Parenteral Nutrition in Patients with Incurable Cancer: Exploring the Heterogenous and Non-Randomised Clinical Landscape
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Ethics
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Nutrition-Related Data
3.3. Indication, Administration, and Discontinuation of Parenteral Nutrition
3.3.1. Indications for Parenteral Nutrition
3.3.2. Dosages and Infusion Rate
3.3.3. Pauses and Duration
3.3.4. Complementary Intake
3.3.5. Discontinuation of Parenteral Nutrition
3.4. Disease and Treatment-Related Complications and Benefits of Parenteral Nutrition Treatment
3.5. Survival
4. Discussion
4.1. Main Findings
4.2. Clinical Implications
4.3. Comparison with Previous Studies
4.4. Future Research
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PN | Parenteral nutrition |
| GI | Gastrointestinal |
| RCTs | Randomised controlled trials |
| SD | Standard deviation |
| IQR | Interquartile range |
| ECOG | Eastern Cooperative Oncology Group |
| BMI | Body Mass Index |
| CRP | C-reactive protein |
| REK | Regional Committee for Health and Research Ethics |
| QoL | Quality of life |
| Kcal | Kilocalories |
| ONS | Oral nutrition supplements |
| IV | Intravenous |
References
- Cotogni, P.; Ossola, M.; Passera, R.; Monge, T.; Fadda, M.; De Francesco, A.; Bozzetti, F. Home parenteral nutrition versus artificial hydration in malnourished patients with cancer in palliative care: A prospective, cohort survival study. BMJ Support. Palliat. Care 2022, 12, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.; Todd, C.; Jayson, G.C.; Teubner, A.; Raftery, A.-M.; Sutton, E.J.; Hardy, L.; et al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst. Rev. 2018, 8, CD012812. [Google Scholar] [CrossRef]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Tobberup, R.; Thoresen, L.; Falkmer, U.G.; Yilmaz, M.K.; Solheim, T.S.; Balstad, T.R. Effects of current parenteral nutrition treatment on health-related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: A systematic literature review. Crit. Rev. Oncol./Hematol. 2019, 139, 96–107. [Google Scholar] [CrossRef]
- Bouleuc, C.; Anota, A.; Cornet, C.; Grodard, G.; Thiery-Vuillemin, A.; Dubroeucq, O.; Crétineau, N.; Frasie, V.; Gamblin, V.; Chvetzoff, G.; et al. Impact on Health-Related Quality of Life of Parenteral Nutrition for Patients with Advanced Cancer Cachexia: Results from a Randomized Controlled Trial. Oncologist 2020, 25, e843–e851. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin. Nutr. 2019, 38, 182–190. [Google Scholar] [CrossRef]
