Health Effects of Ergonomics and Personal Protective Equipment on Chemotherapy Professionals
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
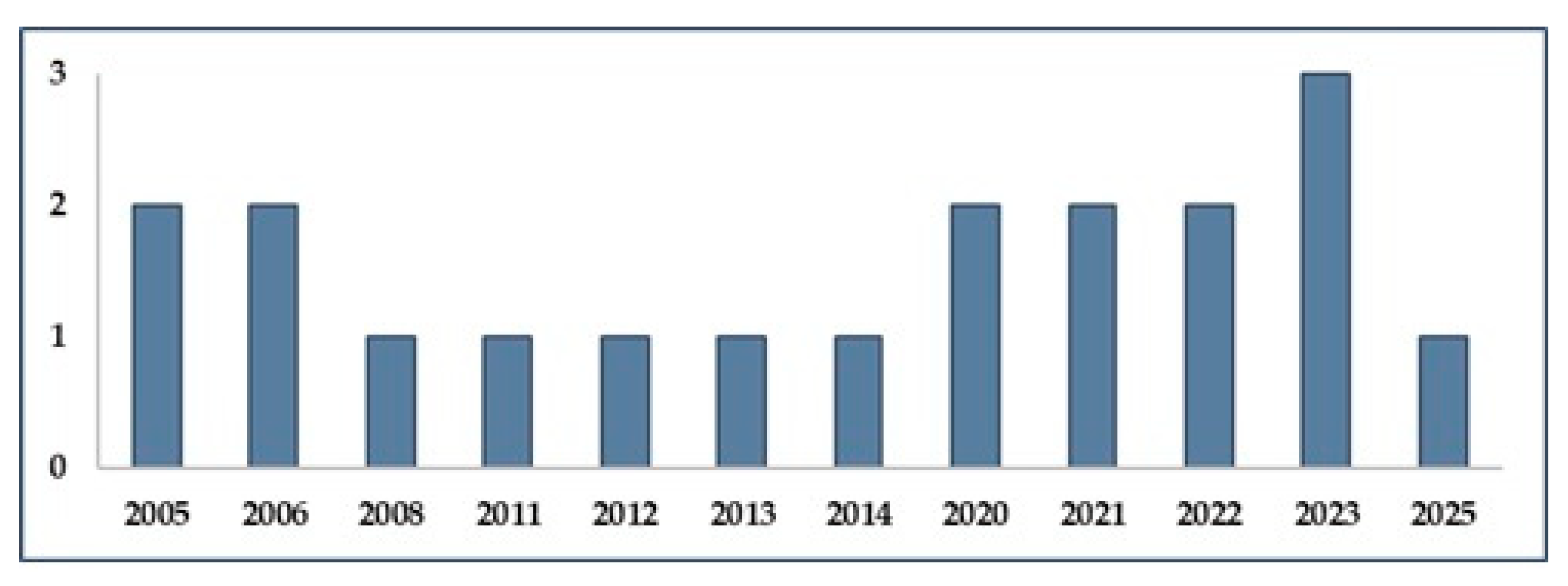
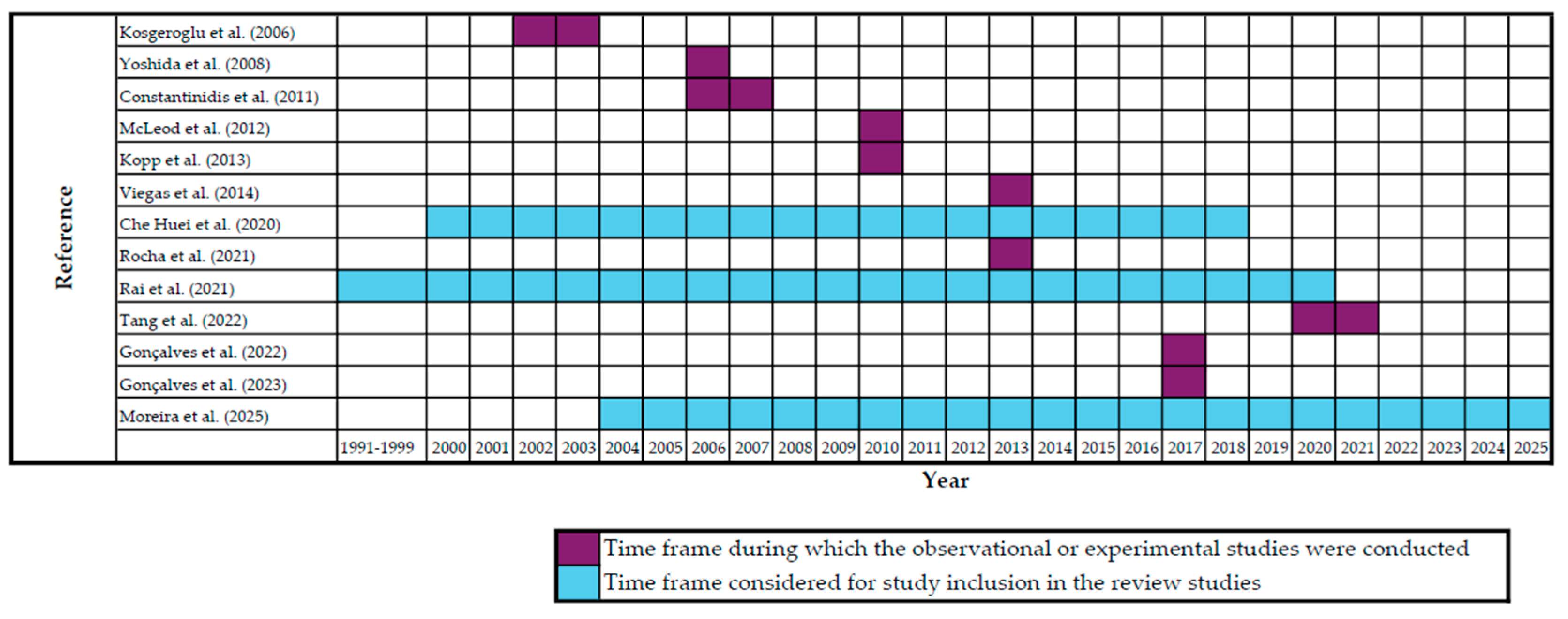
3. Results
4. Discussion
4.1. Personal Protective Equipment (PPE)
4.2. Ergonomics
4.3. Training Gaps
4.4. Psychosocial and Organizational Factors
4.5. Engineering Controls and Recommendations
4.6. Research Gaps, Practical Implications, and Future Directions
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assessment of Repetitive Tasks |
| BSC | Biological Safety Cabinets |
| COSHH | Control of Substances Hazardous to Health Regulations |
| CPE | Chlorinated Polyethylene |
| CSTD | Closed system transfer devices |
| DCT | Docetaxel |
| EMA | European Medicines Agency |
| EPI | Epirubicin |
| ETP | Etoposide |
| FMECA | Failure Modes, Effects, and Criticality Analysis |
| GMP | Good Manufacturing Practices |
| HEPA | High Efficiency Particulate Air |
| HSE | Health and Safety Executive |
| IARC | International Agency for Research on Cancer |
| ISOPP | International Society of Oncology Pharmacy Practitioners |
| LMIC | Low-income or middle-income countries |
| MTX | Methotrexate |
| MSD | Musculoskeletal disorders |
| NIOSH | National Institute for Occupational Safety and Health |
| NT | Nitrile |
| OSHA | Occupational Safety and Health Administration |
| PPE | Personal protective equipment |
| PVC | Polyvinyl Chloride |
| RB | Rubber-based |
| ULD | Upper limb disorder |
| VCR | Vincristine |
References
- Vanderah, T. Katzung’s Basic & Clinical Pharmacology, 16th ed.; MC Graw-Hill: New York, NY, USA, 2023. [Google Scholar]
- Pan American Health Organization. Safe Handling of Hazardous Chemotherapy Drugs in Limited-Resource Settings; Pan American Health Organization: Washington, DC, USA, 2013.
