Simple Summary
Breast cancer is the most common cancer among women. Many patients experience symptoms that affect their ability to function, including feeling tired, having low energy, experiencing anxiety, and an overall sense of not feeling well. While some women report only mild discomfort, many have symptoms that are moderate to severe and may require medical attention. In our study, we asked women who had a range of stages of breast cancer to report their symptoms and daily functioning. We found that about 7 out of 10 had at least one symptom that was strong enough to need extra care. Importantly, symptoms were common for both women with advanced breast cancer and for those with earlier-stage disease, who were expected to be cured. These findings show how important it is for doctors and nurses to ask patients directly about their symptoms during clinic visits. Regular symptom checks can help healthcare teams identify who needs more support, provide timely treatment, and improve quality of life for women living with breast cancer.
Abstract
Background: Symptom burden and functional impairment are common in women with breast cancer, yet their prevalence and clinical significance across the disease spectrum remain underexplored. We sought to describe symptom burden and performance status using patient-reported outcome measures and to identify patient characteristics associated with symptoms requiring clinical intervention. Methods: In this cross-sectional study, women with stage I–IV breast cancer completed the Edmonton Symptom Assessment System (ESAS) and the Patient-Reported Functional Status tool. We assessed the prevalence and severity of symptoms and calculated summary distress scores. Multivariable logistic regression was used to identify patient characteristics associated with clinically significant symptoms (ESAS ≥ 4). Results: Among 381 women (mean age 56.8 years; 27% metastatic; 72% with no comorbidities), 70% reported at least one moderate to severe symptom. The most common were tiredness (31%), lack of well-being (30%), and anxiety (21%). Mean summary distress scores were low overall. Most patients reported functional status scores of 0 or 1, and 43% of those with scores ≥2 had metastatic disease. Compared with metastatic patients, women within the first year after diagnosis were less likely to report a symptom requiring intervention (OR 0.49, 95% CI 0.24–0.90). Conclusions: Clinically significant symptoms are common among women with breast cancer, including those with potentially curable disease. Threshold-based use of ESAS, rather than reliance on mean scores, provides a more accurate assessment of patient needs. These findings support the routine integration of patient-reported outcomes into oncology care and underscore the importance of targeted multidisciplinary interventions.
1. Introduction
Breast cancer is the most frequently diagnosed malignancy among women across the world and, in high-income countries, the second leading cause of cancer-related mortality [1]. Breast cancer survival has improved significantly in recent decades, as a result of screening programmes and therapeutic strategies. For example, in Canada, the five-year net survival for breast cancer in women is 89%, ranging from nearly 100% for women with stage I disease to 23% in women with stage IV disease [2]. With increasing survival has come the need for a better understanding of the frequency and impact of symptoms that women experience– due to both disease sequelae and treatment [3,4,5,6]. These symptoms, which may be common, can fluctuate over time and substantially impair functional capacity [7]. For example, depression has been reported in up to 66% of breast cancer survivors, with risk peaking one year after diagnosis [8]. Symptom clusters, particularly those encompassing emotional distress, can adversely affect functional status and quality of life [9,10,11]. Accurate identification of symptom burden is necessary for the development of tailored management strategies.
Patient-reported outcomes (PROs) are direct accounts from patients about their health status; patient-reported outcome measures (PROMs) are validated instruments designed for the systematic collection of such data [12]. The Edmonton Symptom Assessment System (ESAS) is a PROM that measures common cancer-related symptoms including pain, fatigue, nausea, depression, and anxiety [13,14]. Patients with high physical and total symptom scores, as assessed by ESAS, have shorter survival [15]. In Ontario, routine collection of ESAS at cancer centres was initiated in 2007. Results are immediately available to clinical teams for potential discussion during outpatient visits. Another PROM, the Patient-Reported Functional Status tool, has also been incorporated into routine assessments since 2013 [16]. Approximately 60% of eligible breast cancer patients routinely complete these symptom questionnaires during clinic visits [17].
Routine use of PROMs has been associated with improved symptom identification and monitoring, enhanced communication between patients and their care team, better quality of life, and, in some studies, improved survival [18,19,20,21,22,23,24]. Nevertheless, few studies have reported ESAS outcomes in breast cancer populations [25,26]. To our knowledge, only one previous study has used ESAS to characterize the symptom burden of breast cancer patients across all stages and with a range of times since diagnosis [27].
