Simple Summary
Chemotherapy drugs are vital for treating cancer, but the professionals who prepare and administer them may be exposed to small amounts that can harm their health over time. To stay protected, they use personal protective equipment like gloves, gowns, and masks, and must also work under ergonomic conditions that prevent strain from repetitive tasks and awkward postures. This review examined recent studies to see how these protective measures are applied and what risks remain. We found that use of protective equipment is often inconsistent and ergonomic challenges are common, especially for nurses and pharmacy technicians. These findings show the importance of better training, safer workplace design, and stronger institutional support. Improving protective practices and ergonomics can help protect healthcare workers, enhance their wellbeing, and ensure safer patient care.
Abstract
(1) Background: With the increasing incidence of cancer, the need for handling cytotoxic drugs has also grown. However, manipulating these drugs exposes healthcare professionals to significant risks, including occupational exposure to hazardous chemicals. Therefore, it is important to adopt protective measures, including personal protective equipment (PPE) and correct ergonomic practices, to ensure safe drug preparation and minimize health risks for the operators. However, while chemical exposure and PPE have been extensively addressed in the literature, the combined impact of ergonomic practices and protective measures remains insufficiently emphasized, representing a critical gap this review aims to address. Accordingly, the objective of this literature review was to analyze the ergonomic and individual protection practices during the handling of cytostatic drugs and all the implications that bad ergonomic practices and/or poor individual protection have on the operator’s health; (2) Methods: In order to perform this integrative review, a structured literature search was conducted using online databases (Web of Science®, Google Scholar®, and PubMed®) from January 2005 to June 2025. (3) Results: A total of 19 articles were analyzed, with 17 focusing on PPE and 17 on ergonomics. The findings emphasize that PPE, such as gloves, masks, gowns, sleeves and safety glasses, plays a critical role in the safe handling of cytotoxic drugs, particularly when combined with other safety measures. Additionally, maintaining correct ergonomic posture is important in preventing musculoskeletal disorders; (4) Conclusions: This review emphasizes the significance of integrating appropriate PPE use with sound ergonomic procedures. Although PPE is still the secondary line of defense against occupational exposure, ergonomic issues must also be addressed to avoid chronic musculoskeletal problems. Continuous training, rigorous attention to safety procedures, and ergonomic enhancements should be prioritized by healthcare facilities as a key element of occupational safety programs to reduce the short-term and long-term health hazards for personnel handling dangerous drugs.
1. Introduction
Cytotoxic drugs, also known as antineoplastics, are a class of medications that contain chemicals toxic to cells, primarily used in the treatment of cancer. Their use has been increasingly common, not only for the therapy of malignant diseases but also for prophylactic purposes. Furthermore, their use has expanded to an increasing spectrum of benign pathologies, such as autoimmune diseases and chronic inflammatory conditions, particularly in gastroenterology and rheumatology. These agents work by inhibiting cell replication and growth, which is essential in targeting malignant cells [1]. However, their action is not exclusively targeted and, as a result, they can also affect normal, healthy cells, leading to various side effects in patients and potentially exposing others to these toxic effects [2,3], including healthcare professionals who handle these substances [4]. In oncology, the therapeutic use of these drugs requires careful selection of the combination of agents, dosage, and treatment regimen, since the concept of selectivity cannot be applied—all chemotherapy drugs inevitably produce adverse effects and impact normal cells [5]. These harmful effects on healthy tissue may include hair loss, skin rashes, infertility, spontaneous abortions, and congenital malformations [6,7,8,9]. Despite these side effects and associated risks, cytotoxic drugs remain highly beneficial for cancer patients, as they help eliminate malignant cells, prolong survival, and improve quality of life. Thus, chemotherapy remains the first-line treatment for most types of cancer [10].
