Comparative Prognostic Roles of β-Catenin Expression and Tumor–Stroma Ratio in Pancreatic Cancer: Neoadjuvant Chemotherapy vs. Upfront Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Materials
2.2. Histological Analysis
2.3. Immunohistochemstry (IHC)
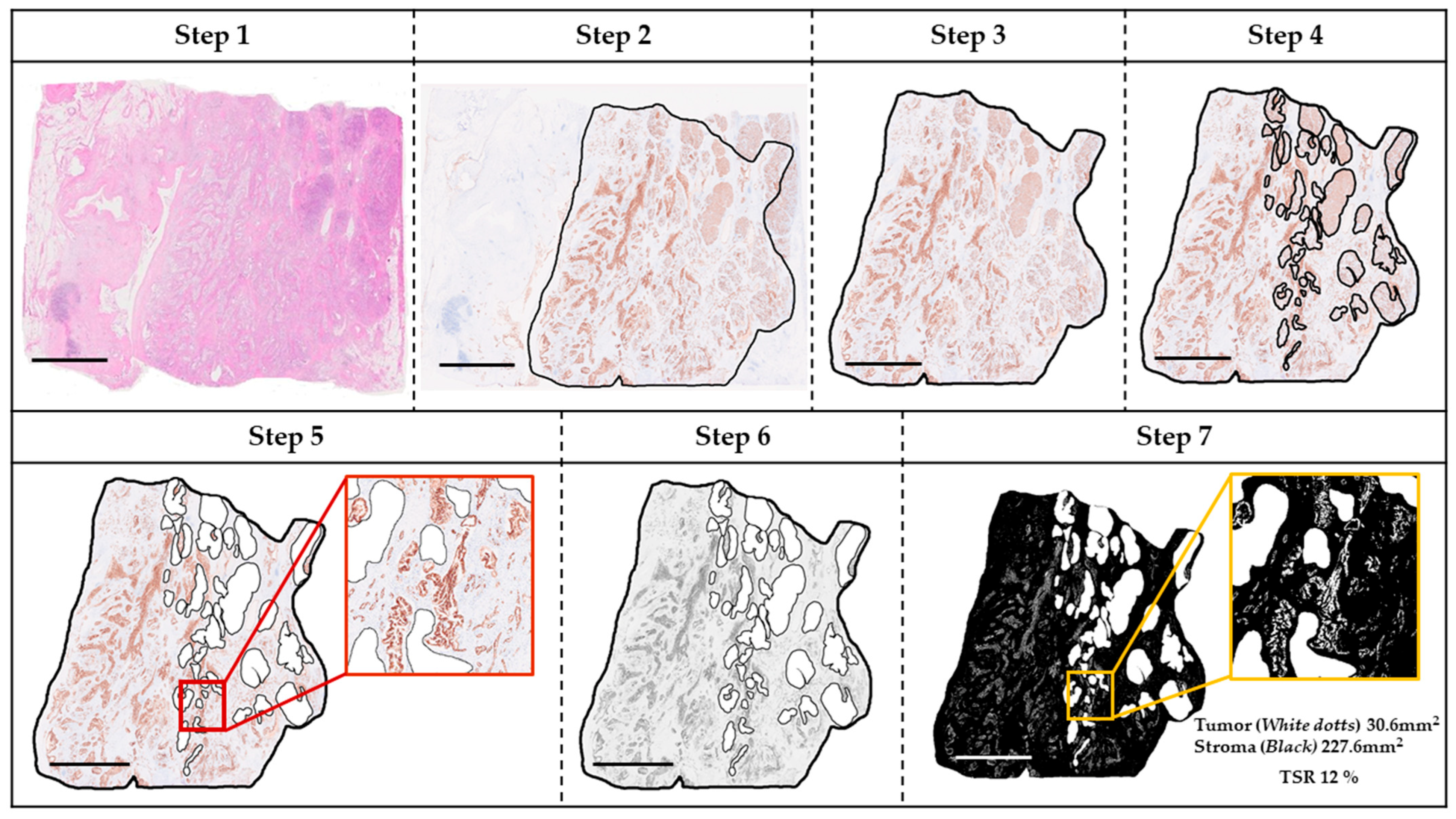
2.4. TSR
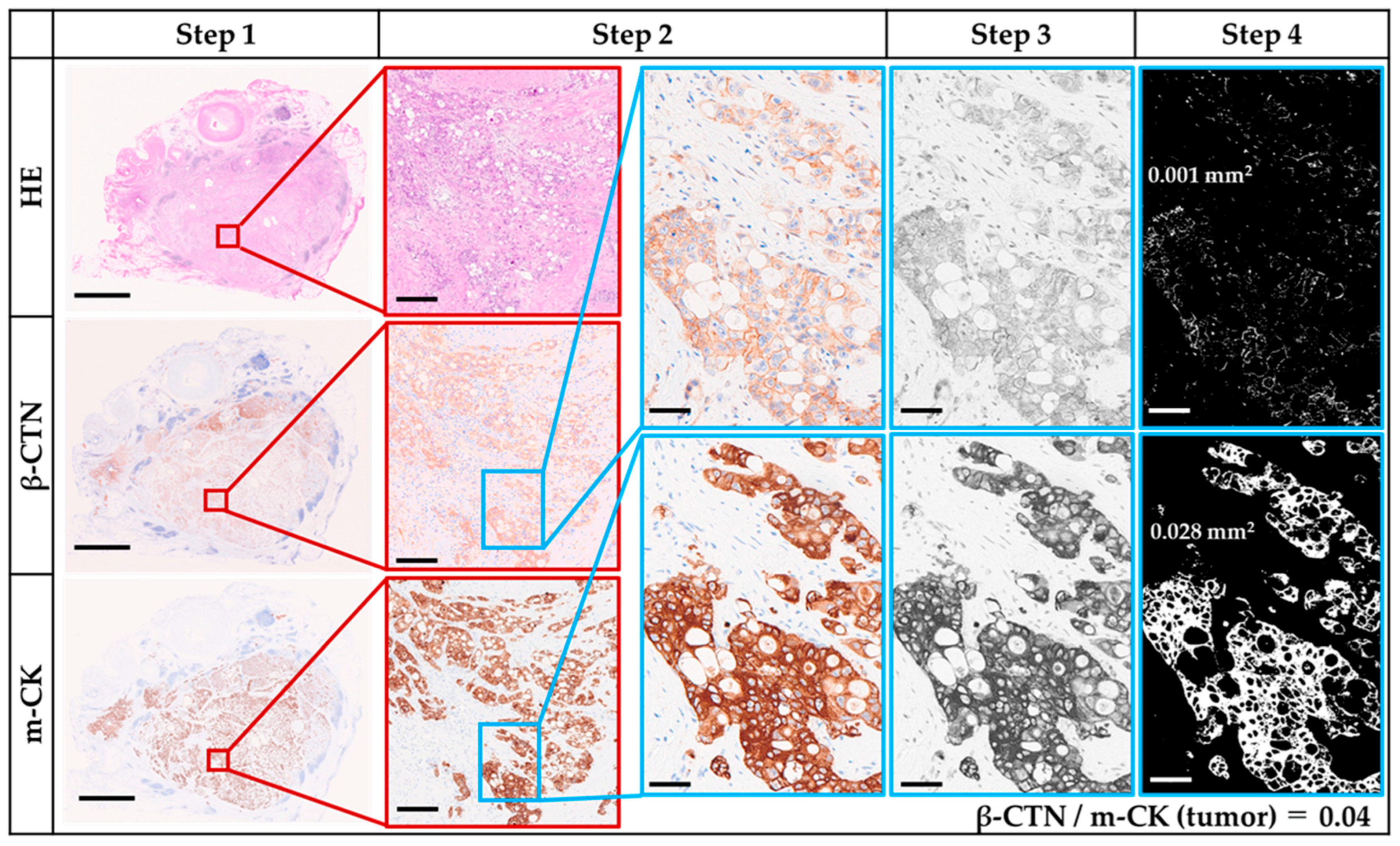
2.5. Assessment of β-CTN IHC
2.6. β-CTN/m-CK Index
2.7. TB
2.8. Statistical Analysis
3. Results
3.1. Clinicopathological Differences Between NAC and UFS
3.2. β-CTN and m-CK IHC
3.3. Baseline Values of TSR, β-CTN/m-CK Index, and TB
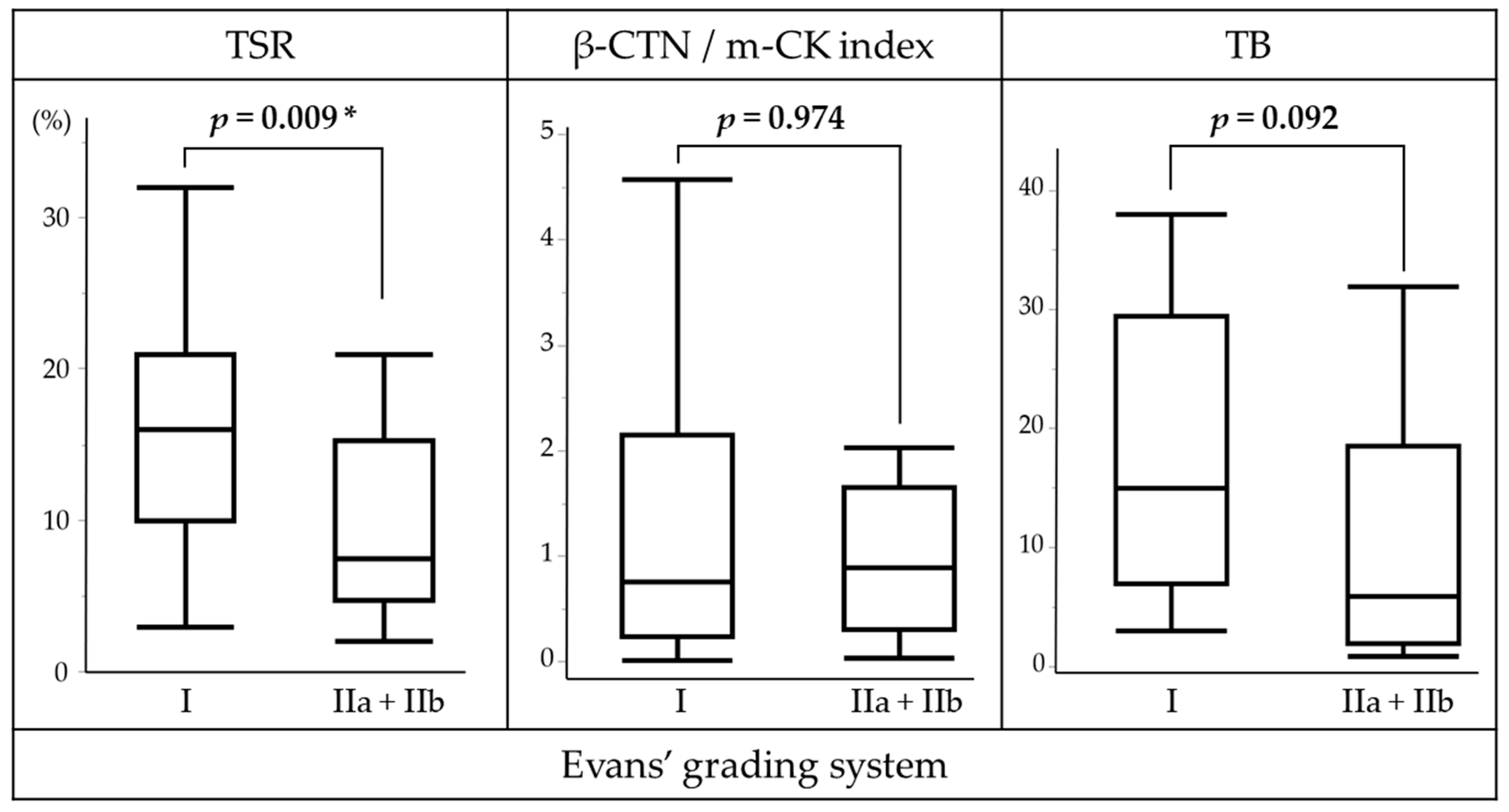
3.4. Association of TSR with Clinicopathological Features
3.5. Association of β-CTN/m-CK Index with Clinicopathological Features
3.6. Association of TB with Clinicopathological Features
3.7. Interrelationships Among β-CTN/m-CK Index, TSR, and TB in PDAC
3.8. NAC Response in Relation to TSR, β-CTN/m-CK Index, and TB
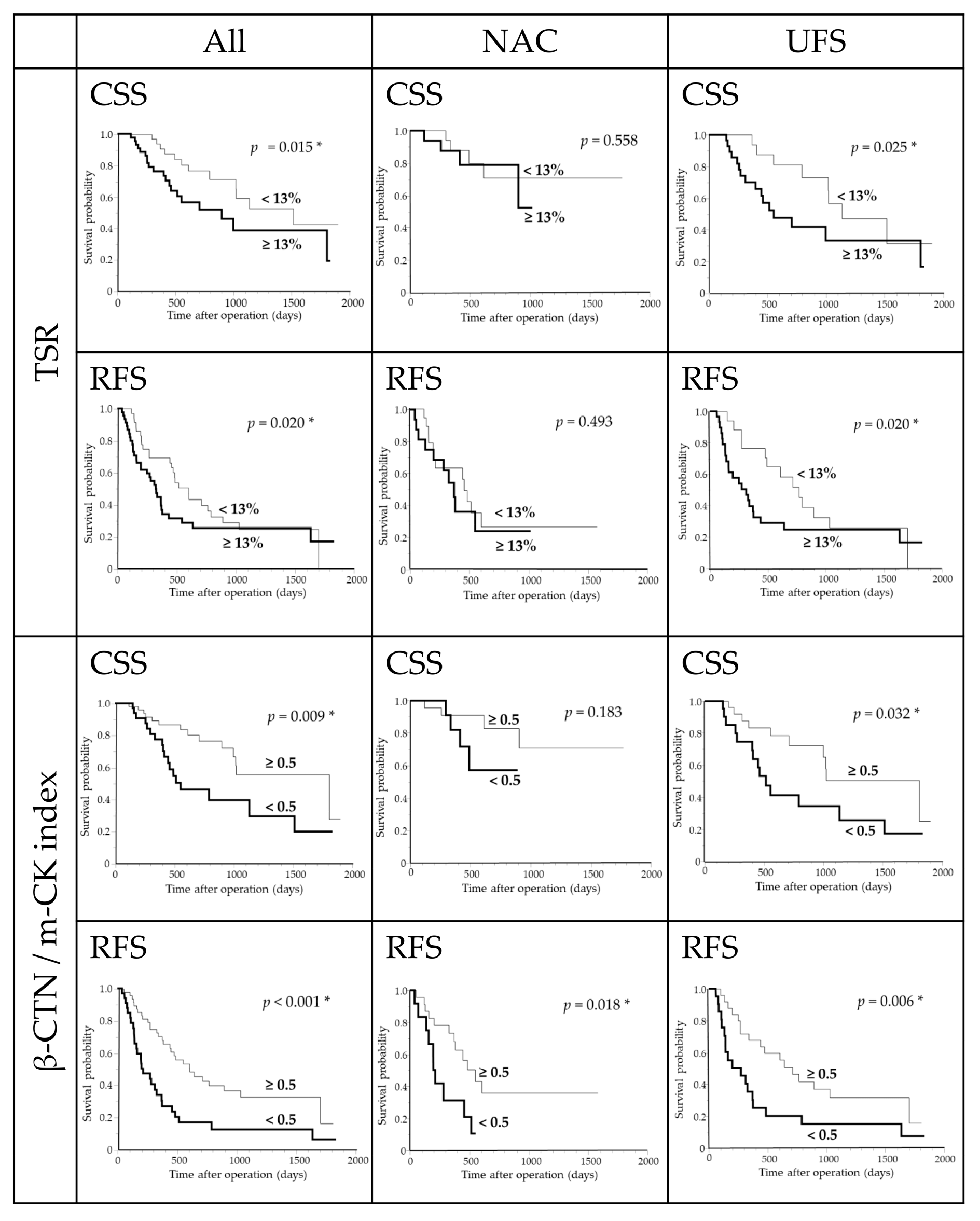
3.9. Survival Analysis in the Overall Cohort
3.10. Survival Analysis in the NAC Subgroup
3.11. Survival Analysis in the UFS Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-CTN | β-catenin |
| CI | confidence interval |
| CSS | cancer-specific survival |
| IHC | immunohistochemistry |
| m-CK | multi-cytokeratin |
| NAC | neoadjuvant chemotherapy |
| PC | pancreatic cancer |
| PDAC | pancreatic ductal adenocarcinoma |
| RFS | relapse-free survival |
| TB | tumor budding |
| TSR | tumor–stroma ratio |
| UFS | upfront surgery |
| WSI | whole-slide image |
References
- Zhao, Z.; Liu, W. Pancreatic cancer: A review of risk factors, diagnosis, and treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Zottl, J.; Sebesta, C.G.; Tomosel, E.; Sebesta, M.C.; Sebesta, C. Unraveling the burden of pancreatic cancer in the 21st century: Trends in incidence, mortality, survival, and key contributing factors. Cancers 2025, 17, 1607. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Ei, S.; Takahashi, S.; Ogasawara, T.; Mashiko, T.; Masuoka, Y.; Nakagohri, T. Neoadjuvant and adjuvant treatments for resectable and borderline resectable pancreatic ductal adenocarcinoma: The current status of pancreatic ductal adenocarcinoma treatment in Japan. Gut Liver 2023, 17, 698–710. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Q.; Chen, Y.; Fu, Q.; Li, X.; Bai, X.; Liang, T. Neoadjuvant chemotherapy for primary resectable pancreatic cancer: A systematic review and meta-analysis. HPB 2020, 22, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Rodriguez Franco, S.; Sherman, S.; Torphy, R.J.; Colborn, K.; Franklin, O.; Ishida, J.; Grandi, S.; Al-Musawi, M.H.; Gleisner, A.; et al. Neoadjuvant chemotherapy versus upfront surgery for resectable pancreatic adenocarcinoma: An updated nationwide study. Ann. Surg. 2024, 279, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Nakamura, K.; Yamaguchi, T. S-1 in the treatment of pancreatic cancer. World J. Gastroenterol. 2014, 20, 15110–15118. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Toyama, H.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; et al. Neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer: Results of the randomized phase II/III Prep-02/JSAP05 trial. Ann. Surg. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y.; et al. Clinical practice guidelines for pancreatic cancer 2022 from the Japan Pancreas Society: A synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef]
- Winther, S.B.; Bjerregaard, J.K.; Schonnemann, K.R.; Ejlsmark, M.W.; Krogh, M.; Jensen, H.A.; Pfeiffer, P. S-1 (Teysuno) and gemcitabine in Caucasian patients with unresectable pancreatic adenocarcinoma. Cancer Chemother. Pharmacol. 2018, 81, 573–578. [Google Scholar] [CrossRef]
- McDonald, O.G. The biology of pancreatic cancer morphology. Pathology 2022, 54, 236–247. [Google Scholar] [CrossRef]
- Di Maggio, F.; El-Shakankery, K.H. Desmoplasia and biophysics in pancreatic ductal adenocarcinoma: Can we learn from breast cancer? Pancreas 2020, 49, 313–325. [Google Scholar] [CrossRef]
- Pyo, J.S.; Kim, N.Y.; Min, K.W.; Kang, D.W. Significance of tumor–stroma ratio (TSR) in predicting outcomes of malignant tumors. Medicina 2023, 59, 1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, S.; Zhao, J.; Yu, S.; Chen, J.; Chen, X. Tumor–stroma ratio combined with PD-L1 identifies pancreatic ductal adenocarcinoma patients at risk for lymph node metastases. Br. J. Cancer 2025, 132, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Yuan, J.; Liu, C.; Zhang, J.; Yang, Y.; Liang, H.; Jiang, S.; Chen, S.; Li, Y.; Liu, Y.; et al. Feasibility and effectiveness of automatic deep learning network and radiomics models for differentiating tumor–stroma ratio in pancreatic ductal adenocarcinoma. Insights Imaging 2023, 14, 223. [Google Scholar] [CrossRef]