- Saha, D.; Anderson, A.; Cisneros, L.; Maley, C. In Silico Investigations of Adaptive Therapy Using Two Cytotoxic or Two Cytostatic Drugs. bioRxiv 2023. [Google Scholar] [CrossRef]
- de Lemos, M.L.; Badry, N.; Conklin, J.; Koberinski, M. Defining Cytotoxic Drugs—You Know It When You See It? J. Oncol. Pharm. Pract. 2021, 27, 1958–1962. [Google Scholar] [CrossRef]
- Winkler, G.C.; Barle, E.L.; Galati, G.; Kluwe, W.M. Functional Differentiation of Cytotoxic Cancer Drugs and Targeted Cancer Therapeutics. Regul. Toxicol. Pharmacol. 2014, 70, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Constantinidis, T.C.; Vagka, E.; Dallidou, P.; Basta, P.; Drakopoulos, V.; Kakolyris, S.; Chatzaki, E. Occupational Health and Safety of Personnel Handling Chemotherapeutic Agents in Greek Hospitals. Eur. J. Cancer Care 2011, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, P.R. Handling of Cytotoxic Drugs by Healthcare Workers. A Review of the Risks of Exposure. Drug Saf. 1992, 7, 374–380. [Google Scholar] [CrossRef]
- Sessink, P.J.; Kroese, E.D.; van Kranen, H.J.; Bos, R.P. Cancer Risk Assessment for Health Care Workers Occupationally Exposed to Cyclophosphamide. Int. Arch. Occup. Environ. Health 1995, 67, 317–323. [Google Scholar] [CrossRef]
- Sorsa, M.; Hemminki, K.; Vainio, H. Occupational Exposure to Anticancer Drug—Potential and Real Hazards. Mutat. Res. 1985, 154, 135–149. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Falck, K.; Gröhn, P.; Sorsa, M.; Vainio, H.; Heinonen, E.; Holsti, L. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet 1979, 313, 1250–1251. [Google Scholar] [CrossRef]
- The National Institute for Occupational Safety and Health. Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings; The National Institute for Occupational Safety and Health: Washington, DC, USA, 2004.
- van Huizen, P.; Russo, P.L.; Manias, E.; Kuhn, L.; Connell, C.J. Knowledge and Safe Handling Practices Affecting the Occupational Exposure of Nurses and Midwives to Hazardous Drugs: A Mixed Methods Systematic Review. Int. J. Nurs. Stud. 2024, 160, 104907. [Google Scholar] [CrossRef]
- Alehashem, M.; Baniasadi, S. Important Exposure Controls for Protection against Antineoplastic Agents: Highlights for Oncology Health Care Workers. Work 2018, 59, 165–172. [Google Scholar] [CrossRef]
- Topçu, S.; Beşer, A. Oncology Nurses’ Perspectives on Safe Handling Precautions: A Qualitative Study. Contemp. Nurse 2017, 53, 271–283. [Google Scholar] [CrossRef]
- Campbell, K.; Afseth, J.; Dunham, M.; King, M.; Dicksit, D. Global Cancer Nurse’s Experiences and Perceptions of Potential Occupational Exposure to Cytotoxic Drugs: Mixed Method Systematic Review with Framework Synthesis. J. Clin. Nurs. 2024, 33, 4585–4601. [Google Scholar] [CrossRef]
- Simons, A.; Toland, S. Perceived Adverse Effects from Handling Systemic Anti-Cancer Therapy Agents. Br. J. Nurs. 2017, 26, S38–S44. [Google Scholar] [CrossRef]
- Connor, T.H.; Lawson, C.C.; Polovich, M.; McDiarmid, M.A. Reproductive Health Risks Associated with Occupational Exposures to Antineoplastic Drugs in Health Care Settings: A Review of the Evidence. J. Occup. Environ. Med. 2014, 56, 901–910. [Google Scholar] [CrossRef]
- Ratner, P.A.; Spinelli, J.J.; Beking, K.; Lorenzi, M.; Chow, Y.; Teschke, K.; Le, N.D.; Gallagher, R.P.; Dimich-Ward, H. Cancer Incidence and Adverse Pregnancy Outcome in Registered Nurses Potentially Exposed to Antineoplastic Drugs. BMC Nurs. 2010, 9, 15. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Nucci, D.; Villarini, M.; Moretti, M. [Genotoxic risk in nurses handling antiblastic drugs: Systematic review of literature and meta-analysis]. Recenti. Prog. Med. 2017, 108, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Grollino, M.G.; Pavanello, S.; Bonfiglioli, R.; Villarini, M.; Appolloni, M.; Carrieri, M.; Sabatini, L.; Dominici, L.; Stronati, L.; et al. Micronuclei and Chromosome Aberrations in Subjects Occupationally Exposed to Antineoplastic Drugs: A Multicentric Approach. Int. Arch. Occup. Environ. Health 2015, 88, 683–695. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, M.A.; Rogers, B.; Oliver, M.S. Chromosomal Effects of Non-Alkylating Drug Exposure in Oncology Personnel. Environ. Mol. Mutagen. 2014, 55, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Soleyman-Jahi, S.; Zendehdel, K.; Mozdarani, H.; Azimi, C.; Farzanfar, F.; Safari, Z.; Mohagheghi, M.-A.; Khaleghian, M.; Divsalar, K.; et al. Chromosomal Aberrations, Sister Chromatid Exchanges, and Micronuclei in Lymphocytes of Oncology Department Personnel Handling Anti-Neoplastic Drugs. Drug Chem. Toxicol. 2017, 40, 235–240. [Google Scholar] [CrossRef]
- Goodman, J.; Lynch, H. Improving the International Agency for Research on Cancer’s Consideration of Mechanistic Evidence. Toxicol. Appl. Pharmacol. 2017, 319, 39–46. [Google Scholar] [CrossRef]
- Crul, M.; Simons-Sanders, K. Carry-over of Antineoplastic Drug Contamination in Dutch Hospital Pharmacies. J. Oncol. Pharm. Pract. 2018, 24, 483–489. [Google Scholar] [CrossRef]
- Crul, M.; Hilhorst, S.; Breukels, O.; Bouman-d’Onofrio, J.R.C.; Stubbs, P.; van Rooij, J.G. Occupational Exposure of Pharmacy Technicians and Cleaning Staff to Cytotoxic Drugs in Dutch Hospitals. J. Occup. Environ. Hyg. 2020, 17, 343–352. [Google Scholar] [CrossRef]
- Kieffer, C.; Verhaeghe, P.; Lagrassa, S.; Grégoire, R.; Moussaoui, Z.; Casteras-Ducros, C.; Clark, J.E.; Vanelle, P.; Rathelot, P. Preventing the Contamination of Hospital Personnel by Cytotoxic Agents: Evaluation and Training of the Para-Professional Healthcare Workers in Oncology Units. Eur. J. Cancer Care 2015, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, E.; Creta, M.; Vanoirbeek, J.; Zakia, M.; Abdesselam, T.; Lebegge, R.; Poels, K.; Duca, R.-C.; Godderis, L. Environmental Contamination and Occupational Exposure of Algerian Hospital Workers. Front. Public Health 2020, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Pádua, M.; Veiga, A.C.; Carolino, E.; Gomes, M. Antineoplastic Drugs Contamination of Workplace Surfaces in Two Portuguese Hospitals. Environ. Monit. Assess 2014, 186, 7807–7818. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; de Oliveira, A.C.; Carolino, E.; Pádua, M. Occupational Exposure to Cytotoxic Drugs: The Importance of Surface Cleaning to Prevent or Minimise Exposure. Arch. Ind. Hyg. Toxicol. 2018, 69, 238–249. [Google Scholar] [CrossRef]
- ISOPP Standards for the Safe Handling of Cytotoxics. J. Oncol. Pharm. Pract. 2022, 28, S1–S126. [CrossRef]
- Easty, A.C.; Coakley, N.; Cheng, R.; Cividino, M.; Savage, P.; Tozer, R.; White, R.E. Safe Handling of Cytotoxics: Guideline Recommendations. Curr. Oncol. 2015, 22, e27–e37. [Google Scholar] [CrossRef]
- Yoshida, J.; Kosaka, H.; Nishida, S.; Kumagai, S. Actual Conditions of the Mixing of Antineoplastic Drugs for Injection in Hospitals in Osaka Prefecture, Japan. J. Occup. Health 2008, 50, 86–91. [Google Scholar] [CrossRef]
- Working Committee on the Safe Handling of Hazardous Drugs. Prevention Guide: Safe Handling of Hazardous Drugs; IRSST: Montreal, QC, Canada, 2008. [Google Scholar]
- Korczowska, E.; Crul, M.; Tuerk, J.; Meier, K. Environmental Contamination with Cytotoxic Drugs in 15 Hospitals from 11 European Countries—Results of the MASHA Project. Eur. J. Oncol. Pharm. 2020, 3, e24. [Google Scholar] [CrossRef]