Concerns about occupational exposure to cytotoxic drugs were first raised by Falck et al., in 1979 [11]. In the context of occupational health, these drugs are classified as hazardous drugs—agents whose inherent toxicity poses risks to healthcare professionals [12]. Nevertheless, occupational exposure is often not adequately controlled or prevented. The likelihood of occupational exposure increases when control measures are inadequate. The main routes of exposure include skin contact, skin absorption, inhalation of aerosols and drug particles, ingestion, and needlestick injuries. These risks can arise during drug preparation, administration, handling of patient waste, transport, waste disposal, or cleaning of spills [13]. Inadequate or insufficient control measures can lead to health issues such as abdominal pain, hair loss, nasal sores, vomiting, contact dermatitis, localized allergic reactions and liver damage [14,15,16,17]. Other adverse effects include fetal loss in pregnant women, and congenital malformations in their children [18,19]. Furthermore, exposure to cytotoxic drugs can alter normal blood cell counts and induce mutagenic activity, leading to abnormal cell formation [20,21,22,23]. Corroborating these findings, the International Agency for Research on Cancer (IARC) has classified many of these drugs as carcinogenic, mutagenic, and/or toxic for reproduction [24].
Those at risk include medical, pharmacy technicians, pharmacists, nursing staff, laboratory workers, cleaning personnel, patients’ family members and caregivers, as well as the patients themselves [25,26,27]. Healthcare professionals involved in the preparation and administration of these drugs (pharmacists, pharmacy technicians and nurses) are at increased risk of experiencing adverse health effects. Previous studies have demonstrated widespread contamination of the environment and work surfaces in healthcare settings [4,28,29,30]. Therefore, it is essential to implement and maintain effective control measures to protect these professionals from exposure.
Biological Safety Cabinets (BSC) are a key engineering control that provide containment during the preparation of cytotoxic drugs [31]. The BSCs help minimize the risk of aerosolization and environmental contamination, and thus reduce exposure to hazardous drug vapors and particles while also providing an aseptic environment [32]. When used in conjunction with appropriate PPE, BSCs form part of a comprehensive safety strategy for mitigating occupational risks.
Regular training regarding the risks and safe handling of cytotoxic drugs is also an important consideration. Healthcare workers, especially pharmacy technicians, pharmacists, and nurses, who are often responsible for the reconstitution, preparation, dilution, and mixing of cytotoxic drugs, must receive proper training and be adequately equipped to work under aseptic conditions [31,32]. PPE plays a pivotal role in reducing the probability of exposure to cytotoxic agents, particularly through dermal contact [33]. Appropriate PPE includes chemical-resistant gloves, impervious gowns, P2 or P3 masks, safety goggles or face shields, caps, and shoe covers—each designed to prevent skin and mucosal exposure to hazardous substances. Since the exposure to cytotoxic drugs can occur even in very small quantities; PPE represents one of the most critical lines of defense in protecting professionals from potentially serious health effects [29,34,35].
Control measures focus on reducing the quantities of drugs used, limiting the number of exposed employees, and minimizing the duration of exposure [6]. Safe handling practices, proper storage, and appropriate disposal of cytotoxic drugs along with contaminated waste are necessary to prevent accidental exposure [36]. Maintaining good hygiene practices is also crucial—for example, prohibiting eating, drinking, and smoking in areas where these drugs are handled, as well as ensuring access to adequate washing facilities [31,37,38].
Clinical practice and oncology hospitals, pharmacies, and caregiver organizations have established strict guidelines to protect healthcare professionals from occupational exposure to cytotoxic drugs and to ensure that contaminated materials are correctly disposed of [4,39]. These recommendations are particularly important given the risks of harmful exposure during the preparation, administration, and waste management of such agents. In addition, while pharmaceutical companies and regulatory agencies receive continuous updates regarding the classification and management of cytotoxic drugs, scientific data and risk assessments have increasingly served as a foundation for establishing exposure limits, safety standards, and handling protocols [4,40,41].
