 mg + Ezetimibe Rosuvastatin 20 mg + Ezetimibe Atorvastatin 10 mg + Ezetimibe Atorvastatin 20 mg + Ezetimibe Atorvastatin 40 mg + Ezetimibe |
References
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Puglisi, S.; Kimpel, O.; Terzolo, M. Adrenocortical carcinoma: A practical guide for clinicians. Lancet Diabetes Endocrinol. 2025, 13, 438–452. [Google Scholar] [CrossRef]
- Else, T.; Williams, A.R.; Sabolch, A.; Jolly, S.; Miller, B.S.; Hammer, G.D. Adjuvant Therapies and Patient and Tumor Characteristics Associated With Survival of Adult Patients With Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 455–461. [Google Scholar] [CrossRef]
- Puglisi, S.; Calabrese, A.; Basile, V.; Ceccato, F.; Scaroni, C.; Altieri, B.; Della Casa, S.; Loli, P.; Pivonello, R.; De Martino, M.C.; et al. Mitotane Concentrations Influence Outcome in Patients with Advanced Adrenocortical Carcinoma. Cancers 2020, 12, 740. [Google Scholar] [CrossRef]
- Basile, V.; Puglisi, S.; Calabrese, A.; Pia, A.; Perotti, P.; Berruti, A.; Reimondo, G.; Terzolo, M. Unwanted Hormonal and Metabolic Effects of Postoperative Adjuvant Mitotane Treatment for Adrenocortical Cancer. Cancers 2020, 12, 2615. [Google Scholar] [CrossRef]
- Daffara, F.; De Francia, S.; Reimondo, G.; Zaggia, B.; Aroasio, E.; Porpiglia, F.; Volante, M.; Termine, A.; Di Carlo, F.; Dogliotti, L.; et al. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr. Relat. Cancer 2008, 15, 1043–1053. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Gentilin, E.; Daffara, F.; Tagliati, F.; Reimondo, G.; Carandina, G.; Ambrosio, M.R.; Terzolo, M.; Degli Uberti, E.C. Therapeutic Concentrations of Mitotane (o,p′-DDD) Inhibit Thyrotroph Cell Viability and TSH Expression and Secretion in a Mouse Cell Line Model. Endocrinology 2010, 151, 2453–2461. [Google Scholar] [CrossRef]
- Vikner, M.E.; Krogh, J.; Daugaard, G.; Andreassen, M. Metabolic and hormonal side effects of mitotane treatment for adrenocortical carcinoma: A retrospective study in 50 Danish patients. Clin. Endocrinol. 2021, 94, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.; Puliani, G.; Chiefari, A.; Mormando, M.; Lauretta, R.; Appetecchia, M. Metabolic and Endocrine Toxicities of Mitotane: A Systematic Review. Cancers 2021, 13, 5001. [Google Scholar] [CrossRef]
