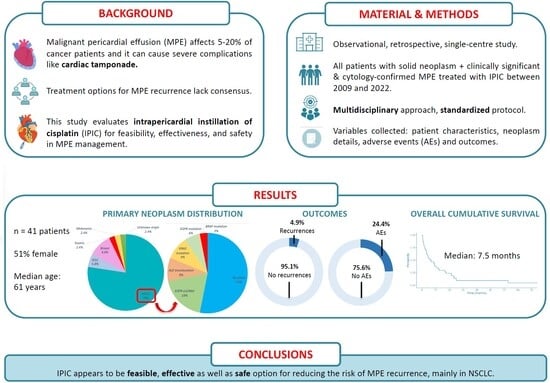
Retrospective Cohort Study of Intrapericardial Cisplatin for Risk Reduction of Malignant Pericardial Effusion Recurrence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
2.3. Assessing Clinical Outcomes
2.4. Statistical Analysis
2.5. Bias Reduction Measures
3. Results
3.1. Description of the Cohort Patients
3.2. Somatic Alterations in NSCLC
3.3. MPE and Pericardiocentesis Characteristics
3.4. Intrapericardial Cisplatin Instillation—Safety
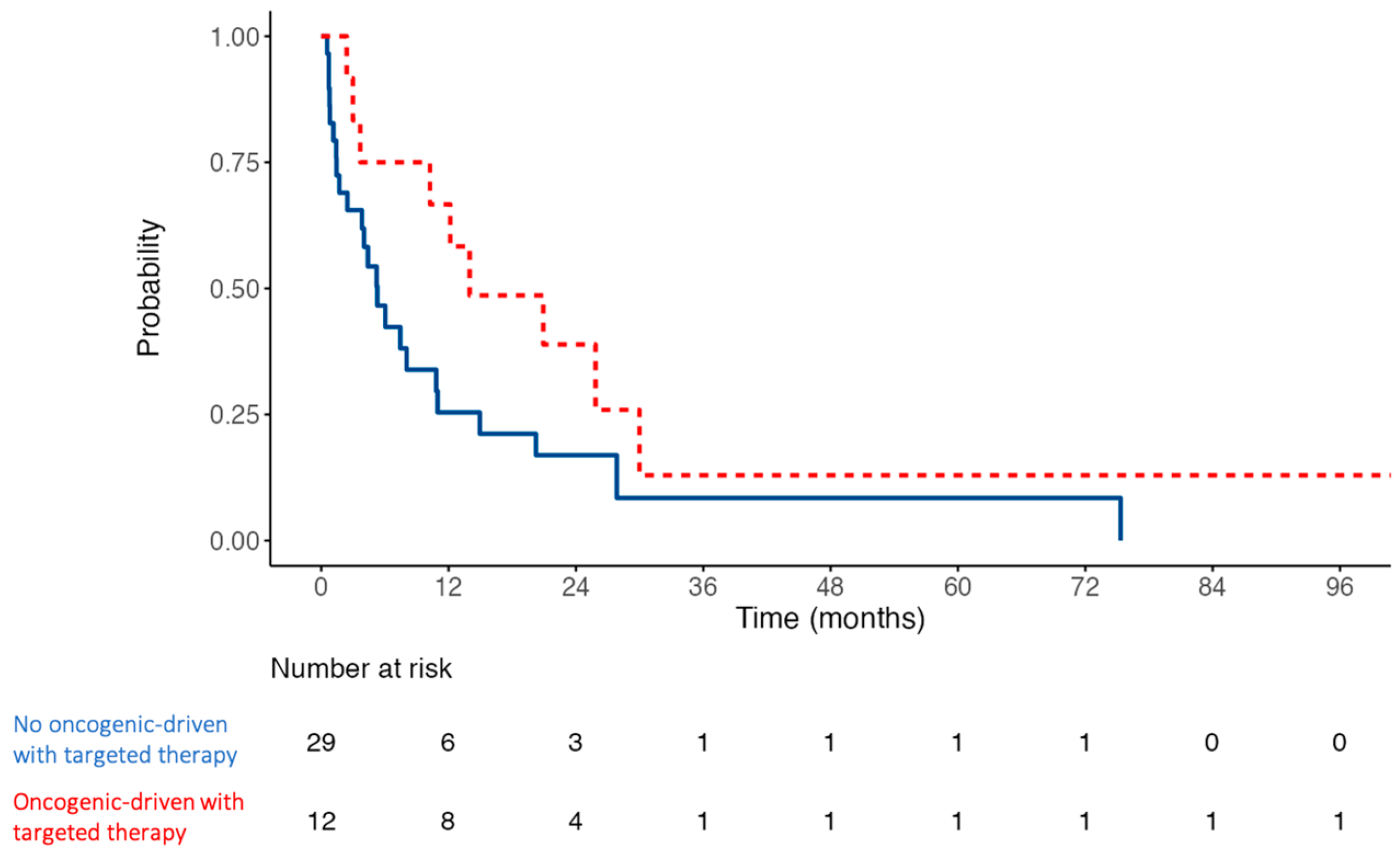
3.5. Recurrence and Survival Outcomes of the Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| AF | Atrial Fibrillation |
| CT | Cardiac Tamponade |
| ECOG | Eastern Cooperative Oncology Group |
| ICU | Intensive Care Unit |
| IPIC | Intrapericardial Instillation of Cisplatin |
| IQR | Interquartile Range |
| MPE | Malignant Pericardial Effusion |
| NSCLC | Non-Small Cell Lung Cancer |
| PS | Performance Status |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TTE | Transthoracic Echocardiography |
Appendix A
| Item No. | Recommendation | Page No. | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide an informative and balanced summary of what was performed and what was found in the abstract | 1–2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 3 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 3 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 3 |
| Participants | 6 | (a) Give the eligibility criteria and the sources and methods of the selection of participants. Describe methods of follow-up | 3–4 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 4 |
| Data sources/measurement | 8 * | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 5 |
| Study size | 10 | Explain how the study size was chosen | 5 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 5 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 5 |
| (b) Describe any methods used to examine subgroups and interactions | 5 | ||
| (c) Explain how missing data were addressed | 5 | ||
| (d) If applicable, explain how loss to follow-up was addressed | 5 | ||
| (e) Describe any sensitivity analyses | 5 | ||
| Results | |||
| Participants | 13 * | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed | 5 |
| (b) Give reasons for non-participation at each stage | 5–6 | ||
| (c) Consider use of a flow diagram | 5–6 | ||
| Descriptive data | 14 * | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 6–7 |
| (b) Indicate number of participants with missing data for each variable of interest | 6–7 | ||
| (c) Summarize follow-up time (e.g., average and total amount) | 6–7 | ||