In the context of occupational exposure to cytotoxic drugs, several international and European frameworks provide overarching guidance. Globally, agencies such as the National Institute for Occupational Safety and Health (NIOSH) and the Occupational Safety and Health Administration (OSHA) in the United States have issued foundational recommendations on the safe handling of hazardous drugs, focusing on engineering controls, safe work practices, and appropriate use of PPE [12,42]. Complementing these, the International Society of Oncology Pharmacy Practitioners (ISOPP) has developed practice standards specifically tailored to oncology pharmacy settings [31]. In Europe, regulatory emphasis is placed on preventing exposure through manufacturing and workplace controls. The European Medicines Agency (EMA) provides Good Manufacturing Practice (GMP) guidelines addressing containment and cross-contamination [43], while the EU-OSHA Carcinogens and Mutagens Directive (2004/37/EC, amended by 2022/431) requires employers to minimize workers’ exposure to hazardous medicinal products, including cytotoxics [44]. Individual Member States, such as the UK (HSE/COSHH) [45] and Germany (BAuA) [46], further operationalize these principles through national regulations. Taken together, these international and European frameworks share a common goal: minimizing occupational risks associated with cytotoxic drugs by combining risk assessment, containment systems, and protective measures, while differing mainly in scope—practice standards in the US, EU-wide legislation in Europe, and more detailed national adaptations at the country level.
Ergonomics, broadly defined as the alignment of job demands with workers’ capabilities and workplace design, has traditionally focused on preventing work-related musculoskeletal disorders, which remain the most common occupational health issue across sectors [47]. By optimizing efficiency, comfort, and safety, ergonomics not only reduces physical strain and injury but also improves productivity, satisfaction, and workforce retention. Risk factors for work-related musculoskeletal disorders include repetitive or forceful movements, awkward or sustained postures, heavy lifting, and inadequate equipment, with frequent consequences such as back, neck, and upper extremity disorders [48,49]. Evidence highlights that psychosocial factors—such as job stress, work organization, and lack of social support—also interact with physical risks, exacerbating injury rates and contributing to burnout, absenteeism, and psychological distress [50,51]. This is particularly relevant in healthcare, where long working hours, high physical demands, and poor ergonomic arrangements heighten both physical and psychosocial risks. Despite these concerns, ergonomics is often underrepresented in the occupational safety literature compared to chemical hazards and PPE. Addressing this gap is essential, as inadequate ergonomic conditions not only compromise worker health but also undermine overall quality of care and organizational performance.
Accordingly, in addition to the need for strict measures to minimize exposure, ergonomic considerations are also important in ensuring the safety of pharmacy technicians [52]. In particular, poor ergonomics during the preparation of cytotoxic drugs can result in musculoskeletal disorders, including pain and stiffness in the arms, shoulders, neck, and back [26,53,54].
The confined space of a BSC, combined with repetitive and conditioned motions the exertion required for certain tasks, can lead to long-term health issues (such as leading to osteoarticular injuries, muscle pain) [55]. Therefore, ergonomic measures must be implemented to prevent such injuries and improve task efficiency, ensuring both safety and comfort for healthcare professionals [55,56].
Although ISOPP recommendations underscore the relevance of maintaining appropriate temperature, humidity, and ventilation to safeguard the comfort of healthcare professionals handling cytotoxic drugs, the vast majority of international guidelines remain silent on ergonomics [31].
The handling of cytotoxic drugs presents not only chemical hazards but also significant physical and ergonomic challenges for healthcare professionals, particularly pharmacy technicians and nurses. While much attention has been given to chemical exposure risks, the importance of proper use of PPE and ergonomic practices is sometimes underestimated. Incorrect or inconsistent use of PPE can result in direct exposure to hazardous substances, while poor ergonomic conditions—such as prolonged awkward postures, repetitive motions, and working within confined spaces like BSC—can lead to musculoskeletal disorders and chronic health problems. Understanding and improving these factors is critical to ensuring the overall safety and wellbeing of personnel involved in cytotoxic drug preparation and administration, reducing both acute toxic risks and long-term occupational injuries. Therefore, assessing PPE adherence and ergonomic factors in this context is essential to inform effective interventions and promote safer work environments. Previous reviews have mainly focused on chemical hazards and PPE use, overlooking ergonomic risks in the handling of cytotoxic drugs [57,58,59,60]. For example, while some emphasized barriers to PPE compliance or the importance of contamination monitoring, none systematically addressed ergonomics as part of occupational safety. By combining PPE and ergonomics, this review provides a more comprehensive perspective and responds to an important gap in the existing literature.
Therefore, this review aims to discuss the importance of PPE and proper ergonomic practices in safeguarding healthcare workers involved in tasks related to the handling of cytotoxic agents, as well as the potential health risks they may face if appropriate precautions are not followed.