- Jun, E.-M.; Kang, S.-W. Effects of Safe Handling Education on Cognition, Compliance and Stress Handling of Antineoplastic Drugs in Clinical Nurses. Nurs. Open 2023, 10, 4144–4152. [Google Scholar] [CrossRef]
- Cotteret, C.; Secretan, P.-H.; Gilles-Afchain, L.; Rousseau, J.; Vidal, F.; Salguero-Hernandez, G.; Batista, J.; Valverde, V.; Guitton, J.; Cisternino, S.; et al. External Contamination of Antineoplastic Drug Vials: An Occupational Risk to Consider. Eur. J. Hosp. Pharm. 2022, 29, 284–286. [Google Scholar] [CrossRef]
- Sessink, P.J.M.; Connor, T.H.; Jorgenson, J.A.; Tyler, T.G. Reduction in Surface Contamination with Antineoplastic Drugs in 22 Hospital Pharmacies in the US Following Implementation of a Closed-System Drug Transfer Device. J. Oncol. Pharm. Pract. 2011, 17, 39–48. [Google Scholar] [CrossRef]
- Ziegler, E.; Mason, H.J.; Baxter, P.J. Occupational Exposure to Cytotoxic Drugs in Two UK Oncology Wards. Occup. Environ. Med. 2002, 59, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Askarian, M.; Azad, H.; Danaei, M. Exposure To Cytotoxic Drugs Threatens The Health Of Staff In Oncology Wards. Russ. Open Med. J. 2021, 10, e0316. [Google Scholar] [CrossRef]
- Simegn, W.; Dagnew, B.; Dagne, H. Knowledge and Associated Factors towards Cytotoxic Drug Handling among University of Gondar Comprehensive Specialized Hospital Health Professionals, Institutional-Based Cross-Sectional Study. Environ. Health Prev. Med. 2020, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration. Controlling Occupational Exposure to Hazardous Drugs; Occupational Safety and Health Administration: Washington DC, USA, 2016.
- European Medicines Agency (EMA). Good Manufacturing Practice (GMP) Guidelines; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2017.
- European Union. Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers From the Risks Related to Exposure to Carcinogens or Mutagens at Work; European Union: Brussels, Belgium, 2004.
- Health and Safety Executive (HSE). Carcinogens and Mutagens: The Control of Substances Hazardous to Health Regulations; Health and Safety Executive (HSE): Bootle, UK, 2015.
- Federal Institute for Occupational Safety and Health (BAuA). Cytotoxic Drugs: Information for Healthcare Professionals; Federal Institute for Occupational Safety and Health (BAuA): Chemnitz, Germany, 2021. [Google Scholar]
- Edwards, C.; Fortingo, N.; Franklin, E. Ergonomics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Waters, T.R.; Dick, R.B. Evidence of Health Risks Associated with Prolonged Standing at Work and Intervention Effectiveness. Rehabil. Nurs. 2015, 40, 148–165. [Google Scholar] [CrossRef]
- Andersen, L.L.; Vinstrup, J.; Sundstrup, E.; Skovlund, S.V.; Villadsen, E.; Thorsen, S.V. Combined Ergonomic Exposures and Development of Musculoskeletal Pain in the General Working Population: A Prospective Cohort Study. Scand. J. Work Environ. Health 2021, 47, 287–295. [Google Scholar] [CrossRef]
- Carayon, P.; Smith, M.J.; Haims, M.C. Work Organization, Job Stress, and Work-Related Musculoskeletal Disorders. Hum. Factors 1999, 41, 644–663. [Google Scholar] [CrossRef]
- Habibi, E.; Pourabdian, S.; Atabaki, A.K.; Hoseini, M. Evaluation of Work-Related Psychosocial and Ergonomics Factors in Relation to Low Back Discomfort in Emergency Unit Nurses. Int. J. Prev. Med. 2012, 3, 564–568. [Google Scholar]
- Lemma, E.; Taye, B.; Hussen, F. Ergonomic Workstations and Work-Related Musculoskeletal Disorders in the Clinical Laboratory. Lab. Med. 2012, 43, e11–e19. [Google Scholar] [CrossRef]
- Erturk Sengel, B.; Tukenmez Tigen, E.; Bilgin, H.; Dogru, A.; Korten, V. Occupation-Related Injuries Among Healthcare Workers: Incidence, Risk Groups, and the Effect of Training. Cureus 2021, 13, e14318. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, H.; Tang, L.; He, Y.; Tian, S. The Factors of Non-Specific Chronic Low Back Pain in Nurses: A Meta-Analysis. J. Back Musculoskelet. Rehabil. 2021, 34, 343–353. [Google Scholar] [CrossRef]
- Barr, A.E.; Barbe, M.F. Pathophysiological Tissue Changes Associated with Repetitive Movement: A Review of the Evidence. Phys. Ther. 2002, 82, 173–187. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M.; Zochowska, A.; Leonard, D.; Crow, M.; Jacklin, A.; Franklin, B.D. Comparing the Upper Limb Disorder Risks Associated with Manual and Automated Cytotoxic Compounding: A Pilot Study. Eur. J. Hosp. Pharm. 2012, 19, 293. [Google Scholar] [CrossRef]
- Moreira, F.; Jesus, Â.; Pinho, C.; Santos, M.; Serdoura, M.; Cruz, A. Ensuring Safety in Cytotoxic Drug Preparation: A Systematic Review of Guidelines Addressing Education for Pharmacy Professionals. J. Am. Pharm. Assoc. (2003) 2025, 65, 102352. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Martínez, M.A.; Ramos Merino, M.; Santos Gago, J.M.; Álvarez Sabucedo, L.M.; Wanden-Berghe, C.; Sanz-Valero, J. Guidelines for Safe Handling of Hazardous Drugs: A Systematic Review. PLoS ONE 2018, 13, e0197172. [Google Scholar] [CrossRef]
- Fazel, S.S.; Keefe, A.; Shareef, A.; Palmer, A.L.; Brenner, D.R.; Nakashima, L.; Koehoorn, M.W.; McLeod, C.B.; Hall, A.L.; Peters, C.E. Barriers and Facilitators for the Safe Handling of Antineoplastic Drugs. J. Oncol. Pharm. Pract. 2022, 28, 1709–1721. [Google Scholar] [CrossRef]
- Favier, B.; Simonin, C.; Tokatian, S.; Guitton, J.; Darnis, S.; Basset, M.; Chabaud, S.; Gilles, L. Cytotoxic Surface Contamination in Hospitals: Current Practices, Challenges and Perspectives. J. Oncol. Pharm. Pract. 2025, 31, 305–314. [Google Scholar] [CrossRef]
- Wallemacq, P.E.; Capron, A.; Vanbinst, R.; Boeckmans, E.; Gillard, J.; Favier, B. Permeability of 13 Different Gloves to 13 Cytotoxic Agents under Controlled Dynamic Conditions. Am. J. Health Syst. Pharm. 2006, 63, 547–556. [Google Scholar] [CrossRef]
- Kosgeroglu, N.; Ayranci, U.; Ozerdogan, N.; Demirustu, C. Turkish Nurses’ Information about, and Administration of, Chemotherapeutic Drugs. J. Clin. Nurs. 2006, 15, 1179–1187. [Google Scholar] [CrossRef]
- Kopp, B.; Schierl, R.; Nowak, D. Evaluation of Working Practices and Surface Contamination with Antineoplastic Drugs in Outpatient Oncology Health Care Settings. Int. Arch. Occup. Environ. Health 2013, 86, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Che Huei, L.; Ya-Wen, L.; Chiu Ming, Y.; Li Chen, H.; Jong Yi, W.; Ming Hung, L. Occupational Health and Safety Hazards Faced by Healthcare Professionals in Taiwan: A Systematic Review of Risk Factors and Control Strategies. SAGE Open Med. 2020, 8, 2050312120918999. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.D.; Gomes, A.N.H.; Zen, P.R.G.; Bica, C.G. Handling of Antineoplastic Drugs: A Health Concern among Health Care Workers. Rev. Bras. Med. Trab. 2021, 18, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; El-Zaemey, S.; Dorji, N.; Rai, B.D.; Fritschi, L. Exposure to Occupational Hazards among Health Care Workers in Low- and Middle-Income Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2603. [Google Scholar] [CrossRef]
- Tang, Y.; Che, X.; Wang, Y.L.; Ye, X.; Cao, W.L.; Wang, Y. Evaluation of Closed System Transfer Devices in Preventing Chemotherapy Agents Contamination During Compounding Process-A Single and Comparative Study in China. Front. Public Health 2022, 10, 827835. [Google Scholar] [CrossRef]