| Outcome data | 15 * | Report numbers of outcome events or summary measures over time | 7–8 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 7–8 |
| (b) Report category boundaries when continuous variables were categorized | 7–8 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | 7–8 | ||
| Other analyses | 17 | Report other analyses performed—e.g., analyses of subgroups and interactions, and sensitivity analyses | 7–8 |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives | 9 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 9–10 |
| Interpretation | 20 | Give a cautious overall interpretation of results, considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 10–11 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 10–11 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study upon which the present article is based | 12 |
References
- Burazor, I.; Imazio, M.; Markel, G.; Adler, Y. Malignant pericardial effusion. Cardiology 2013, 124, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Colopi, M.; De Ferrari, G.M. Pericardial diseases in patients with cancer: Contemporary prevalence, management and outcomes. Heart 2020, 106, 569–574. [Google Scholar] [CrossRef]
- Maisch, B.; Ristic, A.; Pankuweit, S. Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog. Cardiovasc. Dis. 2010, 53, 157–163. [Google Scholar] [CrossRef]
- Tsang, T.S.M.; Seward, J.B.; Barnes, M.E.; Bailey, K.R.; Sinak, L.J.; Urban, L.H.; Hayes, S.N. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin. Proc. 2000, 75, 248–253. [Google Scholar] [CrossRef]
- Gornik, H.L.; Gerhard-Herman, M.; Beckman, J.A. Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J. Clin. Oncol. 2005, 23, 5211–5216. [Google Scholar] [CrossRef]
- Ilerhunmwuwa, N.; Sedeta, E.; Wasifuddin, M.; Hakobyan, N.; Aiwuyo, H.O.; Perry, J.C.; Uche, I.; Okhawere, K.; Torere, B.E.; Burak, E.; et al. Cardiac Tamponade in Patients with Breast Cancer: A Systematic Review. Cureus 2022, 14, e33123. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- European Society of Medical Oncology (ESMO). ESMO Handbook of Oncological Emergencies, 2nd ed.; ESMO: Lugano, Switzerland, 2016. [Google Scholar]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology—Part 1: Acute coronary syndromes and acute pericardial diseases. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 947–959. [Google Scholar] [CrossRef]
- Vakamudi, S.; Ho, N.; Cremer, P.C. Pericardial Effusions: Causes, Diagnosis, and Management. Prog. Cardiovasc. Dis. 2017, 59, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Dybowska, M.; Szturmowicz, M.; Błasińska, K.; Gatarek, J.; Augustynowicz-Kopeć, E.; Langfort, R.; Kuca, P.; Tomkowski, W. Large Pericardial Effusion—Diagnostic and Therapeutic Options, with a Special Attention to the Role of Prolonged Pericardial Fluid Drainage. Diagnostics 2022, 12, 1453. [Google Scholar] [CrossRef]