- Oh, S.Y.; Jun, H.J.; Park, S.J.; Park, I.K.; Lim, G.J.; Yu, Y.; Cho, S.-J.; Song, A. A randomized phase II study to assess the effectiveness of fluid therapy or intensive nutritional support on survival in patients with advanced cancer who cannot be nourished via enteral route. J. Palliat. Med. 2014, 17, 1266–1270. [Google Scholar] [CrossRef]
- Wallace, S.S.; Barak, G.; Truong, G.; Parker, M.W. Hierarchy of Evidence Within the Medical Literature. Hosp. Pediatr. 2022, 12, 745–750. [Google Scholar] [CrossRef]
- Wilson, B.E.; Booth, C.M. Real-world data: Bridging the gap between clinical trials and practice. eClinicalMedicine 2024, 78, 102915. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Intention-to-treat versus as-treated versus per-protocol approaches to analysis. Korean J. Anesthesiol. 2023, 76, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Vassar, M.; Holzmann, M. The retrospective chart review: Important methodological considerations. J. Educ. Eval. Health Prof. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Worster, A. Advanced statistics: Understanding Medical Record Review (MRR) Studies. Acad. Emerg. Med. 2004, 11, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Santarpia, L.; Alfonsi, L.; Pasanisi, F.; De Caprio, C.; Scalfi, L.; Contaldo, F. Predictive factors of survival in patients with peritoneal carcinomatosis on home parenteral nutrition. Nutrition 2006, 22, 355–360. [Google Scholar] [CrossRef]
- Bozzetti, F.; Santarpia, L.; Pironi, L.; Thul, P.; Klek, S.; Gavazzi, C.; Tinivella, M.; Joly, F.; Jonkers, C.; Baxter, J.; et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: A multi-centre observational study with prospective follow-up of 414 patients. Ann. Oncol. 2014, 25, 487–493. [Google Scholar] [CrossRef]
- Bozzetti, F.; Cotogni, P.; Lo Vullo, S.; Pironi, L.; Giardiello, D.; Mariani, L. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann. Oncol. 2015, 26, 2335–2340. [Google Scholar] [CrossRef]
- Cotogni, P.; Shaw, C.; Jimenez-Fonseca, P.; Partridge, D.; Pritchett, D.; Webb, N.; Crompton, A.; Garcia-Lorda, P.; Shepelev, J. High-protein home parenteral nutrition in malnourished oncology patients: A systematic literature review. Support. Care Cancer 2023, 32, 52. [Google Scholar] [CrossRef]
- Goodrose-Flores, C.; Schedin, A.; Nelander, J.; Almerud, A.; Trolle-Lagerros, Y.; Bonn, S.; Björkhem-Bergman, L. High-protein compared with standard parenteral nutrition in palliative cancer care. BMJ Support. Palliat. Care 2022, 12, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Argüeso, M.; Gómez-Bayona, E.; Ugalde, B.; Vega-Piñero, B.; Gil-Díaz, M.; Longo, F.; Pintor, R.; Botella-Carretero, J.I. Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study. Nutrients 2024, 16, 457. [Google Scholar] [CrossRef] [PubMed]