- Gonçalves, A.; de Oliveira, R.A.F.; Fernandes, P.O. Protective Equipment Applicable to a Centralized Cytostatic Preparation Unit. Procedia Comput. Sci. 2022, 196, 663–672. [Google Scholar] [CrossRef]
- Gonçalves, A.; Oliveira, R.; Fernandes, P. The Occupational Risks and Health Effects Resulting from Exposition to Cytotoxic Drugs Preparation. Procedia Comput. Sci. 2023, 219, 1420–1429. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Xu, F. Comparison of Permeabilities of Eight Different Types of Cytotoxic Drugs to Five Gloves with Different Materials by LC-MS/MS Methods to Reduce Occupational Exposure of Medical Personnel. J. Oncol. Pharm. Pract. 2023, 29, 1548–1554. [Google Scholar] [CrossRef]
- Bhirich, N.; Chefchaouni, A.C.; Medkouri, S.E.; Shytry, O.; Belahcen, M.J.; Rahali, Y. Risk Assessment of Personnel Exposure in a Central Cytotoxic Preparation Unit Using the FMECA Method. J. Oncol. Pharm. Pract. 2023, 29, 1884–1892. [Google Scholar] [CrossRef]
- McDiarmid, M.A.; Condon, M. Organizational Safety Culture/Climate and Worker Compliance with Hazardous Drug Guidelines: Lessons from the Blood-Borne Pathogen Experience. J. Occup. Environ. Med. 2005, 47, 740–749. [Google Scholar] [CrossRef]
- Fransman, W.; Vermeulen, R.; Kromhout, H. Dermal Exposure to Cyclophosphamide in Hospitals during Preparation, Nursing and Cleaning Activities. Int. Arch. Occup. Environ. Health 2005, 78, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Villain, A.; Sakji, I.; Bogart, E.; Strobbe, G.; Marliot, G.; Feutry, F. Optimisation of the Preparation of Chemotherapy Based on 5-Fluorouracil by the Use of Peristaltic Pumps. Pharm. Technol. Hosp. Pharm. 2020, 5, 20200003. [Google Scholar] [CrossRef]
- SHPA Committee of Specialty Practice in Oncology. SHPA Standards of Practice for the Safe Handling of Cytotoxic Drugs in Pharmacy Departments. J. Pharm. Pract. Res. 2005, 35, 44–52. [Google Scholar] [CrossRef]
- Yuki, M.; Sekine, S.; Takase, K.; Ishida, T.; Sessink, P.J.M. Exposure of Family Members to Antineoplastic Drugs via Excreta of Treated Cancer Patients. J. Oncol. Pharm. Pract. 2013, 19, 208–217. [Google Scholar] [CrossRef]
- Böhlandt, A.; Sverdel, Y.; Schierl, R. Antineoplastic Drug Residues inside Homes of Chemotherapy Patients. Int. J. Hyg. Environ. Health 2017, 220, 757–765. [Google Scholar] [CrossRef]
- Bláhová, L.; Kuta, J.; Doležalová, L.; Kozáková, Š.; Hojdarová, T.; Bláha, L. Levels and Risks of Antineoplastic Drugs in Households of Oncology Patients, Hospices and Retirement Homes. Environ. Sci. Eur. 2021, 33, 104. [Google Scholar] [CrossRef]
- Çınar, D.; Karadakovan, A. Investigation of Occupational Safety in Oncology Nurses. Int. J. Occup. Saf. Ergon. 2022, 28, 1750–1755. [Google Scholar] [CrossRef]
- Soheili, M.; Taleghani, F.; Jokar, F.; Eghbali-Babadi, M.; Sharifi, M. Oncology Nurses’ Needs Respecting Healthy Work Environment in Iran: A Descriptive Exploratory Study. Asia Pac. J. Oncol. Nurs. 2021, 8, 188–196. [Google Scholar] [CrossRef]
- Abbasi, K.; Hazrati, M.; Mohammadbeigi, A.; Ansari, J.; Sajadi, M.; Hosseinnazzhad, A.; Moshiri, E. Protection Behaviors for Cytotoxic Drugs in Oncology Nurses of Chemotherapy Centers in Shiraz Hospitals, South of Iran. Indian J. Med. Paediatr. Oncol. 2016, 37, 227–231. [Google Scholar] [CrossRef]
- Ramphal, R.; Bains, T.; Goulet, G.; Vaillancourt, R. Occupational Exposure to Chemotherapy of Pharmacy Personnel at a Single Centre. Can. J. Hosp. Pharm. 2015, 68, 104–112. [Google Scholar] [CrossRef]

| Database | Search String |
|---|---|
| PubMed | (“Cytotoxic Drugs”[MeSH Terms] OR “Antineoplastic Agents”[MeSH Terms] OR “Hazardous Drugs”) AND (“Handling” OR “Exposure”) AND (“Ergonomics” OR “Musculoskeletal Injuries” OR “Personal Protective Equipment” OR PPE) |
| Web of Science | Cytotoxic Handling Musculoskeletal Injuries Personal Protective Equipment Ergonomics |
| Google Scholar | Cytotoxic Handling Musculoskeletal Injuries Personal Protective Equipment Ergonomics |
| Reference | Year of Publication | Study Type | Country | Journal | Sample | Limitations |
|---|---|---|---|---|---|---|
| Fransman et al. [73] | 2005 | Experimental | The Netherlands | Int Arch Occup Environ Health | Total of 389 samples of dermal exposure from healthcare professionals (pharmacy technicians, oncology nurses and cleaning personnel), surfaces and materials (e.g., gloves) from four hospitals | The entire glove was analyzed; contamination might have been on the inside, indicating actual rather than potential exposure. Recovery efficiency was assumed equal across different glove types (based on a prior study with only one glove brand), possibly causing exposure misclassification. Multiple dermal exposure assessment techniques were used (e.g., glove analysis, skin wipes), each with differing efficiency. Surrogate sampling methods (e.g., gloves and patches) may overestimate exposure, particularly on forearms. Hospital layout differences (e.g., cleaning the whole bathroom vs. just the toilet) influenced exposure results. |
| Wallemacq et al. [61] | 2006 | Experimental | The medical school of the Catholic University of Louvain in Brussels, Belgium. | Am J Health Syst Pharm | Different glove types: Neoprene, nitrile, and natural rubber latex gloves. Total of 13 gloves testing 13 cytotoxic agents used by pharmacy personnel (undisclosed professions) | Lab-based exposure conditions (controlled temperature and time) may underestimate real-life permeation, as higher temperatures (body heat), motion, and humidity increase risks. No standardized prediction possible:
|
| Kosgeroglu et al. [62] | 2006 | Cross-Sectional Observational | Hospitals in Eskisehir, west Turkey | J Clin Nurs | A total of 121 nurses responded to a questionnaire concerning exposure to chemotherapy | Study only included 121 nurses from Turkey, limiting its generalizability Observational bias may exist as behavior was partly self-reported |
| Yoshida et al. [33] | 2008 | Cross-sectional Observational | Osaka Prefecture, Japan | J Occup Health | Total of 107 questionnaires answered by doctors, nurses and pharmacists, depicting the conditions of preparation of cytotoxic drugs, in their respective hospitals | Self-reported data gathered through questionnaires may be subject to bias or underreporting. No statistical analysis was conducted to compare awareness levels by job role due to insufficient sample size. Exposure levels were not measured directly (no environmental sampling or biological monitoring). Waste and excreta handling practices were poorly documented and implemented, especially regarding patient excreta, which may pose delayed exposure risks. |
| Constantinidis et al. [6] | 2011 | Cross-sectional Observational | Greece | Eur J Cancer Care (Engl) | Questionnaire distributed by 24 Greek hospitals with 353 answers by healthcare workers (96% corresponding to assistant nurses, and the remaining to pharmacist assistants and technicians) enrolled in handling chemotherapeutic drugs | Most data were collected through self-assessment questionnaires, introducing possible subjectivity or recall bias. Reported symptoms (e.g., headaches, skin irritation) were not clinically diagnosed. Causal relationships between exposure, PPE use, and health outcomes cannot be firmly established Lack of institutional health and safety infrastructure (e.g., occupational physicians, safety officers) reduced formal evaluation or reporting mechanisms. |