2. Materials and Methods
This study was designed as an integrative review, as it combines evidence from original research and review articles, synthesizing findings across diverse study designs to provide a comprehensive understanding of the use of PPE and ergonomic challenges in the handling of cytotoxic drugs. The aim was to capture and integrate the most relevant evidence to contextualize current practices and knowledge gaps. The primary databases used were PubMed®, Google Scholar®, and Web of Science®. Combinations of the following keywords were used to select relevant references: “Cytotoxic Drugs,” “Handling”, “Musculoskeletal injuries”, “Personal Protective Equipment,” and “Ergonomics”. The complete search strings presented in Table 1 were applied.

Table 1.
Search strings used in each database.
The inclusion criteria comprised articles published from 1 January 2005 to 30 June 2025—written in English and focused on the handling of cytotoxic drugs, the importance of PPE, or ergonomic challenges. The last search through all databases was performed on 1 July 2025. Peer-reviewed original research studies were prioritized, but systematic and narrative reviews relevant to the topic were also included to provide a broader synthesis of existing evidence. Articles that lacked scientific relevance to the research question were excluded based on title and abstract analysis. Additionally, non–peer-reviewed publications (such as conference abstracts), editorials, guidelines and opinion pieces, were also excluded.
To minimize the risk of omitting relevant studies, the reference lists of selected articles were reviewed.
The results were synthesized using a thematic analysis approach. Studies were classified into two main thematic axes: (1) Personal Protective Equipment (PPE), including training, compliance, and use of specific protective devices; and (2) Ergonomics, encompassing musculoskeletal risks, workspace design, and preventive measures.
No formal critical appraisal of methodological quality was performed; however, studies were selected based on scientific relevance, peer-reviewed status, and methodological transparency to ensure reliable synthesis.
3. Results
After the identification of 1046 articles during the primary search, a total of 19 articles were ultimately included (Figure 1).
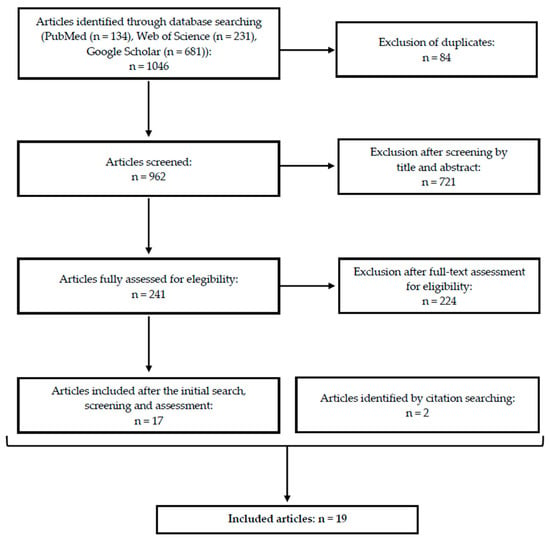
Figure 1.
Simplified flow diagram of the literature search and selection process—number of records identified, screened, excluded, and finally included in the integrative review.
Fifteen of the 19 included studies (79%) addressed both PPE use and ergonomics [6,29,33,57,61,62,63,64,65,66,67,68,69,70,71], two (10.5%) focused solely on PPE [72,73], and the remaining two (10.5%) exclusively on ergonomics [56,74]. Most of the studies described observational or experimental methodologies that are depicted on Table 2.

Table 2.
Characteristics of the observational and experimental studies included in the review, detailing study type, country, journal, year of publication, and sample characteristics.
Different types of reviews were deemed relevant during the selection of published papers, including narrative, scoping and systematic reviews (Table 3).

Table 3.
Characteristics of the review studies included in the review, including review type, journal, number of studies included, languages of eligible studies, year of publication, and databases searched.
The temporal distribution of the included studies is presented in Figure 2, which display the publication years.
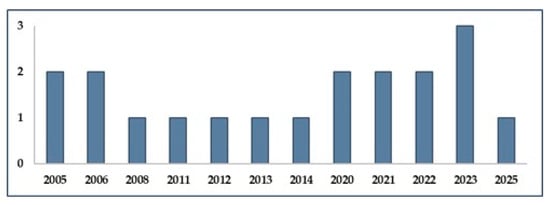
Figure 2.