- Lu, M.; Zou, Y.; Fu, P.; Li, Y.; Wang, P.; Li, G.; Luo, S.; Chen, Y.; Guan, G.; Zhang, S.; et al. The tumor–stroma ratio and the immune microenvironment improve the prognostic prediction of pancreatic ductal adenocarcinoma. Discov. Oncol. 2023, 14, 124. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, H.; Li, Q.; Liu, F.; Fang, X.; Li, J.; Yu, J.; Feng, X.; Lu, J.; Bian, Y.; et al. Magnetic resonance radiomics and machine-learning models: An approach for evaluating tumor–stroma ratio in patients with pancreatic ductal adenocarcinoma. Acad. Radiol. 2022, 29, 523–535. [Google Scholar] [CrossRef]
- Kawahara, K.; Takano, S.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Ohtsuka, M. The effect of the low stromal ratio induced by neoadjuvant chemotherapy on recurrence patterns in borderline resectable pancreatic ductal adenocarcinoma. Clin. Exp. Metastasis 2022, 39, 311–322. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Jiang, H.; Li, B.; Shi, X.; Gao, S.; Ni, C.; Zhang, Z.; Guo, S.; Xu, J.; et al. Pros and cons: High proportion of stromal component indicates better prognosis in patients with pancreatic ductal adenocarcinoma—A research based on the evaluation of whole-mount histological slides. Front. Oncol. 2020, 10, 1472. [Google Scholar] [CrossRef]
- Leppänen, J.; Lindholm, V.; Isohookana, J.; Haapasaari, K.M.; Karihtala, P.; Lehenkari, P.P.; Saarnio, J.; Kauppila, J.H.; Karttunen, T.J.; Helminen, O.; et al. Tenascin C, fibronectin, and tumor–stroma ratio in pancreatic ductal adenocarcinoma. Pancreas 2019, 48, 43–48. [Google Scholar] [CrossRef]
- Matsuda, Y.; Inoue, Y.; Hiratsuka, M.; Kawakatsu, S.; Arai, T.; Matsueda, K.; Saiura, A.; Takazawa, Y. Encapsulating fibrosis following neoadjuvant chemotherapy is correlated with outcomes in patients with pancreatic cancer. PLoS ONE 2019, 14, e0222155. [Google Scholar] [CrossRef]
- Koay, E.J.; Lee, Y.; Cristini, V.; Lowengrub, J.S.; Kang, Y.; Lucas, F.A.S.; Hobbs, B.P.; Ye, R.; Elganainy, D.; Almahariq, M.; et al. Visually apparent quantifiable CT imaging and feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2018, 24, 5883–5894. [Google Scholar] [CrossRef]
- Vendittelli, P.; Bokhorst, J.M.; Smeets, E.M.M.; Kryklyva, V.; Brosens, L.A.A.; Verbeke, C.; Litjens, G. Automatic quantification of tumor–stroma ratio as a prognostic marker for pancreatic cancer. PLoS ONE 2024, 19, e0301969. [Google Scholar] [CrossRef]