- Persani, L.; Brabant, G.; Dattani, M.; Bonomi, M.; Feldt-Rasmussen, U.; Fliers, E.; Gruters, A.; Maiter, D.; Schoenmakers, N.; van Trotsenburg, A.S.P. 2018 European Thyroid Association (ETA) Guidelines on the Diagnosis and Management of Central Hypothyroidism. Eur Thyroid J. 2018, 7, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. Atherosclerosis 2025, 409, 120479. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M.; Santos, R.D.; Corsini, A. Fixed Combination for the Treatment of Dyslipidaemia. Curr. Atheroscler. Rep. 2023, 25, 691–699. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Poirier, J.; Godemel, S.; Mourot, A.; Grunenwald, S.; Olney, H.J.; Le, X.K.; Lacroix, A.; Caron, P.; Bourdeau, I. Central Hypothyroidism is Frequent During Mitotane Therapy in Adrenocortical Cancer Patients: Prevalence and Timeline. J. Clin. Endocrinol. Metab. 2023, 108, 2336–2342. [Google Scholar] [CrossRef]
- Faglia, G.; Bitensky, L.; Pinchera, A.; Ferrari, C.; Paracchi, A.; Beck-Peccoz, P.; Ambrosi, B.; Spada, A. Thyrotropin Secretion in Patients with Central Hypothyroidism: Evidence for Reduced Biological Activity of Immunoreactive Thyrotropin. J. Clin. Endocrinol. Metab. 1979, 48, 989–998. [Google Scholar] [CrossRef]
- Persani LFerretti, E.; Borgato, S.; Faglia, G.; Beck-Peccoz, P. Circulating Thyrotropin Bioactivity in Sporadic Central Hypothyroidism. J. Clin. Endocrinol. Metab. 2000, 85, 3631–3635. [Google Scholar] [PubMed]
- Shawa, H.; Deniz, F.; Bazerbashi, H.; Hernandez, M.; Vassilopoulou-Sellin, R.; Jimenez, C.; Habra, M.A. Mitotane-Induced Hyperlipidemia: A Retrospective Cohort Study. Int. J. Endocrinol. 2013, 2013, 624962. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, D. Update on dyslipidemia in hypothyroidism: The mechanism of dyslipidemia in hypothyroidism. Endocr. Connect. 2022, 11, e210002. [Google Scholar] [CrossRef]
- Del Rivero, J.; Else, T.; Hallanger-Johnson, J.; Kiseljak-Vassiliades, K.; Raj, N.; Reidy-Lagunes, D.; Srinivas, S.; Gilbert, J.; Vaidya, A.; Aboujaoude, E.; et al. A review of mitotane in the management of adrenocortical cancer. Oncologist 2024, 29, 747–760. [Google Scholar] [CrossRef]
- Vodanović, I.D.; Barač Nekić, A.; Šambula, L.; Zibar Tomšić, K.; Dušek, T.; Kaštelan, D. Adverse Events of Adjuvant Mitotane Treatment for Adrenocortical Carcinoma. Endocr. Res. 2025, 50, 50–56. [Google Scholar] [CrossRef] [PubMed]