Year of publication of the 19 studies included in the review, showing the temporal distribution of research on PPE and ergonomics in the handling of cytotoxic drugs.
Thirteen of the 19 included studies described their occurrence over time, which is further demonstrated in Figure 3, according to the research periods covered.
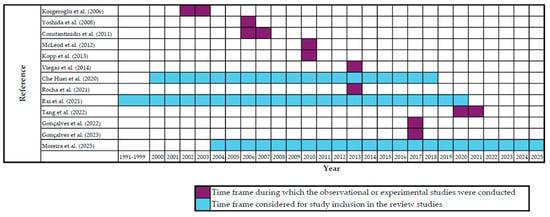
Figure 3.
Time frames covered by the included studies, distinguishing observational/experimental studies from review studies [6,29,33,56,57,62,63,64,65,66,67,68,69].
The main findings of the included studies regarding the use of personal protective equipment and ergonomic aspects are summarized in Table 4 and Table 5, respectively.

Table 4.
Main findings from the studies included in this review regarding the use of personal protective equipment (PPE).

Table 5.
Main findings from the studies included in this review regarding ergonomics.
4. Discussion
4.1. Personal Protective Equipment (PPE)
PPE remains a cornerstone in the protection of healthcare workers against cytotoxic drug exposure, but significant lapses in correct selection, use, and replacement persist across studies [61,70]. Gloves are the most consistently used item, especially during drug preparation and administration [33,63]. However, issues remain regarding glove type, thickness, permeation resistance, and replacement frequency. Vinyl gloves, for instance, allow rapid permeation of several cytotoxic agents, whereas latex, nitrile, or neoprene gloves offer superior resistance, particularly when at least 0.2 mm thick. To ensure adequate protection, gloves should be changed every 15–30 min, or immediately if torn or contaminated, with double-gloving recommended during high-risk tasks such as drug preparation [2,31,61,63,70]. Despite these guidelines, adherence is inconsistent. Kopp et al. [63] found that while gloves were worn by 92.5% of staff during drug preparation, use dropped sharply during unpacking (2.5%) and surface cleaning (35%). Similarly, Yoshida et al. [33] reported that only 29.2% of facilities practiced double-gloving, and 10.1% reported not using PPE at all for certain tasks.
Gowns are another critical component, intended to be single-use, fluid-resistant, and made from non-woven, low-permeability materials with long sleeves and tight cuffs [32]. Nonetheless, Gonçalves et al. [69] observed instances of gowns being reused to reduce costs, particularly in resource-limited hospitals, directly contravening safety standards. Respiratory protection shows similar gaps: although FFP2 (or higher) respirators are recommended for aerosol-generating procedures, many facilities rely on surgical masks, which are ineffective in this context [63,66,68,69,73]. Moreover, staff are often unaware of the importance of proper fit when using respirators [68,69,72]. Eye and face protection is also underused, often due to discomfort. When worn, devices are sometimes inadequate—for example, simple plastic visors with poor lateral coverage—leaving workers insufficiently protected from splashes [12,31,33,63,68]. Foot protection, such as overshoes, is rarely implemented despite recommendations to reduce contamination risks [32,75].
Another widespread issue is the extended use or reuse of disposable PPE, reported in both high- and low-resource settings [6,69]. This practice compromises barrier integrity and increases the likelihood of cross-contamination between environments and patients. Training deficiencies compound these risks: Kopp et al. [63] reported that only 25% of institutions provided hands-on training in PPE use, and even fewer had procedures for safe disposal or spill response. Consequently, healthcare workers often rely on informal instruction or habits rather than standardized protocols [57].
Several studies also highlighted institutional and organizational variation influencing PPE use. For example, McDiarmid & Condon [72] and Yoshida et al. [33] reported wide differences in the implementation of safety practices across hospitals, particularly smaller ones. Fransman et al. [73] further noted that hospital layouts and glove availability limited comparability across settings, while Kopp et al. [63] described preparation practices outside of recommended engineering controls. These findings underscore the importance of institutional commitment and infrastructure in ensuring that PPE use is consistent and effective.