| McLeod et al. [56] | 2012 | Prospective Observational | United Kingdom hospital pharmacy | Eur J Hosp Pharm | Observation of 21 manual preparation sessions of cytotoxic drugs by 6 operators and 4 automated preparation sessions by 2 operators (exact professions undisclosed) | Small sample size, particularly for automated sessions (n = 4), limits generalizability. Sessions were not matched for number/type of drugs compounded—ULD risk likely increases with volume and complexity. Observer bias risk due to long observation periods (up to 148 min); inter-rater reliability could not be statistically confirmed. Psychosocial risk factors were not included in the ART assessment, although these are known contributors to ULDs. |
| Kopp et al. [63] | 2013 | Cross-sectional Observational and Experimental | Germany | Int Arch Occup Environ Health | Forty institutions (17 day hospitals and 23 private clinics) completed a questionnaire on cytotoxic handling A total of 375 surface wipe samples were collected from 28 facilities, including floors of medical and therapy rooms, toilets, infusion poles, and infusion pumps, testing for 8 cytotoxic agents. | Only 40 out of 137 contacted facilities participated, potentially introducing selection bias. Small sample size for correlation analysis limited statistical power. Cross-contamination and cleaning variability: Difficult to attribute contamination to specific work practices. |
| Viegas et al. [29] | 2014 | Experimental | Portugal | Environ Monit Assess | A total of 327 surface wipe samples were collected from 2 Portuguese hospitals, testing for 3 cytotoxic agents, prepared by pharmacy technicians. | The study is based on only two hospital settings, which limits the generalizability of the findings. Lack of detailed reporting on PPE practices: while environments imply proper use, the actual compliance, comfort, or training aspects are not explored. Ergonomic assessment is indirect—findings are inferred from infrastructure and workflow design rather than direct ergonomic evaluation or staff feedback. No quantitative data on incidents, exposures, or ergonomic strain are provided, limiting statistical analysis. |
| Villain et al. [74] | 2020 | Experimental and Cross-sectional Observational | France | Pharmaceutical Technology in Hospital Pharmacy | Comparison of 24 measurements in manual filling and 24 in automated mode Six pharmacy technicians completed a questionnaire for each of the two elastomeric pumps, both with automated and manual filling (24 questionnaires in total) | Calibration method depends on visual reading by staff and is operator-dependent, requiring strict training to minimize risk—volume delivery accuracy may be affected by poor calibration, although mitigated by a gravimetric control system with a 5% detection threshold. Economic limitation: Automation introduces a higher upfront cost, though this cost decreases significantly when multiple preparations are made. Cost-effectiveness depends on routine use and may improve with bulk pricing negotiations. |
| Rocha et al. [65] | 2021 | Cross-sectional Observational | Brazil. | Rev Bras Med Trab | A total of 40 healthcare workers handling antineoplastic drugs (11 nurses, 14 pharmacists, and 15 technicians) from the 3 major referral centers for chemotherapy treatment | Small sample size (n = 40) from only three referral centers in a single city (Porto Alegre), limiting generalizability. Self-reported data on accidents and PPE use may introduce recall or reporting bias. Underreporting of accidents: Only 44.4% of those who experienced exposure submitted official Work Accident Statements. No direct ergonomic assessments (e.g., posture analysis, workload evaluation) were performed—risks were inferred rather than measured. Lack of documentation of training activities limits the ability to assess the effectiveness of safety programs. Knowledge gaps among healthcare workers suggest training content may be insufficient or inconsistently delivered. |
| Tang et al. [67] | 2022 | Experimental | China | Front Public Health | A total of 96 wipe samples were collected from protective equipment (gloves, masks) and other sites (door handles, air-intake vents, transfer containers, etc.) in 1 hospital in Shanghai, testing for 2 cytotoxic agents, handled by 8 pharmacists and 6 pharmacy technicians. | Single-center study, which limits the generalizability of the findings. Small sample size, potentially reducing the statistical power and robustness of the results. |
| Gonçalves et al. [68] | 2022 | Cross-sectional Observational | Portugal | Procedia Computer Science | A questionnaire was applied to 83 professionals involved in cytotoxic handling (“most pharmacy technicians”—undisclosed percentage or representation by other professions) from 18 hospital institutions | Exploratory and descriptive design; lacks analytical or inferential scope Included studies collected data restricted to specific hospitals, limiting international generalizability, and displayed high difficulty obtaining responses, leading to potential non-response bias. |
| Gonçalves et al. [69] | 2023 | Cross-sectional Observational | Portugal | Procedia Computer Science | A questionnaire was applied to 83 professionals involved in cytotoxic handling (“most pharmacy technicians”—undisclosed percentage or representation by other professions) from 18 hospital institutions | Low response rate from hospitals hindered data collection and required persistent follow-up from researchers—Limited generalizability of the findings to the entire population of cytotoxic handlers. Potential selection bias, as not all invited professionals participated. Use of self-reported questionnaires, which may be subject to underreporting or recall bias |
| Zhou et al. [70] | 2023 | Experimental | China | J Oncol Pharm Pract | A total of 240 samples from 5 different glove materials were tested for permeation of 8 cytotoxic drugs (no specific professionals involved as the study targeted the gloves) | High variability in repeated tests for the same glove/drug/time combination, possibly due to inconsistencies in glove material composition. The experiment was in vitro; actual clinical conditions (e.g., hand movement, temperature, sweat, and friction) were not simulated. Only single-use gloves were tested per trial, limiting real-world generalizability where gloves may be worn for extended periods. The solvent used (ethanol) may have enhanced drug permeation in certain cases (e.g., DCT, ETP), limiting applicability to drugs prepared with different solvents. Ergonomic and psychosocial factors related to glove use (e.g., discomfort, fatigue) were not addressed. The study was conducted in a controlled laboratory setting, not in a live clinical environment, which may limit extrapolation of results. |