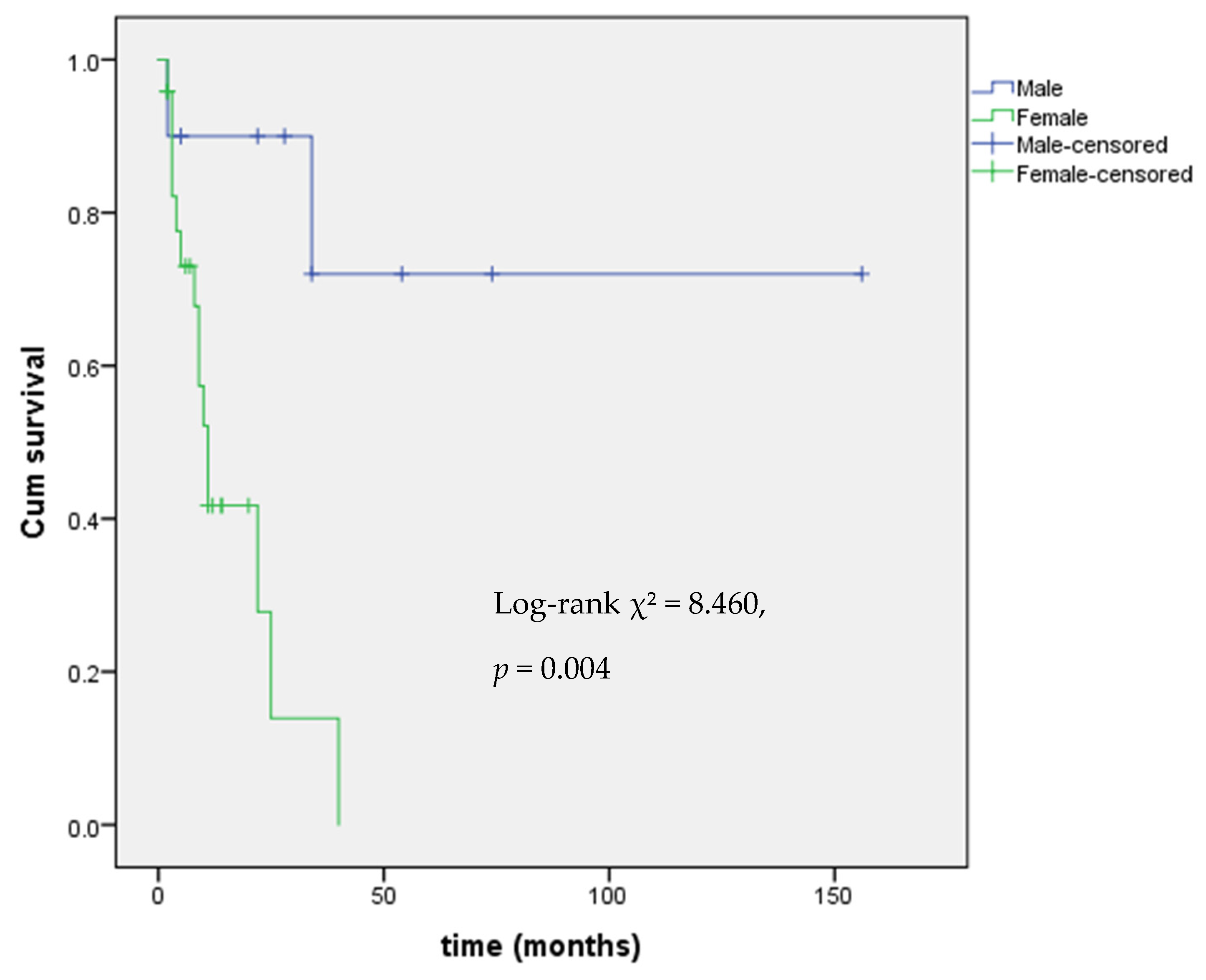
| HR (95% CI) | p Value | |
|---|---|---|
| Sex (F vs. M) | 7.73 (1.63–36.64) | 0.010 |
| Age at diagnosis (years) | 0.99 (0.96–1.03) | 0.657 |
| Hormonal secretion (cortisol/androgen secreting vs. non-secreting) | 2.63 (0.85–8.11) | 0.092 |
| TTR months (%) | 1.02 (1.002–1.043) | 0.034 |
| HR (95% CI) | p Value | |
|---|---|---|
| Sex (F vs. M) | 6.41 (1.36–30.2) | 0.019 |
| TTR months (%) | 1.01 (1.002–1.043) | 0.102 |
| HR (95% CI) | p Value | |
|---|---|---|
| Sex (F vs. M) | 0.16 (0.03–0.93) | 0.042 |
| Age at diagnosis (years) | 0.99 (0.94–1.05) | 0.854 |
| Hormonal secretion (non-secreting vs. cortisol/androgen secreting) | 0.96 (0.22–4.06) | 0.960 |
| TTR months (%) | 0.99 (0.96–1.02) | 0.842 |
| Central hypothyroidism (yes vs. no) | 2.01 (0.75–5.96) | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tizianel, I.; Beber, A.; Madinelli, A.; Caccese, M.; Barollo, S.; Bertazza, L.; Ruggiero, E.; Censi, S.; Mian, C.; Ceccato, F. Mitotane-Induced Hypothyroidism and Dyslipidemia in Adrenocortical Carcinoma: Sex Differences and Novel Evidence from a Thyroid Cell Model. Curr. Oncol. 2025, 32, 700. https://doi.org/10.3390/curroncol32120700
Tizianel I, Beber A, Madinelli A, Caccese M, Barollo S, Bertazza L, Ruggiero E, Censi S, Mian C, Ceccato F. Mitotane-Induced Hypothyroidism and Dyslipidemia in Adrenocortical Carcinoma: Sex Differences and Novel Evidence from a Thyroid Cell Model. Current Oncology. 2025; 32(12):700. https://doi.org/10.3390/curroncol32120700
Chicago/Turabian StyleTizianel, Irene, Arianna Beber, Alberto Madinelli, Mario Caccese, Susi Barollo, Loris Bertazza, Elena Ruggiero, Simona Censi, Caterina Mian, and Filippo Ceccato. 2025. "Mitotane-Induced Hypothyroidism and Dyslipidemia in Adrenocortical Carcinoma: Sex Differences and Novel Evidence from a Thyroid Cell Model" Current Oncology 32, no. 12: 700. https://doi.org/10.3390/curroncol32120700
APA StyleTizianel, I., Beber, A., Madinelli, A., Caccese, M., Barollo, S., Bertazza, L., Ruggiero, E., Censi, S., Mian, C., & Ceccato, F. (2025). Mitotane-Induced Hypothyroidism and Dyslipidemia in Adrenocortical Carcinoma: Sex Differences and Novel Evidence from a Thyroid Cell Model. Current Oncology, 32(12), 700. https://doi.org/10.3390/curroncol32120700