- Obling, S.R.; Wilson, B.V.; Kjeldsen, J. Home parenteral support in patients with incurable cancer. Patient characteristics of importance for catheter related complications and overall survival. Clin. Nutr. ESPEN 2018, 28, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Dahlk, S.; Popiel, B.; Lammersfeld, C.A.; Ireton-Jones, C.; Gupta, D. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014, 15, 593. [Google Scholar]
- Ruggeri, E.; Giannantonio, M.; Agostini, F.; Ostan, R.; Pironi, L.; Pannuti, R. Home artificial nutrition in palliative care cancer patients: Impact on survival and performance status. Clin. Nutr. 2020, 39, 3346–3353. [Google Scholar] [CrossRef]
- Ruggeri, E.; Giannantonio, M.; Ostan, R.; Agostini, F.; Sasdelli, A.S.; Valeriani, L.; Pironi, L.; Pannuti, R. Choice of access route for artificial nutrition in cancer patients: 30 y of activity in a home palliative care setting. Nutrition 2021, 90, 111264. [Google Scholar] [CrossRef]
- Cotogni, P.; De Carli, L. Near-Death Quality of Life in Cancer Patients on Home Parenteral Nutrition. Nutrients 2025, 17, 271. [Google Scholar] [CrossRef]
- Rabben, J.; Vivat, B.; Fossum, M.; Rohde, G. Shared decision-making in palliative cancer care: A systematic review and meta-synthesis. Palliat. Med. 2024, 38, 406–422. [Google Scholar] [CrossRef]
- Omerovic, E.; Petrie, M.; Redfors, B.; Fremes, S.; Murphy, G.; Marquis-Gravel, G.; Lansky, A.; Velazquez, E.; Perera, D.; Reid, C.; et al. Pragmatic randomized controlled trials: Strengthening the concept through a robust international collaborative network: PRIME-9-Pragmatic Research and Innovation through Multinational Experimentation. Trials 2024, 25, 80. [Google Scholar] [CrossRef]
- Orsso, C.E.; Ford, K.L.; Kiss, N.; Trujillo, E.B.; Spees, C.K.; Hamilton-Reeves, J.M.; Prado, C.M. Optimizing clinical nutrition research: The role of adaptive and pragmatic trials. Eur. J. Clin. Nutr. 2023, 77, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Missing data. BMJ 2007, 334, 424. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Lin, N.; Zhou, Y.; He, W.; Liu, J.; Ma, X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front. Immunol. 2021, 12, 800879. [Google Scholar] [CrossRef]

| Descriptive Characteristics | n = 507, n (%) | Mean (SD) or Median (IQR) |
|---|---|---|
| Age, years, mean (SD) | 65 (13) | |
| Sex, females | 270 (53%) | |
| Time since diagnosis, months, mean (SD) | 507 (100%) | 11 (3–24) |
| BMI, kg/m2, mean (SD) 1 | 389 (77%) | 21.4 (4.2) |
| Height, cm, mean (SD) | 464 (92%) | 171.6 (8.9) |
| Weight, kg, mean (SD) | 400 (79%) | 63.1 (13.9) |
| Weight loss, % 2 | ||
| 2 weeks–1 month, mean (SD) | 146 (29%) | 6.5 (4.0) |