| Bhirich et al. [71] | 2023 | Qualitative Risk Analysis/Observational Study | Morocco | J Oncol Pharm Pract | Multidisciplinary group of 7 pharmacy professionals (1 professor of pharmacotechnics, 3 resident pharmacists, 1 pharmacist in charge of unit of cytotoxic preparation, and 2 pharmacy operators) | The FMECA analysis was based on subjective assessments from a multidisciplinary working group; ratings of severity, frequency, and detectability may vary with different participants. No quantitative exposure measurements (e.g., surface or biological contamination) were performed; results are based on perceived risk, not direct monitoring. The study was single-centered, conducted in a single institution, limiting generalizability. Despite identifying 12 failure modes, only three were deemed critical, and their CI was partially influenced by group discussion biases. |
| Reference | Year of Publication | Review Type | Journal | Number of Included Studies | Languages of Included Studies | Searched Databases | Limitations |
|---|---|---|---|---|---|---|---|
| McDiarmid & Condon [72] | 2005 | Narrative Review | Journal of Occupational and Environmental Medicine | Not revealed | Not revealed | Not revealed | There is a lack of systematic implementation of safety standards and feedback mechanisms. |
| Che Huei et al. [64] | 2020 | Systematic Review | SAGE Open Medicine | 30 | Not revealed | MEDLINE (Ovid), PubMed, PMC, TOXLINE, CINAHL, PLOS One, and Access Pharmacy | Selection bias: although published studies and some of the gray literature were included, potentially valuable unverifiable reports may have been excluded. Reliance on observational studies: no intervention studies were included, which may limit causal inferences. Heterogeneity of included studies: variability in methodologies across the 30 studies raises challenges for standardization and comparison. |
| Rai et al. [66] | 2021 | Scoping Review | Int J Environ Res Public Health | 99 | English | Five electronic databases using MEDLINE, Scopus, CINAHL, Embase, and PsycINFO | Very few studies focused specifically on LMIC healthcare workers, despite the high burden of MSDs observed. Use of different sampling methods (gloves, handwash, wipes, patches) with variable efficiencies; surrogate methods may overestimate exposure. Possible overestimation of glove protection due to sampling entire glove (both inside and outside contamination included). |
| Moreira et al. [57] | 2025 | Systematic Review | J Am Pharm Assoc | 20 | English, Spanish, or Portuguese | PubMed, Cochrane, and LILACS (Literatura Latino-Americana e do Caribe em Ciências da Saúde) | Heterogeneity in the scope, structure, and content of the reviewed guidelines limited comparability. Most guidelines lacked detailed educational content or operational definitions regarding training. Limited geographic representation, with an overrepresentation of high-income countries. The assessment relied heavily on AGREE II scores, which may not capture all nuances of guideline applicability. |
| Reference | Key Findings on the Use of PPE |
|---|---|
| McDiarmid & Condon [72] | Historically, compliance with PPE use has been inconsistent and suboptimal. Gloves were the most used PPE, but even their use was low in early decades. Use of masks, gowns, and goggles was significantly lower than recommended. Although glove usage reached near 100% in recent years, usage of other PPE still falls below OSHA guidelines. Lack of availability of PPE; poor training and education regarding hazardous drugs risks and correct PPE practices; and inadequate written protocols are important barriers to PPE use |
| Fransman et al. [73] | Pharmacy technicians always wore gloves (mostly latex), with 27% using double gloving. Gloves generally provide good protection, with minimal contamination of hands underneath. Oncology nurses had inconsistent glove use: 100% wore gloves when handling patients’ urine, yet hands were still contaminated—suggesting poor glove integrity or accidental exposure. Only 36% used gloves while washing patients and 29% when removing bed sheets, resulting in higher dermal exposure. Cleaning personnel wore gloves (26% nitrile, 74% latex; 26% used double gloves). Gloves effectively prevented hand contamination despite high surface contamination on cleaning materials. Regarding other PPE: Pharmacy technicians also used aprons, surgical masks, and hair covers. Head and forearm contamination was occasional but present, likely due to lack of full-body coverage. |
| Wallemacq et al. [61] | Neoprene, nitrile, and certain natural rubber latex gloves provided the highest resistance to cytotoxic drug permeation. Vinyl gloves were the most permeable, with some showing unacceptable permeability within 15 min for multiple drugs. Permeation rates increased over time, with a mean 5-fold increase from 15 to 60 min. Some drugs (e.g., cyclophosphamide, docetaxel) showed a 10-fold increase. Thicker gloves (≥0.24 mm for natural rubber, ≥0.16 mm for nitrile) showed better protection, whereas the thinnest glove (vinyl, 0.12 mm) had the highest permeability. Carmustine was the most permeative drug, affecting various glove materials. ASTM D6978-05 permeation limit (10 ng/cm2/min) was exceeded by several gloves for several drugs, especially with vinyl and thinner gloves. Gloves should be changed at least every 30 min, ideally every 15–20 min when handling cytotoxic agents. |
| Kosgeroglu et al. [62] | Although nurses demonstrated high awareness of safety protocols, actual use of PPE was consistently lower. Although 74.8% of the participants knew gloves should be discarded in special containers, only 57.6% did so in practice. Other PPE components (e.g., masks, goggles, aprons) showed low use rates. |
| Yoshida et al. [33] | Availability and usage rates of PPE was very variable with gloves being the most frequently used item—Gloves (82.7%), masks (69.0%), gowns (62.1%), goggles (36.8%). Double gloving was practiced in 29.2% of hospitals. 10.1% of hospitals reported no PPE usage at all. Goggles underused despite the risk of ocular contamination. Discomfort while wearing goggles was cited as a common reason for non-use. Spill kits and sterilized sheets were more frequently used in hospitals where staff wore double gloves. Small-scale hospitals showed significantly lower availability and adoption of PPE |
| Constantinidis et al. [6] | While most employees reported using PPE, many used only gloves. Masks, gowns, goggles, caps, and shoe covers were used sporadically. PPE use was higher among those involved in preparation/reconstitution than in transportation/storage, likely reflecting better awareness or training. Only 51% of those handling drug preparation used gloves specifically designed for cytotoxic agents, despite evidence that glove material and thickness influence permeability. Morning-shift workers were more likely to comply with PPE use than shift workers. Many workers did not receive formal training; PPE use often correlated with experience or workload rather than age or education. No clear correlation with health outcomes: Although adverse health effects were reported, they did not statistically correlate with PPE use—possibly due to the inadequacy of the PPE used (only partial protection). |