As part of a broader investigation into quality of life among women with a history of breast cancer, we aimed to both describe and interpret ESAS scores across the disease spectrum. Our interpretation is based on applying clinically significant thresholds to ESAS scores, specifically, levels that may warrant clinical intervention and are therefore a clinically meaningful assessment of patients’ needs. In addition, we measured patient-reported functional status to characterize functional impact in parallel with symptom burden. To our knowledge, this is the first study to use this approach across the full disease spectrum, offering novel insights to guide supportive care strategies and inform future clinical practice.
2. Materials and Methods
2.1. Research Design and Procedures
We conducted a cross-sectional study with a convenience sample of women with invasive breast cancer who were recruited during outpatient visits between November 2016 and June 2017. Participants completed ESAS and the Patient-Reported Functional Status tool, either in waiting room kiosks or on electronic tablets (iPads®, Apple Inc., Cupertino, CA, USA). Clinical and demographic data were obtained from patient self-report and supplemented by chart review. The study was reported in accordance with the STROBE guidelines (Table S1) [28].
2.2. Setting and Participants
The study was conducted at two tertiary cancer centres in Toronto, Canada: the Louise Termerty Breast Cancer Centre and the Princess Margaret Cancer Centre. Eligible participants were women aged ≥18 years with a histologically confirmed diagnosis of invasive breast cancer (stage I to IV), who were undergoing treatment or active surveillance at the time of recruitment and were able to read and complete the questionnaires in English. Women were excluded if they could not provide informed consent or complete the questionnaires independently. Each participant was enrolled only once. Patients were recruited consecutively during the study period.
2.3. Symptom and Functional Status Assessment
ESAS is a validated self-reported instrument that measures nine common cancer-related symptoms: pain, tiredness, drowsiness, nausea, lack of appetite, shortness of breath, depression, anxiety, and overall well-being [13,14]. It requires approximately 3 min to complete [17]. Each symptom is scored from 0 (no symptoms) to 10 (worst possible symptom). For clinical interpretation, scores can be categorized as none (0), mild (1–3), moderate (4–6) and severe (7–10) [29,30,31]. Moderate to severe scores (≥4) are considered clinically significant, generally warranting intervention such as a prescription, additional investigation, or referral to a specialist [29,31,32].
In addition to symptoms scores, we calculated three ESAS summary scores: (i) a physical distress score (0 to 60; sum of pain, tiredness, nausea, drowsiness, lack of appetite, and shortness of breath), (ii) an emotional distress score (0 to 20; sum of anxiety and depression), and (iii) a total symptom distress score (0–90; sum of physical and emotional scores plus overall well-being) [30,33,34,35].
Functional status was assessed using the Patient-Reported Functional Status tool, a validated patient-completed version of the Eastern Cooperative Oncology Group (ECOG) performance status instrument. [16] This tool is a single-item measure with which patients rate their activity over the past month on a five-point scale ranging from 0 (“normal with no limitation”) to 4 (“pretty much bedridden, rarely out of bed”). The ECOG performance status is widely used by oncologists to quantify functional capacity and to estimate prognosis [36].
2.4. Sample Size
We did not calculate sample size a priori because our study was a secondary analysis of a fixed convenience sample. We included all consecutive eligible patients approached during the study period.
2.5. Statistical Analysis
We used descriptive statistics to characterize the study population as a whole and stratified by ESAS symptom severity and Patient-Reported Functional Status tool level. Comparisons between patients who completed ESAS and patients who did not were made using chi-squared or Fisher’s exact test, as appropriate.
Multivariable logistic regression was used to identify socio-demographic and clinical characteristics independently associated with having at least one clinically significant symptom (ESAS score ≥4). Candidate predictors were selected a priori based on clinical relevance, including age, Charlson Comorbidity Index, breast cancer subtype, disease status (metastatic vs. non-metastatic), and treatment type.
All statistical analyses were conducted using SAS® 9.4 (SAS Institute, Cary, NC, USA). All tests were two-sided, with p < 0.05 considered statistically significant.
2.6. Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Sunnybrook Research Ethics Board (protocol number: 2551; approval date: 28 October 2016) and the University Health Network Research Ethics Boards (protocol number: 06-639: 6 December 2006). Written informed consent was obtained from all participants prior to participation. Confidentiality was ensured through secure storage, restricted access, and data de-identification before analysis.