- Shi, S.; Liang, C.; Xu, J.; Meng, Q.; Hua, J.; Yang, X.; Ni, Q.; Yu, X.M. The strain ratio as obtained by endoscopic ultrasonography elastography correlates with the stroma proportion and the prognosis of local pancreatic cancer. Ann. Surg. 2020, 271, 559–565. [Google Scholar] [CrossRef]
- Bever, K.M.; Sugar, E.A.; Bigelow, E.; Sharma, R.; Laheru, D.; Wolfgang, C.L.; Jaffee, E.M.; Anders, R.A.; De Jesus-Acosta, A.; Zheng, L.; et al. The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB 2015, 17, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shi, S.; Meng, Q.; Liang, D.; Ji, S.; Zhang, B.; Qin, Y.; Xu, J.; Ni, Q.; Yu, X.; et al. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: Where we are and where we are going. Exp. Mol. Med. 2017, 49, e406. [Google Scholar] [CrossRef] [PubMed]
- Koay, E.J.; Truty, M.J.; Cristini, V.; Thomas, R.M.; Chen, R.; Chatterjee, D.; Kang, Y.; Bhosale, P.R.; Tamm, E.P.; Crane, C.H.; et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J. Clin. Investig. 2014, 124, 1525–1536. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Yamada, S.; Fujii, T.; Tanaka, C.; Kanda, M.; Nakayama, G.; Sugimoto, H.; Koike, M.; Fujiwara, M.; Kodera, Y. S-1 plus nab-paclitaxel is a promising regimen for pancreatic cancer in a preclinical model. J. Surg. Oncol. 2016, 113, 413–419. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takada, K.; Nakano, A.; Takeuchi, O.; Watanabe, K.; Hirohara, M.; Masuda, Y. Combination of S-1 and the oral ATR inhibitor ceralasertib is effective against pancreatic cancer cells. Cancer Chemother. Pharmacol. 2024, 94, 763–774. [Google Scholar] [CrossRef]
- Lin, W.H.; Cooper, L.M.; Anastasiadis, P.Z. Cadherins and catenins in cancer: Connecting cancer pathways and tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1137013. [Google Scholar] [CrossRef]
- Borcherding, N.; Cole, K.; Kluz, P.; Jorgensen, M.; Kolb, R.; Bellizzi, A.; Zhang, W. Re-evaluating E-cadherin and β-catenin: A pan-cancer proteomic approach with an emphasis on breast cancer. Am. J. Pathol. 2018, 188, 1910–1920. [Google Scholar] [CrossRef]
- Rosova, B.; Proks, J.; Filipova, A.; Hadzi Nikolov, D.; Chloupkova, R.; Richter, I.; Szabo, A.; Rozsypalova, A.; Matej, R.; Melichar, B.; et al. Favorable prognostic significance of membranous β-catenin expression and negative prognostic significance of cytoplasmic β-catenin expression in pancreatic cancer. Neoplasma 2023, 70, 796–803. [Google Scholar] [CrossRef]