- Sánchez-Enrique, C.; Nuñez-Gil, I.J.; Viana-Tejedor, A.; De Agustín, A.; Vivas, D.; Palacios-Rubio, J.; Vilchez, J.P.; Cecconi, A.; Macaya, C.; Fernández-Ortiz, A. Cause and Long-Term Outcome of Cardiac Tamponade. Am. J. Cardiol. 2016, 117, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Ristić, A.D.; Maksimović, R.; Simeunović, D.S.; Milinković, I.; Seferović Mitrović, J.P.; Kanjuh, V.; Pankuweit, S.; Maisch, B. Pericardial syndromes: An update after the ESC guidelines 2004. Heart Fail. Rev. 2013, 18, 255–266. [Google Scholar] [CrossRef]
- Martinoni, A.; Cipolla, C.M.; Cardinale, D.; Civelli, M.; Lamantia, G.; Colleoni, M.; Fiorentini, C. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest 2004, 126, 1412–1416. [Google Scholar] [CrossRef]
- Virk, S.A.; Chandrakumar, D.; Villanueva, C.; Wolfenden, H.; Liou, K.; Cao, C. Systematic review of percutaneous interventions for malignant pericardial effusion. Heart 2015, 101, 1619–1626. [Google Scholar] [CrossRef]
- Jama, G.M.; Scarci, M.; Bowden, J.; Marciniak, S.J. Palliative treatment for symptomatic malignant pericardial effusion. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 1019–1026. [Google Scholar] [CrossRef]
- Defruyt, L.; Özpak, E.; Gevaert, S.; De Buyzere, M.; Vandecasteele, E.; De Pauw, M.; Tromp, F. Malignant cardiac tamponade: Safety and efficacy of intrapericardial bleomycin instillation. Acta Clin. Belg. 2020, 77, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bishiniotis, T.S.; Antoniadou, S.; Katseas, G.; Mouratidou, D.; Litos, A.G.; Balamoutsos, N. Malignant cardiac tamponade in women with breast cancer treated by pericardiocentesis and intrapericardial administration of triethylenethiophosphoramide (thiotepa). Am. J. Cardiol. 2000, 86, 362–364. [Google Scholar] [CrossRef]
- Lestuzzi, C.; Berretta, M.; Tomkowski, W. 2015 update on the diagnosis and management of neoplastic pericardial disease. Expert Rev. Cardiovasc. Ther. 2015, 13, 377–389. [Google Scholar] [CrossRef]
- Kotake, M.; Imai, H.; Kaira, K.; Fujisawa, T.; Yanagita, Y.; Minato, K. Intrapericardial carboplatin in the management of malignant pericardial effusion in breast cancer: A pilot study. Cancer Chemother. Pharmacol. 2019, 84, 655–660. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Cisplatino: Ficha Técnica. 2022. Available online: https://cima.aemps.es/cima/pdfs/es/ft/62107/FT_62107.pdf (accessed on 24 March 2023).