3. Results
Of 549 women recruited for the larger study, 381 (69%) completed ESAS during their clinic visit (Table 1). Respondents had a mean age of 56.8 years (SD 12); most had no comorbidities (Charlson Comorbidity Index of 0), had a post-secondary education, and were born outside Canada. Approximately three-quarters were receiving active treatment, most commonly hormonal therapy. Compared with respondents, women who did not complete ESAS were more likely to be pre-menopausal, never married, and White, and had a different distribution of diagnosis years.

Table 1.
Characteristics of patients who completed ESAS.
Overall, 70% of patients reported at least one moderate to severe symptom, and 19% reported at least one severe symptom (Table 2). Tiredness, lack of well-being, anxiety, and depression were the most frequently reported symptoms, while nausea was the least common (2–8% across groups; Figure 1). Lack of well-being was the most common symptom among patients with metastatic breast cancer (44%), whereas tiredness was most common among women without metastatic disease (25 to 32%).

Table 2.
Frequency of moderate to severe ESAS symptom scores (≥4).
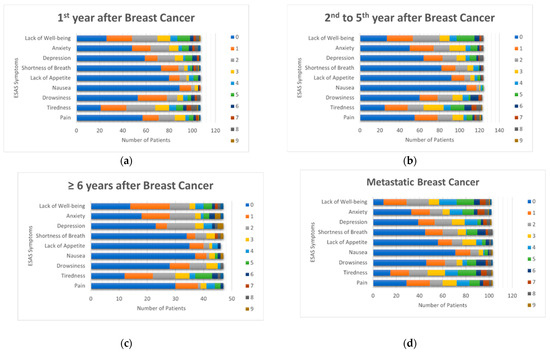
Figure 1.
ESAS Symptom Scores by disease status. (a) First year after breast cancer diagnosis; (b) Second to fifth year after breast cancer diagnosis; (c) Sixth and following years after breast cancer diagnosis; (d) Metastatic breast cancer.
Mean ESAS physical, emotional, and total Symptom Distress Scores (SDS) were low across all disease groups, including patients with metastatic disease (Table 3). Despite this, 81% of patients with metastatic breast cancer reported at least one moderate to severe symptom.

Table 3.
ESAS summary scores by patient group.
Most patients (86%) reported an ECOG performance status of 0 (“normal with no limitation”) or 1 (“not my normal self, but able to be up and about with fairly normal activities”) (Table 4). Nearly half of patients with functional limitations (ECOG ≥ 2) had metastatic disease.

Table 4.
Patient-Reported Functional Status (ECOG) results.
In multivariable analysis, being in the first year after primary breast cancer was associated with a lower probability of reporting at least one clinically significant symptom compared with metastatic disease (Odds ratio [OR] 0.49, 95% CI 0.24–0.90, Table 5). Patients diagnosed ≥6 years earlier also had a reduced likelihood, although this result was less precise (OR 0.50, 95% CI 0.22–1.13).

Table 5.
Logistic regression model for predictors of at least one clinically significant ESAS symptom.
4. Discussion
We evaluated symptom burden and functional status among women with breast cancer using ESAS and the Patient-Reported Functional Status tool. By focusing on clinically significant thresholds rather than mean scores, we aimed to provide a more meaningful assessment of symptoms that require intervention. Our cohort included both patients with potentially curable disease and those with metastatic breast cancer, thus capturing the full spectrum of the disease continuum.
We found that 70% of patients reported at least one moderate to severe symptom, and nearly one in five reported at least one severe symptom. Fatigue, lack of well-being, anxiety, and depression were the most frequently reported symptoms, while nausea was least common. Importantly, two-thirds of patients with curable disease also reported at least one clinically significant symptom, indicating that symptom burden is not confined to those with advanced disease. Even women who were disease-free more than six years after diagnosis frequently reported persistent symptoms, particularly fatigue, lack of well-being, anxiety, and depression.
Our findings are consistent with systematic reviews reporting that fatigue, emotional distress, and depressive symptoms are among the most common and persistent issues faced by breast cancer survivors, sometimes lasting for years after treatment completion [10,11,37]. Prior studies using ESAS in breast cancer populations have identified similar symptom patterns, despite recruiting diverse patient groups with a range of disease durations [27,38]. By applying clinically significant thresholds, our analysis demonstrates how focusing on group-level averages may underestimate the true prevalence of symptoms requiring clinical attention.