| Kopp et al. [63] | Glove use was high but inconsistent: 92.5% of staff wore gloves during drug administration; however, only 17.5% wore gloves when unpacking transport boxes, and 17.5% did not wear them at all during this task. Glove use was sporadic during critical tasks such as connecting infusion sets (17.5%) and cleaning (2.5%). Limited use of additional PPE: Protective garments (e.g., scrubs or coats) were used in 65.5% of cases. Impermeable gowns, goggles, and masks were used only in response to spills or during drug preparation. |
| Viegas et al. [29] | Although PPE usage is not described in detail, the infrastructure implies mandatory use of gloves, gowns, masks, and other barrier protections in the cleanroom environments The highest contamination of paclitaxel was observed during drug transportation, likely due to gloves and metal trays. |
| Che Huei et al. [64] | A range of PPE types are discussed across hazards: face shields, goggles, surgical masks, respirators, gowns, gloves, and protective clothing. PPE is consistently recommended for chemical, and physical hazards, including aerosolized drug handling and needle-stick incidents. PPE effectiveness is emphasized but considered the least effective control in the hierarchy, especially when used in isolation. It is recommended in conjunction with engineering and administrative controls. PPE usage is tied to proper risk assessments and training, highlighting the importance of education on selection and correct usage. |
| Rocha et al. [65] | High exposure rates to cytotoxic drugs were reported: 67.5% of participants had experienced some form of occupational accident related to antineoplastic drugs. Contact exposure was the most common route (96.3%)—further highlighting the relevance of the use of PPE—followed by aerosol (14.8%), ingestion (3.7%), and inhalation (3.7%). A significant proportion of workers were not using PPE correctly during observed work activities. |
| Rai et al. [66] | Included studies with pharmacy technicians from LMIC revealed the frequent use of gloves, aprons, surgical masks, and hair covers during cytotoxics’ preparation. Although gloves were highly contaminated, skin underneath was rarely contaminated, indicating good protection. As to PPE use among nurses, it varied greatly by task (handling urine; patient washing; remotion of bed sheets). Gloves sometimes failed to protect hands; hand contamination occurred even without glove surface contamination, raising concerns about glove integrity or improper donning/doffing. Regarding cleaning personnel, it was described as a constant use of gloves, although the use of double gloves was uncommon. |
| Tang et al. [67] | CSTD significantly reduced contamination with cyclophosphamide and cytarabine on surfaces and PPE compared to traditional systems. |
| Gonçalves et al. [68] | High perceived importance of some PPE items, according to some studies: disposable caps; P3 masks, or, alternatively P2 masks; sterile latex gloves or, at a less extent, other glove types (nitrile, thick latex) Certain PPE undervalued: goggles and surgical masks deemed not relevant or minimally important by many Non-optimal practices identified: Many professionals still use surgical (P1) masks, contrary to guidelines. Some PPE reused (mask, gown, cap), mainly due to perceived stability and cost-saving. Most studies reported the use of two pairs of gloves, changed hourly or after contamination events. PPE comfort is a key criteria for the selection of PPE and involvement of pharmacy services in PPE choice |
| Gonçalves et al. [69] | Handlers using P2 class masks reported fewer health symptoms. There is a statistically significant association between the type of mask used and the presence of health effects. A lack of institutional training (80.7%) may contribute to improper or non-use of PPE, increasing the risk of intoxication, infertility, and other adverse effects. |
| Zhou et al. [70] | Results demonstrated significant differences in protective capacity among glove materials:
Double-layer glove combinations (e.g., CPE + PVC, CPE + NT) improved protection and are recommended for clinical use, though attention is needed for 5-FU. The study provides graded recommendations (A–F) for glove selection based on drug permeability, supporting evidence-based PPE decisions in hazardous drug handling. |
| Bhirich et al. [71] | The study highlighted multiple failure modes related to insufficient or incorrect use of PPE, such as:
Risk mitigation strategies proposed include:
|
| Moreira et al. [57] | Some guidelines explicitly recommend the use of PPE such as gloves, gowns, and respiratory protection during the preparation and administration of cytotoxic drugs. The use of CSTD devices is also emphasized in certain recommendations as a complement to PPE. Regular training on proper donning, doffing, and disposal of PPE is cited as essential to protect healthcare workers. |
| Reference | Key Findings on Ergonomics |
|---|---|
| Wallemacq et al. [61] | Glove performance depends on real-world conditions:
Workers handling hazardous drugs must balance protection with comfort and dexterity. Discomfort with goggles or thick gloves may reduce compliance. |
| Kosgeroglu et al. [62] | Nurses worked 43.1 ± 3.6 h/week and cared for ~24 patients each. High workload and understaffing may impair compliance. Working hours negatively correlated with protective behaviors: Self-protection scores dropped as weekly hours increased (r = –0.535, p < 0.01). Resource gaps and lack of supportive infrastructure (e.g., availability of PPE, time for safe practices) were implied contributors to poor adherence. |
| Yoshida et al. [33] | Only 44.8% of hospitals used exclusive workbenches for antineoplastic drug handling. In 48.3% of hospitals, non-antineoplastic drugs were also handled on the same workbench. In 6.9%, even office work was performed on the same bench, increasing contamination risks. Biological safety cabinets were installed in only 57.4% of hospitals, and significantly fewer in small-scale hospitals. Unsafe ampoule handling led to injuries and possible drug vapor exposure. |
| Constantinidis et al. [6] | 63.6% of employees cited poorly designed workspaces as a contributing factor to accidents. Accidents were more frequent in preparation/administration roles, likely due to high physical and cognitive demands. Workers identified poor ergonomics, time pressure, staff shortages, and high workload as major causes of incidents. Lack of laminar flow hoods, specialized preparation rooms, and appropriate transportation/storage equipment created additional ergonomic and safety risks. |
| McLeod et al. [56] | Manual compounding was associated with significantly higher upper limb disorder exposure scores (median 9.8) than automated compounding (median 2.5). Highest-risk domains in manual sessions included: Force exerted, arm movement, arm posture. Automated compounding eliminated all high-risk scores; all sessions were classified as low risk. Manual sessions lacked breaks, whereas automation allowed passive breaks during compounding cycles. Ergonomic benefits of automation include reduced force and repetition, improved posture, decreased physical strain, even in high-volume sessions Limitations in ergonomics still exist with automation: requires setup/cleaning, though no moderate/high ULD risks were associated with these tasks; restriction to infusion bags ≤500 mL and specific device types, necessitating manual compounding for more complex preparations |