- Maisch, B.; Risti, A.D.; Pankuweit, S.; Neubauer, A.; Moll, R. Neoplastic pericardial effusion: Efficacy and safety of intrapericardial treatment with cisplatin. Eur. Heart J. 2002, 23, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Tomkowski, W.Z.; Wiśniewska, J.; Szturmowicz, M.; Kuca, P.; Burakowski, J.; Kober, J.; Fijałkowska, A. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Support. Care Cancer 2004, 12, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Bischiniotis, T.S.; Lafaras, C.T.; Platogiannis, D.N.; Moldovan, L.; Barbetakis, N.G.; Katseas, G.P. Intrapericardial cisplatin administration after pericardiocentesis in patients with lung adenocarcinoma and malignant cardiac tamponade. Hell. J. Cardiol. 2005, 46, 324–329. [Google Scholar]
- Lestuzzi, C.; Bearz, A.; Lafaras, C.; Gralec, R.; Cervesato, E.; Tomkowski, W.; DeBiasio, M.; Viel, E.; Bishiniotis, T.; Platogiannis, D.N.; et al. Neoplastic pericardial disease in lung cancer: Impact on outcomes of different treatment strategies. A multicenter study. Lung Cancer 2011, 72, 340–347. [Google Scholar] [CrossRef]
- Figoli, F.; Zanette L., Z.M.L.; Tirelli, U.; Sorio, R.; Lestuzzi, C.; Urso, R.; Monfardini, S.; D’Incalci, M. Pharmacokinetics of VM 26 given intrapericardially or intravenously in patients with malignant pericardial effusion. Cancer Chemother. Pharmacol. 1987, 20, 239–242. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef]
- Kunitoh, H.; Tamura, T.; Shibata, T.; Imai, M.; Nishiwaki, Y.; Nishio, M.; Yokoyama, A.; Watanabe, K.; Noda, K.; Saijo, N. A randomised trial of intrapericardial bleomycin for malignant pericardial effusion with lung cancer (JCOG9811). Br. J. Cancer 2009, 100, 464–469. [Google Scholar] [CrossRef]
- Lambert, A.; Salleron, J.; Kieffer, A.; Raymond, P.; Geoffrois, L.; Gavoille, C. Intrapericardial bleomycin instillation prevent recurrence of malignant pericardial effusions: Series of 46 cases and comprehensive literature review. Bull. Cancer 2020, 107, 756–762. [Google Scholar] [CrossRef]
- Wei, J.; Shi, Z.; Song, Z. Effects of intrapericardial administration after catheter drainage on malignant pericardial effusion in non-small cell lung cancer: A real-world study. Cancer Med. 2023, 12, 18211–18218. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Lu, X.; Sumey, C.; Maxson, D.A.; Weickhardt, A.J.; Oton, A.B.; Bunn, P.A.; Barón, A.E.; Franklin, W.A.; Aisner, D.L.; et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012, 118, 4502–4511. [Google Scholar] [CrossRef] [PubMed]
- Fallet, V.; Matton, L.; Schernberg, A.; Canellas, A.; Cornelis, F.H.; Cadranel, J. Local ablative therapy in oncogenic-driven oligometastatic non-small cell lung cancer: Present and ongoing strategies—A narrative review. Transl. Lung Cancer Res. 2021, 10, 3457–3472. [Google Scholar] [CrossRef]
- Guglin, M.; Aljayeh, M.; Saiyad, S.; Ali, R.; Curtis, A.B. Introducing a new entity: Chemotherapy-induced arrhythmia. EP Eur. 2009, 11, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Lafaras, C.; Mavroudi, E.; Platogiannis, D.; Bischiniotis, T. Local chemotherapy with cisplatin in non small lung cancer patients and malignant cardiac tamponade. Intensive Care Med. 2009, 35, S279. [Google Scholar]