ESAS provides a brief, practical tool to screen symptoms such anxiety and depression during oncology visits, and to identify patients in need of further evaluation and support [39,40]. Interventions such as structured exercise programmes have been shown to reduce fatigue, anxiety, and depressive symptoms, in breast cancer survivors and are recommended by clinical guidelines [41,42,43]. Moreover, as patients transition from cancer centres to community-based care, individualized survivorship care plans may facilitate ongoing symptom management and improve long-term outcomes [44].
Effective symptom management requires a multidisciplinary team [45,46]. Oncologists, nurses, psychologists, social workers, physiotherapists, and palliative care specialists all contribute to the identification and treatment of symptom burden. Integrating PROMs such as ESAS into multidisciplinary care pathways can enhance communication, guide individualized interventions, and ensure timely referral to appropriate services.
Our study has several strengths. We drew on a relatively large cohort of consecutively recruited patients from two major cancer centres, which enhances generalizability. We used validated PROMs that are routinely integrated into clinical practice, and we applied clinically relevant thresholds to provide a more accurate assessment of symptoms requiring intervention. The inclusion of ECOG performance status provided additional insight into the relationship between symptom burden and functional capacity.
Limitations include the cross-sectional design, which precludes causal inference, and the exclusion of non-English speakers, which may limit generalizability. ESAS does not capture disease- or treatment-specific symptoms, such as cognitive impairment, lymphedema, or sexual dysfunction, which are nonetheless common and clinically relevant [10,47]. In men, the recognition that some patients reported symptoms not addressed by ESAS led to the implementation of the Expanded Prostate Cancer Index Composite for clinical practice (EPIC-CP) in Ontario [48,49]. A similar approach may be beneficial for addressing the range of symptoms experienced by women with a history of breast cancer. Selection bias is possible, as non-responders differed from responders in some characteristics, including race and ethnicity. The vast majority of ESAS studies includes mostly White patients, and mean scores may vary with ethno-racial background [50]. Moreover, patients too unwell to attend outpatient clinics (for example, patients admitted to hospital or to palliative care) or long-term survivors no longer followed in cancer centres were not included. Finally, the sample size may have been too small to estimate some important effects with precision.
Our results have important implications for clinical practice. Routine use of PROMs with attention to clinically significant thresholds can help clinicians identify patients who are most in need of intervention. Incorporating ESAS into survivorship care planning and community-based follow-up may improve continuity of care. Importantly, multidisciplinary involvement can address the complex and overlapping physical and psychological symptoms experienced by breast cancer patients.
Future research should investigate longitudinal symptom trajectories, evaluate interventions tailored to clinically significant symptom thresholds, and explore differences in symptom burden across ethno-racial groups. Research should also assess the impact of PROM-driven multidisciplinary care on long-term outcomes such as quality of life, healthcare utilization, and survival.
5. Conclusions
In conclusion, 70% of women with breast cancer reported at least one moderate to severe symptom, and nearly 20% reported at least one severe symptom, as measured by ESAS. Fatigue, lack of well-being, anxiety, and depression were the most common symptoms, and importantly, a substantial proportion of patients with potentially curable disease also reported clinically significant burden. These findings highlight that symptom management remains an important need across the entire disease spectrum, not only for patients with metastatic disease.
Our results have important implications for clinical practice. Routine use of PROMs such as ESAS, with attention to clinically significant thresholds, may improve the timely identification of patients in need of intervention. Incorporating symptom assessment into survivorship care planning and multidisciplinary care pathways can enhance quality of life and ensure continuity of care.