- Eurola, A.; Ristimäki, A.; Mustonen, H.; Nurmi, A.M.; Hagström, J.; Kallio, P.; Alitalo, K.; Haglund, C.; Seppänen, H. β-catenin plus PROX1 immunostaining stratifies disease progression and patient survival in neoadjuvant-treated pancreatic cancer. Tumour Biol. 2022, 44, 69–84. [Google Scholar] [CrossRef]
- Saukkonen, K.; Hagström, J.; Mustonen, H.; Juuti, A.; Nordling, S.; Kallio, P.; Alitalo, K.; Seppänen, H.; Haglund, C. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer 2016, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Q.; Li, P.; Sha, H.; Li, X.; Xu, J. Aberrant expression of CXCR4 and β-catenin in pancreatic cancer. Anticancer Res. 2013, 33, 4103–4110. [Google Scholar] [PubMed]
- Zhong, R.L.; Li, Y.; Fang, Z.; Fang, K.F.; Wang, L. PKR2 and β-catenin genes regulates pancreatic cancer chemosensitivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 48–54. [Google Scholar] [PubMed]
- Eurola, A.; Ristimäki, A.; Mustonen, H.; Nurmi, A.M.; Hagström, J.; Haglund, C.; Seppänen, H. Impact of histological response after neoadjuvant therapy on podocalyxin as a prognostic marker in pancreatic cancer. Sci. Rep. 2021, 11, 9896. [Google Scholar] [CrossRef]
- Kokumai, T.; Omori, Y.; Ishida, M.; Ohtsuka, H.; Mizuma, M.; Nakagawa, K.; Maeda, C.; Ono, Y.; Mizukami, Y.; Miura, S.; et al. GATA6 and CK5 stratify the survival of patients with pancreatic cancer undergoing neoadjuvant chemotherapy. Mod. Pathol. 2023, 36, 100102. [Google Scholar] [CrossRef]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2021, 18, 101–115. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Kohler, I.; Bronsert, P.; Timme, S.; Werner, M.; Brabletz, T.; Hopt, U.T.; Schilling, O.; Bausch, D.; Keck, T.; Wellner, U.F. Detailed analysis of epithelial–mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. 1), 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Man, Q.; Li, X.; Xie, Y.; Hou, X.; Wang, H.; Yan, J.; Wei, X.; Bai, W.; Liu, Z.; et al. Artificial intelligence-based comprehensive analysis of immune-stemness-tumor budding profile to predict survival of patients with pancreatic adenocarcinoma. Cancer Biol. Med. 2023, 20, 196–217. [Google Scholar] [CrossRef] [PubMed]