- Davis, S.; Rambotti, P.; Grignani, F. Intrapericardial tetracycline sclerosis in the treatment of malignant pericardial effusion: An analysis of thirty-three cases. J. Clin. Oncol. 1984, 2, 631–636. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Morgan, C.; Evans, W.K.; Ginsberg, J.F.; Watt, D.; Murphy, K. Medical management of malignant pericardial effusion by tetracycline sclerosis. Am. J. Cardiol. 1987, 60, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.; Dalmau, E.; Seguí, M.; García, Y.; Bonfill, T.; Pericay, C.; Fernández, L.; Querol, R.; Pampols, M.; Saigí, E. Efficacy and safety of intrapericardial bleomycin for malignant pericardial effusion. J. Clin. Oncol. 2010, 28, e19687. [Google Scholar] [CrossRef]
- Maruyama, R.; Yokoyama, H.; Seto, T.; Nagashima, S.; Kashiwabara, K.; Araki, J.; Semba, H.; Ichinose, Y. Catheter drainage followed by the instillation of bleomycin to manage malignant pericardial effusion in non-small cell lung cancer: A multi-institutional phase II trial. J. Thorac. Oncol. 2007, 2, 65–68. [Google Scholar] [CrossRef]
- Lee, L.N.; Yang, P.C.; Chang, D.B.; Yu, C.J.; Ko, J.C.; Liaw, Y.S.; Wu, R.G.; Luh, K.T. Ultrasound guided pericardial drainage and intrapericardial instillation of mitomycin C for malignant pericardial effusion. Thorax 1994, 49, 594–595. [Google Scholar] [CrossRef]
- Lentzsch, S.; Reichardt, P.; Gürtler, R.; Dörken, B. Intrapericardial Application of Mitoxantrone for Treatment of Malignant Pericardial Effusion. Oncol. Res. Treat. 1994, 17, 504–507. [Google Scholar] [CrossRef]
- Musch, E.; Gremmler, B.; Nitsch, J.; Rieger, J.; Malek, M.; Chrissafidou, A. Intrapericardial Instillation of Mitoxantrone in Palliative Therapy of Malignant Pericardial Effusion. Oncol. Res. Treat. 2003, 26, 135–139. [Google Scholar] [CrossRef]
- Oida, T.; Mimatsu, K.; Kano, H.; Kawasaki, A.; Kuboi, Y.; Fukino, N.; Amano, S. Pericardiocentesis with cisplatin for malignant pericardial effusion and tamponade. World J. Gastroenterol. 2010, 16, 740–744. [Google Scholar] [CrossRef]
- Colleoni, M.; Martinelli, G.; Beretta, F.; Marone, C.; Gallino, A.; Fontana, M.; Graffeo, R.; Zampino, G.; De Pas, T.; Cipolla, G.; et al. Intracavitary chemotherapy with thiotepa in malignant pericardial effusions: An active and well-tolerated regimen. J. Clin. Oncol. 1998, 16, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K. Management of Malignant Pericardial Effusion with Instillation of Mitomycin C in Non-small Cell Lung Cancer. Jpn. J. Clin. Oncol. 2005, 35, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, S.; Esteve, A.; González, A.; Ibáñez, C.; Martínez, J.; Mesía, R.; Clopés, A. Real-world treatment outcomes of medicines used in special situations (off-label and compassionate use) in oncology and hematology: A retrospective study from a comprehensive cancer institution. Cancer Med. 2023, 12, 17112–17125. [Google Scholar] [CrossRef] [PubMed]
- Zaij, S.; Pereira Maia, K.; Leguelinel-Blache, G.; Roux-Marson, C.; Kinowski, J.M.; Richard, H. Intervention of pharmacist included in multidisciplinary team to reduce adverse drug event: A qualitative systematic review. BMC Health Serv. Res. 2023, 23, 927. [Google Scholar] [CrossRef]
- de Castro, G.; Souza, F.H.; Lima, J.; Bernardi, L.P.; Teixeira, C.H.A.; Prado, G.F. Does Multidisciplinary Team Management Improve Clinical Outcomes in NSCLC? A Systematic Review with Meta-Analysis. JTO Clin. Res. Rep. 2023, 4, 100580. [Google Scholar] [CrossRef]

| Baseline Characteristics (n = 41) | |
|---|---|