Future research should focus on whether symptom-specific interventions are effective and are associated with better functional outcomes and overall quality of life in breast cancer survivors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol32110598/s1, Table S1: The Strobe reporting checklist: completed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist. This checklist details how each item of the STROBE guidelines has been addressed in the manuscript.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S.T. The first draft of the manuscript was written by S.T. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Sunnybrook Health Sciences Centre Research Ethics Board (protocol number: 2551; approval date: 28 October 2016) and the University Health Network Research Ethics Board (protocol number: 06-639: 6 December 2006).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BC | Breast Cancer |
| CI | Confidence Interval |
| ECOG | Eastern Cooperative Oncology Group |
| EPIC-CP | Expanded Prostate Cancer Index Composite for Clinical Practice |
| ESAS | Edmonton Symptom Assessment System |
| Max | Maximum |
| Min | Minimum |
| N | Number |
| OR | Odds ratio |
| PRO | Patient-Reported Outcome |
| PROM | Patient-Reported Outcome Measure |
| REB | Research Ethics Board |
| SD | Standard deviation |
| SDS | Symptom distress scores |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Brenner, D.; Poirier, A.; Smith, L.; Aziz, L.S.; Ellison, L.; Fitzgerald, N.; Woods, R. Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society. Can. Cancer Stat. 2021, 2021, 1–95. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Brenner, D.R.; Gillis, J.; Demers, A.A.; Ellison, L.F.; Billette, J.-M.; Zhang, S.X.; Liu, J.L.; Woods, R.R.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2024. Can. Med. Assoc. J. 2024, 196, E615–E623. [Google Scholar] [CrossRef]
- Xie, L.; Semenciw, R.; Mery, L. Cancer incidence in Canada: Trends and projections (1983–2032). Health Promot. Chronic Dis. Prev. Can. 2015, 35 (Suppl. 1), 2–186. [Google Scholar] [CrossRef]
- Parry, C.; Kent, E.E.; Mariotto, A.B.; Alfano, C.M.; Rowland, J.H. Cancer survivors: A booming population. Cancer Epidemiology Biomarkers Prev. 2011, 20, 1996–2005. [Google Scholar] [CrossRef]
- Cleeland, C.S. Symptom Burden: Multiple Symptoms and Their Impact as Patient-Reported Outcomes. JNCI Monogr. 2007, 2007, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Maass, S.; Roorda, C.; Berendsen, A.; Verhaak, P.; de Bock, G. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Sarenmalm, E.K.; Browall, M.; Gaston-Johansson, F. Symptom Burden Clusters: A Challenge for Targeted Symptom Management. A Longitudinal Study Examining Symptom Burden Clusters in Breast Cancer. J. Pain Symptom Manag. 2014, 47, 731–741. [Google Scholar] [CrossRef] [PubMed]
- So, W.K.W.; Law, B.M.H.; Ng, M.S.N.; He, X.; Chan, D.N.S.; Chan, C.W.H.; McCarthy, A.L. Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Med. 2021, 10, 2531–2565. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Leblanc, M.; Elgbeili, G.; Cordova, M.J.; Marin, M.-F.; Brunet, A. The mental health impacts of receiving a breast cancer diagnosis: A meta-analysis. Br. J. Cancer 2021, 125, 1582–1592. [Google Scholar] [CrossRef]
- Weldring, T.; Smith, S.M. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Nekolaichuk, C.; Watanabe, S.; Beaumont, C. The Edmonton Symptom Assessment System: A 15-year retrospective review of validation studies (1991–2006). Palliat. Med. 2008, 22, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.Y.; Barmala, N.; Zarinehbaf, S.; Rodin, G.; Le, L.W.; Zimmermann, C. The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J. Pain Symptom Manag. 2009, 37, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Green, E.; Molloy, S.; Dudgeon, D.; Howell, D.; Krzyzanowska, M.K.; Mahase, W.; Tabing, R.; Urowitz, S.; Macdougall, L. Population-based standardized symptom screening: Cancer Care Ontario’s Edmonton Symptom Assessment System and performance status initiatives. J. Oncol. Pract. 2014, 10, 212–214. [Google Scholar] [CrossRef]
- Health, O. Cancer System Quality Index 2021–Ontario Cancer System Performance; Ontario Health: Toronto, ON, Canada, 2021. [Google Scholar]
- Chen, J.; Ou, L.; Hollis, S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 2013, 13, 211. [Google Scholar] [CrossRef]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A Systematic Review of Controlled Trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar] [CrossRef]
- Yang, L.Y.; Manhas, D.S.; Howard, A.F.; Olson, R.A. Patient-reported outcome use in oncology: A systematic review of the impact on patient-clinician communication. Support. Care Cancer 2018, 26, 41–60. [Google Scholar] [CrossRef]