- Hayasaki, A.; Mizuno, S.; Usui, M.; Kaluba, B.; Komatsubara, H.; Sakamoto, T.; Maeda, K.; Shinkai, T.; Noguchi, D.; Ito, T.; et al. Tumor budding is an independent adverse prognostic factor of pancreatic ductal adenocarcinoma patients treated by resection after preoperative chemoradiotherapy. Pancreas 2025, 54, e340–e348. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, E.; Kadota, K.; Kimura, N.; Ishikawa, R.; Oshima, M.; Okano, K.; Haba, R. Prognostic significance of tumor budding in patients with pancreatic invasive ductal carcinoma who received neoadjuvant therapy. Heliyon 2023, 10, e23928. [Google Scholar] [CrossRef]
- Argon, A.; Öz, Ö.; Kebat, T.A. Evaluation and prognostic significance of tumor budding in pancreatic ductal adenocarcinomas. Indian J. Pathol. Microbiol. 2023, 66, 38–43. [Google Scholar] [CrossRef]
- Karamitopoulou, E.; Wartenberg, M.; Zlobec, I.; Cibin, S.; Worni, M.; Gloor, B.; Lugli, A. Tumour budding in pancreatic cancer revisited: Validation of the ITBCC scoring system. Histopathology 2018, 73, 137–146. [Google Scholar] [CrossRef]
- Lohneis, P.; Sinn, M.; Klein, F.; Bischoff, S.; Striefler, J.K.; Wislocka, L.; Sinn, B.V.; Pelzer, U.; Oettle, H.; Riess, H.; et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br. J. Cancer 2018, 118, 1485–1491. [Google Scholar] [CrossRef]
- Oba, A.; Del Chiaro, M.; Fujii, T.; Okano, K.; Stoop, T.F.; Wu, Y.H.A.; Maekawa, A.; Yoshida, Y.; Hashimoto, D.; Sugawara, T.; et al. “Conversion surgery” for locally advanced pancreatic cancer: A position paper by the study group at the joint meeting of the International Association of Pancreatology (IAP) & Japan Pancreas Society (JPS) 2022. Pancreatology 2023, 23, 712–720. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Evans, D.B.; Rich, T.A.; Byrd, D.R.; Cleary, K.R.; Connelly, J.H.; Levin, B.; Charnsangavej, C.; Fenoglio, C.J.; Ames, F.C. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. 1992, 127, 1335–1339. [Google Scholar] [CrossRef]
- Neumann, C.C.M.; von Hörschelmann, E.; Reutzel-Selke, A.; Seidel, E.; Sauer, I.M.; Pratschke, J.; Bahra, M.; Schmuck, R.B. Tumor–stromal cross-talk modulating the therapeutic response in pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 461–472. [Google Scholar] [CrossRef]
- Gore, J.; Korc, M. Pancreatic cancer stroma: Friend or foe? Cancer Cell 2014, 25, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021, 39, 548–565.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Roh, J.; Park, C.S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kawakami, H.; Natsuizaka, M.; Kawakubo, K.; Marukawa, K.; Kudo, T.; Abe, Y.; Kubo, K.; Kuwatani, M.; Hatanaka, Y.; et al. CTNNB1 mutational analysis of solid-pseudopapillary neoplasms of the pancreas using endoscopic ultrasound-guided fine-needle aspiration and next-generation deep sequencing. J. Gastroenterol. 2015, 50, 203–210. [Google Scholar] [CrossRef]