| 2–3 months, mean (SD) | 169 (33%) | 10.6 (6.0) |
| 4–6 months, mean (SD) | 173 (34%) | 12.6 (6.7) |
| Albumin, g/L, mean (SD) | 438 (86%) | 31.9 (6.8) |
| CRP, mg/L, median (IQR) | 487 (96%) | 52 (17–107) |
| ECOG (0–4) 3 | 230 (45%) | |
| 0 | 5 (<1%) | |
| 1 | 40 (8%) | |
| 2 | 88 (17%) | |
| 3 | 77 (15%) | |
| 4 | 20 (4%) | |
| Cancer diagnosis | 507 (100%) | |
| Upper gastrointestinal tract 4 | 188 (37%) | |
| Colorectal | 109 (21%) | |
| Gynaecological | 69 (14%) | |
| Lymphoma | 22 (4%) | |
| Lung | 21 (4%) | |
| Head and neck | 16 (3%) | |
| Breast | 15 (3%) | |
| Prostate | 9 (2%) | |
| Other 5 | 62 (12%) | |
| Metastasis | ||
| Metastatic cancer (yes) | 426 (84%) | |
| Current anticancer treatment 6,7 | 506 (100%) | |
| Systemic therapy 8 | 148 (29%) | |
| Radiotherapy | 18 (4%) | |
| None | 340 (67%) | |
| Survival | ||
| Survival from start of PN, days, median (IQR) | 507 (100%) | 70 (33–153) |
| Survival after discontinuation of PN, days, median (IQR) | 507 (100%) | 7 (1–40) |
| Main provision of care 9 | ||
| Hospital/palliative care unit | 332 (66%) | |
| Home | 151 (30%) | |
| Other | 24 (4%) |
| Variable | n = 507, n (%) | Median (Range) |
|---|---|---|
| Indication for start of parenteral nutrition 1 | ||
| Insufficient oral intake or tube feeding | 378 (75%) | |
| Gastrointestinal malfunction 2 | 237 (47%) | |
| Promote tolerance for anticancer treatment | 43 (8%) | |
| Patient wish | 20 (4%) | |
| Other | 119 (23%) | |
| Parenteral nutrition treatment | ||
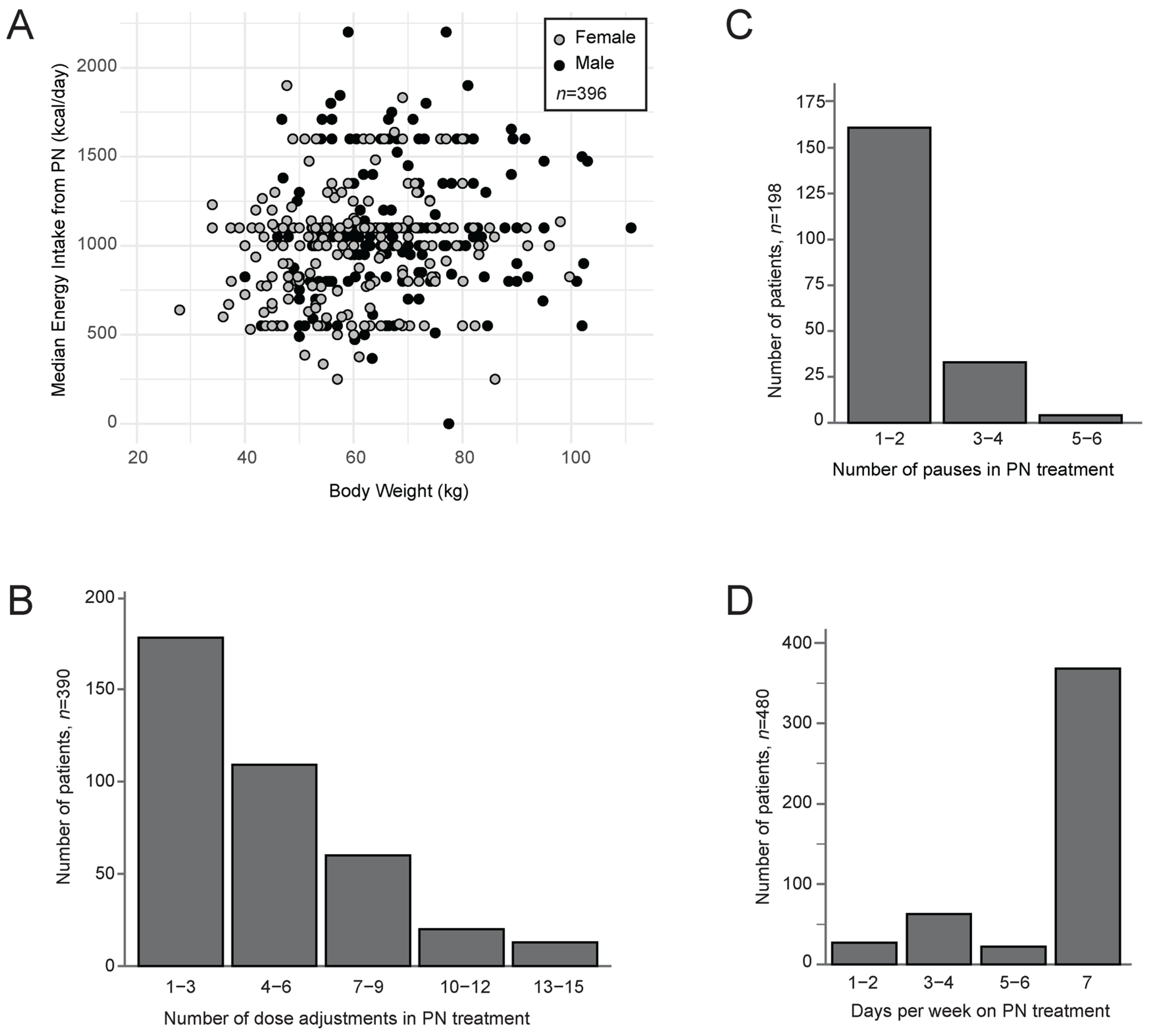
| Starting dose (kcal/day) | 493 (97%) | 1000 (200–2200) |
| Starting infusion rate, mL/h | 131 (26%) | 75 (25–150) |
| Median dose (kcal/day) | 497 (98%) | 1050 (0–2200) |
| Max dose (kcal/day) | 497 (98%) | 1600 (1100–2900) |
| Pauses in treatment (yes) 3 | 208 (41%) | |
| Duration of pauses, days 3 | 2 (1–5) 4 | |
| Duration of treatment (days) | 507 (100%) | 34 (13–84) 4 |
| Complementary energy intake | ||
| Complementary oral/tube feeding/IV energy intake | 475 (94%) | |
| Food intake | 392 (78%) | |
| Caloric liquids | 243 (48%) | |
| Oral nutritional supplements | 240 (47%) | |
| Tube feeding | 32 (6%) | |
| IV glucose | 113 (22%) | |
| No oral/tube feeding or IV intake | 32 (6%) | |
| Reasons for discontinuation 5 | ||
| Patient is terminal | 238 (47%) | |
| Complications related to PN treatment | 87 (17%) | |
| Transition to oral nutritional intake | 86 (17%) | |
| No perceived benefits | 57 (11%) | |
| Transition to tube feeding | 29 (6%) | |
| Recovery of gastrointestinal tract functions | 29 (6%) | |
| Patient wish due to burden of PN treatment | 23 (5%) | |
| Other 6 | 19 (4%) | |
| Unknown | 32 (6%) |