| Kopp et al. [63] | Only 20% of facilities had specific zones for handling cytotoxic drugs, increasing the risk of exposure in multi-use areas due to the lack of designated work areas. In several settings, cytotoxic drugs were transported by hand rather than using trays, increasing physical strain and contamination risk. No mention of ergonomic training or assessment, suggesting this area may be largely overlooked. |
| Viegas et al. [29] | The clear division of responsibilities—pharmacy technicians for preparation and nurses for administration—helps optimize work roles and prevent cognitive overload or procedural errors, aligning with organizational ergonomics. Use of BSCs within Grade B rooms improves physical ergonomic conditions by centralizing high-risk activity to a controlled space. |
| Villain et al. [74] | Significant reduction in MSD risk with automated preparation compared to manual methods. Mean MSD risk score: - Manual filling: 23.5 (SD = 2.8) - Automated filling: 8.7 (SD = 4.5) Improvements observed in almost all evaluated body regions (shoulders, wrists, fingers, forearms), except for neck posture. Automation reduced repetitive motions, manual effort, and postural constraints. |
| Che Huei et al. [64] | Healthcare professionals commonly suffer musculoskeletal disorders, such as low back pain, wrist strain, and neck/shoulder pain. Risk factors include repetitive tasks. Poorly designed workstations, non-ergonomic equipment, and inadequate administrative support also constitute major risk factors. Engineering controls recommended: adjustable workstations and seating, mechanical aids, and automation where feasible. Long working hours, shift work, workplace violence, stress, and burnout were linked to fatigue, mental disorders, hypertension, and chronic illness and, thus, constitute psychosocial hazards that threaten organizational and cognitive ergonomics. Controls emphasized include improved scheduling, conflict training, and supportive organizational policies. |
| Rocha et al. [65] | The study identified the presence of ergonomic risks, defined as physical or mental strain caused by poor working conditions, which may lead to discomfort, illness, or musculoskeletal and psychological disorders. Nurses were highlighted as the group most exposed to physical and mental strain, due to both the nature of their tasks and emotional burden from patient care. Psychosocial risks (e.g., stress from patient suffering, shift work, and emotional fatigue) were recognized, which align with cognitive and organizational ergonomic concerns. There was no mention of ergonomic training, equipment design, or workflow adaptation, suggesting that ergonomic risks remain under-addressed in practice. |
| Rai et al. [66] | It was reported a high prevalence of MSDs: 12 out of 13 studies reported MSDs in at least one body region in the previous 12 months, with prevalence ranging from 50.7% to 95%. The most commonly affected site was the lower back (reported by 35.3–78.2% of participants). Other affected areas included: Neck: 28–49.8%; Shoulders: 23.5–52.1%; Upper back: 20.7–54%; Knees: 11–68.7% Identified occupational physical risk factors included: prolonged static postures, working in bent or twisted positions, lifting or transferring patients, managing high patient volumes, performing repetitive tasks Identified occupational psychosocial risk factors included: high stress and anxiety, mental exhaustion, poor workplace support, low decision-making autonomy, increased workload and monotonous tasks, lack of experience Geographical limitation: Very few studies focused specifically on LMIC healthcare workers, despite the high burden of MSDs observed. |
| Tang et al. [67] | Pharmacists using CSTDs reported higher satisfaction with ergonomics, encumbrance, and safety during the compounding process. |
| Gonçalves et al. [68] | Common self-reported organizational ergonomics and work conditions include: working in double-sided permeable work areas, laminar flow cabinets mostly vertical class II type B; disinfection performed before and after work; HEPA filters replaced mainly every 6–12 months by qualified technicians; aseptic rooms featuring air extraction/filtration and pressure control. |
| Gonçalves et al. [69] | Handlers working 7 h per day reported a higher perceived risk (median risk = 3.6 on a 1–4 scale). A high incidence of occupational accidents (80.7%) indicates potential failures in workflow design, physical layout, or ergonomic practices. Lack of continuous training and absence of standard operating procedures may compromise the proper adaptation of tasks to workers, a core principle of ergonomics. Perceived risk levels were high (mean score = 3.27 out of 4), especially for outcomes like mutagenicity, infertility, and cancer—reflecting mental workload and perceived insecurity (cognitive ergonomics). |
| Zhou et al. [70] | However, glove selection may influence manual dexterity, comfort, and tactile sensitivity, which are critical ergonomic considerations in clinical practice. The results may indirectly inform ergonomic practices by highlighting which glove types can provide both protection and likely maintain dexterity (e.g., avoiding bulky or permeable gloves that compromise task performance or comfort). |
| Bhirich et al. [71] | While ergonomics was not directly evaluated, several human factors and workflow elements emerged:
The team indirectly addressed ergonomic risks by recommending automation to reduce manual workload and improve safety. Stress and workload-related fatigue, may indirectly affect ergonomic health and performance. |
| Moreira et al. [57] | Ergonomics is not a primary focus of the guidelines analyzed; however, the importance of structured workflow and task allocation is mentioned in the context of reducing procedural errors and operator fatigue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.; Silva, V.; Joaquim, J.J.; Valadares, L.; Matos, C.; Valeiro, C.; Mateos-Campos, R.; Moreira, F. Health Effects of Ergonomics and Personal Protective Equipment on Chemotherapy Professionals. Curr. Oncol. 2025, 32, 563. https://doi.org/10.3390/curroncol32100563
Reis A, Silva V, Joaquim JJ, Valadares L, Matos C, Valeiro C, Mateos-Campos R, Moreira F. Health Effects of Ergonomics and Personal Protective Equipment on Chemotherapy Professionals. Current Oncology. 2025; 32(10):563. https://doi.org/10.3390/curroncol32100563
Chicago/Turabian StyleReis, Ana, Vítor Silva, João José Joaquim, Luís Valadares, Cristiano Matos, Carolina Valeiro, Ramona Mateos-Campos, and Fernando Moreira. 2025. "Health Effects of Ergonomics and Personal Protective Equipment on Chemotherapy Professionals" Current Oncology 32, no. 10: 563. https://doi.org/10.3390/curroncol32100563
APA StyleReis, A., Silva, V., Joaquim, J. J., Valadares, L., Matos, C., Valeiro, C., Mateos-Campos, R., & Moreira, F. (2025). Health Effects of Ergonomics and Personal Protective Equipment on Chemotherapy Professionals. Current Oncology, 32(10), 563. https://doi.org/10.3390/curroncol32100563