| Sex, women | 21 (51.2%) |
| Age | 61 (51–69) |
| Current or former smoker | 26 (63.4%) |
| Alcohol consumption | 5 (12.2%) |
| Comorbidities | |
| Arterial hypertension | 18 (43.9%) |
| Dyslipidaemia | 8 (19.5%) |
| Type-2 diabetes mellitus | 6 (14.6%) |
| Chronic heart disease | 5 (12.2%) |
| Chronic lung disease | 4 (9.8%) |
| Chronic kidney disease | 2 (4.9%) |
| Chronic neurological disease | 2 (4.9%) |
| Primary neoplasm | |
| Non-small cell lung cancer | 32 (78%) |
| Small cell lung cancer | 2 (5.8%) |
| Breast cancer | 4 (9.8%) |
| Gastric cancer | 1 (2.4%) |
| Malignant melanoma | 1 (2.4%) |
| Cancer of unknown origin | 1 (2.4%) |
| Main Symptom or Sign at Malignant Pericardial Effusion Diagnosis | |
|---|---|
| Dyspnoea | 30 (73.1%) |
| Hypotension without symptoms | 4 (9.8%) |
| Asthenia | 3 (7.3%) |
| Chest pain or discomfort | 2 (4.9%) |
| Syncope | 1 (2.4%) |
| Constitutional syndrome | 1 (2.4%) |
| Pericardiocentesis characteristics | |
| Initial amount of pericardial fluid drained (mL) 1 | 886 (560–1000) |
| Duration of pericardial drainage (days) | 11 (2–25) |
Adverse events related to pericardiocentesis:
| 7 (17.1%) 5 (12.2%) 1 (2.4%) 1 (2.4%) |
| Data regarding intrapericardial instillation of cisplatin | |
| Days of cisplatin instillation | 4 (3–5) |
| Total of adverse events related to intrapericardial instillation of cisplatin | 10 (24.4%) |
Immediate adverse events (5 first days, those in which instillations are performed):
| 7 (17.1%) 3 (7.3%) 3 (7.3%) 1 (2.4%) 1 (2.4%) |
Early adverse events (from sixth day to fourteen days since the first instillation):
| 3 (7.3%) 2 (4.9%) 1 (2.4%) |
| Late adverse events (two weeks from the first instillation) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Carrillo, F.J.; Reyes, R.M.; Pesántez, D.; Carrera, G.; Cascos, E.; Castro, P.; Fernández-Méndez, S.; Font, C.; González-Aguado, L.; Grafiá, I.; et al. Retrospective Cohort Study of Intrapericardial Cisplatin for Risk Reduction of Malignant Pericardial Effusion Recurrence. Curr. Oncol. 2025, 32, 568. https://doi.org/10.3390/curroncol32100568
Muñoz-Carrillo FJ, Reyes RM, Pesántez D, Carrera G, Cascos E, Castro P, Fernández-Méndez S, Font C, González-Aguado L, Grafiá I, et al. Retrospective Cohort Study of Intrapericardial Cisplatin for Risk Reduction of Malignant Pericardial Effusion Recurrence. Current Oncology. 2025; 32(10):568. https://doi.org/10.3390/curroncol32100568
Chicago/Turabian StyleMuñoz-Carrillo, Francisco Javier, Roxana Maribel Reyes, David Pesántez, Gemma Carrera, Enric Cascos, Pedro Castro, Sara Fernández-Méndez, Carme Font, Laura González-Aguado, Ignacio Grafiá, and et al. 2025. "Retrospective Cohort Study of Intrapericardial Cisplatin for Risk Reduction of Malignant Pericardial Effusion Recurrence" Current Oncology 32, no. 10: 568. https://doi.org/10.3390/curroncol32100568
APA StyleMuñoz-Carrillo, F. J., Reyes, R. M., Pesántez, D., Carrera, G., Cascos, E., Castro, P., Fernández-Méndez, S., Font, C., González-Aguado, L., Grafiá, I., Llavata, L., Monge-Escartín, I., Padrosa, J., Reguart, N., Téllez, A., Tuca, A., Viladot, M., Zamora-Martínez, C., Amorós-Reboredo, P., & Marco-Hernández, J. (2025). Retrospective Cohort Study of Intrapericardial Cisplatin for Risk Reduction of Malignant Pericardial Effusion Recurrence. Current Oncology, 32(10), 568. https://doi.org/10.3390/curroncol32100568