Although few data are available regarding potential contamination of family members of healthcare professionals handling cytotoxic drugs, the possibility of “take-home exposure” cannot be entirely excluded, further highlight the relevance of the use of PPE. Several studies have demonstrated exposure of relatives of oncology patients to antineoplastic agents [76,77,78]. While absorption and transfer of these agents from healthcare professionals to their households at concentrations comparable to those of patients is unlikely—since contamination in occupational settings typically occurs at residual levels far below therapeutic doses—the reality is that professionals may be exposed repeatedly and cumulatively over many years. This raises the need for greater awareness and further research into the possible indirect risks posed to the acquaintances and household members of oncology healthcare professionals.
4.2. Ergonomics
Ergonomics plays a critical but often underrecognized role in hazardous drug handling. Tasks such as drug compounding, patient care, and cleaning require repetitive movements, static postures, and high cognitive demand—conditions conducive to musculoskeletal disorders (MSDs). Multiple studies reported high prevalence rates of MSDs among healthcare workers, especially in the lower back, shoulders, neck, and upper limbs [64,66].
Manual compounding workstations are often not ergonomically designed. As noted by McLeod et al. [56], manual compounding sessions had significantly higher upper limb disorder scores than automated systems. Similarly, Villain et al. [74] found that automation significantly reduced MSD risks, especially in the shoulders, wrists, and fingers.
Organizational ergonomics is equally essential. Poor workflow design, staff shortages, and lack of standard procedures were repeatedly cited as contributors to physical and cognitive overload [6,69]. For instance, nurses in under-resourced facilities reported emotional and physical fatigue, often exacerbated by high patient volumes and poor team support [62,65].
Even infrastructure elements affect ergonomic safety. For example, only 20% of facilities reported in the study by Kopp et al. [63] had designated cytotoxic drug preparation areas, leading to tasks being performed on therapy counters or multipurpose benches. These layouts increase both contamination and injury risk due to physical strain.
Preliminary evidence suggests that automation may help reduce ergonomic risks, although broader studies are needed to confirm its effectiveness across diverse settings. Villain et al. [74] and McLeod et al. [56] both demonstrated improved ergonomic conditions through automation, including reductions in static posture duration and repetitive movements. However, these systems are costly and require standardization, which limits widespread adoption, especially in lower-resourced settings.
4.3. Training Gaps
The consistent theme across studies is a disconnection between knowledge of PPE/ergonomics and actual behavior. Nurses, pharmacy technicians and pharmacists often demonstrated high theoretical awareness but failed to translate this into consistent safe practices [6,62]. In the studies included in this review, the evaluation of the impact of PPE use and/or ergonomics in handling cytotoxic drugs was most frequently reported in relation to pharmacy technicians (n = 8) [6,65,67,68,69,71,73,74] and nurses (n = 5) [6,33,62,65,73]. This finding highlights the central role of these professionals in cytotoxic drug handling and, consequently, their greater likelihood of experiencing both chemical and ergonomic occupational exposures. Indeed, for different reasons, these two professional groups are at heightened risk of developing adverse health effects or musculoskeletal disorders. Nurses, for instance, have direct contact with patients, which generates significant ergonomic challenges [79,80], and often handle cytotoxic drugs in clinical settings where primary engineering controls, such as laminar airflow hoods, are not available [81]. Pharmacy technicians, on the other hand, face ergonomic challenges related to repetitive and precise movements carried out for prolonged periods in restricted spaces [65]. From a chemical exposure perspective, although their work environments are generally better controlled than those of nurses (e.g., use of laminar airflow hoods), pharmacy technicians handle the concentrated formulations of cytotoxic drugs, whereas nurses usually work with diluted solutions [82]. For any of the mentioned professional groups, the aforementioned training gap may be attributed to a lack of time, institutional support, or practical training.
Hands-on training is especially important. Kopp et al. [63] reported that only 25% of facilities provided practical instruction, even though 83% had some form of training program. Similarly, Gonçalves et al. [69] observed that 80.7% of handlers lacked institutional training, which correlated with higher accident rates and improper PPE use.