- Howell, D.; Molloy, S.; Wilkinson, K.; Green, E.; Orchard, K.; Wang, K.; Liberty, J. Liberty Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. Ann. Oncol. 2015, 26, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef]
- Denis, F.; Basch, E.; Septans, A.-L.; Bennouna, J.; Urban, T.; Dueck, A.C.; Letellier, C. Two-Year Survival Comparing Web-Based Symptom Monitoring vs Routine Surveillance Following Treatment for Lung Cancer. JAMA 2019, 321, 306–307. [Google Scholar] [CrossRef]
- Barbera, L.; Sutradhar, R.; Seow, H.; Mittmann, N.; Howell, D.; Earle, C.C.; Li, Q.; Thiruchelvam, D. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: Results of a population-based retrospective matched cohort analysis. Cancer Med. 2020, 9, 7107–7115. [Google Scholar] [CrossRef]
- Ecclestone, C.; Chow, R.; Pulenzas, N.; Zhang, L.; Leahey, A.; Hamer, J.; DeAngelis, C.; Bedard, G.; McDonald, R.; Bhatia, A.; et al. Quality of life and symptom burden in patients with metastatic breast cancer. Support. Care Cancer 2016, 24, 4035–4043. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Pulenzas, N.; Zhang, L.; Ecclestone, C.; Leahey, A.; Hamer, J.; DeAngelis, C.; Bedard, G.; McDonald, R.; Bhatia, A.; et al. Quality of life and symptom burden in patients with breast cancer treated with mastectomy and lumpectomy. Support. Care Cancer 2016, 24, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.; Sussman, J.; Martelli-Reid, L.; Pond, G.; Bainbridge, D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J. Oncol. Pract. 2012, 8, e142–e148. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Selby, D.; Cascella, A.; Gardiner, K.; Do, R.; Moravan, V.; Myers, J.; Chow, E. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010, 39, 241–249. [Google Scholar] [CrossRef]
- Serlin, R.C.; Mendoza, T.R.; Nakamura, Y.; Edwards, K.R.; Cleeland, C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. PAIN 1995, 61, 277–284. [Google Scholar] [CrossRef]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Khamash, O.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Arthur, J.; Bruera, E. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2016, 51, 262–269. [Google Scholar] [CrossRef]
- Zimmermann, C.; Burman, D.; Bandukwala, S.; Seccareccia, D.; Kaya, E.; Bryson, J.; Rodin, G.; Lo, C. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support. Care Cancer 2010, 18, 609–616. [Google Scholar] [CrossRef]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Yennu, S.; Kang, J.H.; Bruera, E. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015, 121, 3027–3035. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Pilevarzadeh, M.; Amirshahi, M.; Afsargharehbagh, R.; Rafiemanesh, H.; Hashemi, S.-M.; Balouchi, A. Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Bubis, L.D.; Mahar, A.L.; Li, Q.; Sussman, J.; Moody, L.; Barbera, L.; Holloway, C.M.; Coburn, N.G. Patient-reported symptoms after breast cancer diagnosis and treatment: A retrospective cohort study. Eur. J. Cancer 2018, 101, 1–11. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Bandieri, E.; Pessi, M.A.; Maruelli, A.; Buonaccorso, L.; Miccinesi, G. The Edmonton Symptom Assessment System (ESAS) as a screening tool for depression and anxiety in non-advanced patients with solid or haematological malignancies on cure or follow-up. Support. Care Cancer 2014, 22, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Caruso, R.; Riba, M.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; Campos-Ródenas, R.; Zachariae, R.; Santini, D.; et al. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 101155. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Kunthur, A.; Xiang, Z.; Kaur, H.; Jewell, S.; Mehta, P. Updates on Cancer Survivorship Care Planning. Fed. Pract. 2015, 32 (Suppl. 7), 64s–69s. [Google Scholar] [PubMed]
- Alfieri, S.; Brunelli, C.; Capri, G.; Caraceni, A.; Bianchi, G.V.; Borreani, C. A Qualitative Study on the Needs of Women with Metastatic Breast Cancer. J. Cancer Educ. 2022, 37, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Visvanathan, K.; Wolff, A.C. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast 2015, 24 (Suppl. 2), S149–S153. [Google Scholar] [CrossRef]
- Brundage, M.D.; Barbera, L.; McCallum, F.; Howell, D.M. A pilot evaluation of the expanded prostate cancer index composite for clinical practice (EPIC-CP) tool in Ontario. Qual. Life Res. 2019, 28, 771–782. [Google Scholar] [CrossRef]
- Haji, F.; Barbera, L.C.; Bedford, C.; Nichols, B.; Brundage, M.D. Standardized symptom screening: Cancer Care Ontario’s expanded prostate cancer index composite for clinical practice (EPIC-CP) provincial implementation approach. J. Clin. Oncol. 2017, 35, 100. [Google Scholar] [CrossRef]
- Richardson, L.A.; Jones, G.W. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr. Oncol. 2009, 16, 53–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).