| n = 507, n (%) | |
|---|---|
| Reported complications during PN 1 | |
| Nausea | 262 (52%) |
| Vomiting | 235 (46%) |
| Oedema | 187 (37%) |
| Dyspnoea | 154 (30%) |
| Ascites | 142 (28%) |
| Infections 2 | 122 (24%) |
| Diarrhoea | 109 (22%) |
| Elevated liver enzymes | 108 (21%) |
| Tachycardia | 61 (12%) |
| Feeling cold | 58 (12%) |
| Hypotension | 42 (8%) |
| Dizziness | 31 (6%) |
| Sepsis 2 | 29 (6%) |
| Feeling warm | 14 (3%) |
| Hypertension | 17 (3%) |
| Headache | 7 (1%) |
| Thrombophlebitis | 5 (1%) |
| No complications reported | 74 (15%) |
| Other 3 | 233 (46%) |
| Consequences or reactions to complications 4 | |
| Pause in PN treatment | 54 (10%) |
| Reduction in PN infusion or dose | 34 (7%) |
| Termination of PN | 22 (4%) |
| Diuretic drugs | 56 (11%) |
| Drainage of accumulated liquid | 70 (14%) |
| Other medical interventions 5 | 29 (6%) |
| None | 235 (46%) |
| Positive observations reported in relation to PN 6 | |
| Increased wellbeing | 76 (15%) |
| Reduced stress related to food intake | 16 (3%) |
| Increased hope | 3 (<1%) |
| Other 7 | 41 (8%) |
| Not reported 8 | 380 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erichsen, M.; Solheim, T.S.; Ottestad, I.; Paur, I.; Sande, R.F.; Nygaard, A.; Markhus, E.H.; Thoresen, L.; Thronæs, M.; Tangvik, R.J.; et al. Parenteral Nutrition in Patients with Incurable Cancer: Exploring the Heterogenous and Non-Randomised Clinical Landscape. Curr. Oncol. 2025, 32, 644. https://doi.org/10.3390/curroncol32110644
Erichsen M, Solheim TS, Ottestad I, Paur I, Sande RF, Nygaard A, Markhus EH, Thoresen L, Thronæs M, Tangvik RJ, et al. Parenteral Nutrition in Patients with Incurable Cancer: Exploring the Heterogenous and Non-Randomised Clinical Landscape. Current Oncology. 2025; 32(11):644. https://doi.org/10.3390/curroncol32110644
Chicago/Turabian StyleErichsen, Marianne, Tora S. Solheim, Inger Ottestad, Ingvild Paur, Rikka F. Sande, Astrid Nygaard, Emilie H. Markhus, Lene Thoresen, Morten Thronæs, Randi J. Tangvik, and et al. 2025. "Parenteral Nutrition in Patients with Incurable Cancer: Exploring the Heterogenous and Non-Randomised Clinical Landscape" Current Oncology 32, no. 11: 644. https://doi.org/10.3390/curroncol32110644
APA StyleErichsen, M., Solheim, T. S., Ottestad, I., Paur, I., Sande, R. F., Nygaard, A., Markhus, E. H., Thoresen, L., Thronæs, M., Tangvik, R. J., Sygnestveit, K., Hansson, P., Vestnor, C., Jakobsen, G., Paulsen, Ø., Løhre, E. T., & Balstad, T. R. (2025). Parenteral Nutrition in Patients with Incurable Cancer: Exploring the Heterogenous and Non-Randomised Clinical Landscape. Current Oncology, 32(11), 644. https://doi.org/10.3390/curroncol32110644