This issue is not exclusive to PPE. Ergonomic training and assessments are virtually nonexistent across many healthcare facilities. Most ergonomic findings in the reviewed studies were inferred from infrastructure and workflow design rather than direct assessment or staff feedback [29,65].
This lack of institutional engagement compromises safety culture. As noted by McDiarmid & Condon [72], poor enforcement of safety guidelines and the absence of feedback loops reduce PPE adherence and increase exposure risks. Facilities with stronger safety climates consistently showed better compliance and fewer adverse health effects.
4.4. Psychosocial and Organizational Factors
The ergonomic risks extend beyond physical strain to psychosocial stressors, including long working hours, shift work, patient suffering, and mental exhaustion. These factors contribute to burnout and can impair judgment, increasing the risk of procedural errors [64,65].
In addition, many workers operate under cognitive overload due to multitasking, poor workflow design, and a lack of breaks [66,71]. High-risk tasks like cytotoxic drug preparation often demand concentration and precision, making cognitive fatigue particularly hazardous.
The organizational environment plays a pivotal role. A culture of safety, supported by regular training, transparent incident reporting, and ergonomic design, can significantly reduce exposure and injury rates [69,72]. Unfortunately, many settings lack such systemic support, especially in low- and middle-income countries where resource limitations exacerbate risks [66].
4.5. Engineering Controls and Recommendations
Engineering controls such as laminar flow hoods, biosafety cabinets, and CSTDs provide foundational protection and should be integrated with PPE use. Studies confirmed that when properly used, these systems reduce environmental and personal contamination [29,67]. Yet, inconsistencies in maintenance and usage compromise their effectiveness [71].
The adoption of CSTDs, though effective, remains uneven due to cost, lack of training, and resistance to workflow changes. Tang et al. [67] found that CSTDs improved both safety and ergonomic satisfaction, while Bhirich et al. [71] highlighted their role in minimizing aerosolized contamination during drug transfer.
Moreover, engineering controls can ease ergonomic strain. Adjustable workstations, mechanical aids for transport, and automation of repetitive tasks reduce the need for prolonged static postures and awkward movements [64,74].
To maximize safety, PPE must be viewed as part of a broader safety system—complemented by engineering controls, administrative policies, and a proactive safety culture [57,72]. Isolated reliance on PPE, especially when misused or poorly selected, offers insufficient protection against cytotoxic drug exposure.
4.6. Research Gaps, Practical Implications, and Future Directions
This review also highlights important gaps in the existing literature. Most studies have been conducted in high-income countries, while evidence from low- and middle-income countries remains scarce, despite these settings often facing greater structural and financial barriers to implementing safety measures. Furthermore, the majority of available studies rely on cross-sectional and observational designs, limiting the ability to establish causal links between protective practices, ergonomic conditions, and health outcomes. Longitudinal studies are particularly lacking, which prevents a comprehensive understanding of the long-term impact of occupational exposure and ergonomic risks on healthcare professionals.
From a practical perspective, the findings underscore the need for institutional commitment in three key areas. First, continuous and hands-on training should be prioritized to ensure correct use of PPE and adoption of ergonomic strategies. Second, healthcare organizations should implement clear policies regarding PPE replacement, environmental monitoring, and psychosocial support, reinforcing a culture of safety. Third, investment in ergonomic infrastructure and technological solutions—such as adjustable workstations, closed-system transfer devices, and automated compounding systems—should be encouraged, as these interventions can simultaneously reduce chemical exposure and musculoskeletal strain.
Looking ahead, future research should include well-designed intervention studies assessing the effectiveness of structured training programs, ergonomic redesign of workspaces, and adoption of automation technologies. Cost–benefit analyses of protective measures are also warranted, as they can demonstrate the broader economic advantages of investing in worker safety, including reductions in absenteeism, occupational illness, and staff turnover. By addressing these gaps, the field can move towards evidence-based strategies that improve both worker protection and organizational performance.