- Dolled-Filhart, M.; McCabe, A.; Giltnane, J.; Cregger, M.; Camp, R.L.; Rimm, D.L. Quantitative in situ analysis of β-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006, 66, 5487–5494. [Google Scholar] [CrossRef]
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 2020, 10, 648–656. [Google Scholar] [CrossRef]
| Variable | NAC | UFS | p-Value |
|---|---|---|---|
| Number of cases | 35 | 49 | |
| Age (years) | |||
| Mean/Median (range) | 69.5/71 (39–85) | 74.8/75 (50–91) | 0.040 * |
| <75 | 22 | 24 | 0.268 |
| ≥75 | 13 | 25 | |
| Sex | 0.664 | ||
| Male | 19 | 24 | |
| Female | 16 | 25 | |
| Tumor location | 1.000 | ||
| Head | 21 | 29 | |
| Body/Tail | 14 | 20 | |
| Adjuvant therapy | 0.264 | ||
| Yes | 23 | 25 | |
| No | 12 | 24 | |
| pT category | 0.060 | ||
| 1 | 13 | 10 | |
| 2 | 13 | 31 | |
| 3 | 9 | 8 | |
| pN category | 0.564 | ||
| N0 | 18 | 21 | |
| N1 | 11 | 15 | |
| N2 | 6 | 13 | |
| Tumor differentiation | 0.787 | ||
| Well | 14 | 23 | |
| Moderate | 18 | 23 | |
| Poor | 3 | 3 | |
| Lymphovascular invasion | 0.468 | ||
| Absent | 8 | 15 | |
| Present | 27 | 34 | |
| Perineural invasion | 0.199 | ||
| Absent | 11 | 9 | |
| Present | 24 | 40 | |
| Peripancreatic fat invasion | 0.409 | ||
| Absent | 9 | 8 | |
| Present | 26 | 41 | |
| Resection margin | 0.067 | ||
| Negative | 33 | 39 | |
| Positive | 2 | 10 | |
| Variable | CSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Adjuvant therapy (Yes vs. No) | 5.395 | 2.233–13.031 | <0.001 * | 2.210 | 1.230–3.972 | 0.008 * |
| pT category (1 + 2 vs. 3) | N/A | N/A | N/A | 1.785 | 0.909–3.505 | 0.093 |
| pN category (N0 vs. N1 + N2) | 1.477 | 0.651–3.350 | 0.350 | 1.953 | 1.093–3.492 | 0.024 * |
| Tumor differentiation (Well vs. Moderate + Poor) | 7.776 | 2.832–21.349 | <0.001 * | 2.394 | 1.260–4.551 | 0.008 * |
| Lymphovascular invasion (Absent vs. Present) | N/A | N/A | N/A | 2.546 | 1.218–5.321 | 0.013 * |
| Peripancreatic fat invasion (Absent vs. Present) | 6.972 | 1.911–25.428 | 0.003 * | N/A | N/A | N/A |
| TSR (<13% vs. ≥13%) | 2.414 | 1.071–5.439 | 0.034 * | N/A | N/A | N/A |
| β-CTN/m-CK index (≥0.5 vs. <0.5) | N/A | N/A | N/A | 2.028 | 1.120–3.670 | 0.020 * |
| Variable | CSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex (Female vs. Male) | 18.054 | 1.614–201.926 | 0.019 * | N/A | N/A | N/A |
| Resection margin (Negative vs. Positive) | 63.446 | 2.871–1402.272 | 0.009 * | 4.033 | 0.817–19.922 | 0.087 |
| β-CTN/m-CK index (≥0.5 vs. <0.5) | N/A | N/A | N/A | 2.516 | 1.031–6.138 | 0.043 * |
| TB (Low vs. High) | 6.008 | 0.847–42.627 | 0.073 | N/A | N/A | N/A |
| Variable | CSS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Adjuvant therapy (Yes vs. No) | 5.539 | 1.882–16.306 | 0.002 * | 2.013 | 0.980–4.136 | 0.057 |
| Tumor differentiation (Well vs. Moderate + Poor) | 7.632 | 2.471–23.575 | <0.001 * | 5.006 | 2.178–11.508 | <0.001 * |
| Peripancreatic fat invasion (Absent vs. Present) | 4.933 | 1.104–22.033 | 0.037 * | N/A | N/A | N/A |
| TSR (<13% vs. ≥13%) | 2.536 | 0.947–6.790 | 0.064 | N/A | N/A | N/A |
| β-CTN/m-CK index (≥0.5 vs. <0.5) | N/A | N/A | N/A | 2.230 | 1.106–4.500 | 0.025 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikawa, S.; Mitomi, H.; Murai, S.; Nakayama, A.; Chiba, S.; Nishihara, S.; Ishii, Y.; Yamochi, T.; Yoshida, H. Comparative Prognostic Roles of β-Catenin Expression and Tumor–Stroma Ratio in Pancreatic Cancer: Neoadjuvant Chemotherapy vs. Upfront Surgery. Curr. Oncol. 2025, 32, 578. https://doi.org/10.3390/curroncol32100578
Oikawa S, Mitomi H, Murai S, Nakayama A, Chiba S, Nishihara S, Ishii Y, Yamochi T, Yoshida H. Comparative Prognostic Roles of β-Catenin Expression and Tumor–Stroma Ratio in Pancreatic Cancer: Neoadjuvant Chemotherapy vs. Upfront Surgery. Current Oncology. 2025; 32(10):578. https://doi.org/10.3390/curroncol32100578
Chicago/Turabian StyleOikawa, Shu, Hiroyuki Mitomi, So Murai, Akihiro Nakayama, Seiya Chiba, Shigetoshi Nishihara, Yu Ishii, Toshiko Yamochi, and Hitoshi Yoshida. 2025. "Comparative Prognostic Roles of β-Catenin Expression and Tumor–Stroma Ratio in Pancreatic Cancer: Neoadjuvant Chemotherapy vs. Upfront Surgery" Current Oncology 32, no. 10: 578. https://doi.org/10.3390/curroncol32100578
APA StyleOikawa, S., Mitomi, H., Murai, S., Nakayama, A., Chiba, S., Nishihara, S., Ishii, Y., Yamochi, T., & Yoshida, H. (2025). Comparative Prognostic Roles of β-Catenin Expression and Tumor–Stroma Ratio in Pancreatic Cancer: Neoadjuvant Chemotherapy vs. Upfront Surgery. Current Oncology, 32(10), 578. https://doi.org/10.3390/curroncol32100578