4.7. Limitations
This review has several limitations that should be acknowledged. First, the number of included studies was relatively small, and many of them relied on cross-sectional or self-reported data, which introduces risks of recall and reporting bias. Second, the heterogeneity of study designs, populations, and outcome measures limited the possibility of direct comparisons or meta-analytic synthesis. Third, our search was restricted to three major databases (PubMed®, Google Scholar®, and Web of Science®). Although these are widely used sources, it is possible that relevant studies indexed elsewhere were not captured, particularly those published in non-English journals or reported in the gray literature. Finally, the geographical distribution of the included studies was skewed towards high-income countries, reducing the generalizability of findings to low- and middle-income settings. These limitations suggest that our synthesis, while informative, should be interpreted with caution.
5. Conclusions
The safe handling of cytotoxic drugs hinges on the effective and consistent use of PPE in conjunction with sound ergonomic practices. While PPE remains a necessary line of defense, it is only effective when selected appropriately, used correctly, and supported by systemic measures including training, engineering controls, and organizational commitment. Ergonomic risks—particularly musculoskeletal and cognitive stress—remain under-addressed but are critical to long-term health outcomes. A multidimensional safety strategy is essential to protect healthcare workers from the complex hazards posed by cytotoxic drug handling.
Author Contributions
Conceptualization, C.M. and J.J.J.; methodology, A.R., V.S., L.V. and F.M.; validation, R.M.-C. and C.M.; formal analysis, J.J.J.; investigation, A.R., V.S. and F.M.; resources, C.M.; data curation, A.R., V.S., C.V., L.V. and F.M.; writing—original draft preparation, A.R. and V.S.; writing—review and editing, F.M., L.V., J.J.J. and R.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
Associação Portuguesa de Licenciados em Farmácia (APLF) have received an unrestricted Education grant from Beckton Dickinson® for the development of the project “Portuguese recommendations for the safety of pharmacy technicians handling chemotherapy”. This work received financial support from the PT national funds (FCT/MECI, Fundação para a Ciência e Tecnologia and Ministério da Educação, Ciência e Inovação) through the project UID/50006—Laboratório Associado para a Química Verde—Tecnologias e Processos Limpos.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ART | Assessment of Repetitive Tasks |
| BSC | Biological Safety Cabinets |
| COSHH | Control of Substances Hazardous to Health Regulations |
| CPE | Chlorinated Polyethylene |
| CSTD | Closed system transfer devices |
| DCT | Docetaxel |
| EMA | European Medicines Agency |
| EPI | Epirubicin |
| ETP | Etoposide |
| FMECA | Failure Modes, Effects, and Criticality Analysis |
| GMP | Good Manufacturing Practices |
| HEPA | High Efficiency Particulate Air |
| HSE | Health and Safety Executive |
| IARC | International Agency for Research on Cancer |
| ISOPP | International Society of Oncology Pharmacy Practitioners |
| LMIC | Low-income or middle-income countries |
| MTX | Methotrexate |
| MSD | Musculoskeletal disorders |
| NIOSH | National Institute for Occupational Safety and Health |
| NT | Nitrile |
| OSHA | Occupational Safety and Health Administration |
| PPE | Personal protective equipment |
| PVC | Polyvinyl Chloride |
| RB | Rubber-based |
| ULD | Upper limb disorder |
| VCR | Vincristine |
References
- Vanderah, T. Katzung’s Basic & Clinical Pharmacology, 16th ed.; MC Graw-Hill: New York, NY, USA, 2023. [Google Scholar]
- Pan American Health Organization. Safe Handling of Hazardous Chemotherapy Drugs in Limited-Resource Settings; Pan American Health Organization: Washington, DC, USA, 2013.
- Saha, D.; Anderson, A.; Cisneros, L.; Maley, C. In Silico Investigations of Adaptive Therapy Using Two Cytotoxic or Two Cytostatic Drugs. bioRxiv 2023. [Google Scholar] [CrossRef]
- de Lemos, M.L.; Badry, N.; Conklin, J.; Koberinski, M. Defining Cytotoxic Drugs—You Know It When You See It? J. Oncol. Pharm. Pract. 2021, 27, 1958–1962. [Google Scholar] [CrossRef]
- Winkler, G.C.; Barle, E.L.; Galati, G.; Kluwe, W.M. Functional Differentiation of Cytotoxic Cancer Drugs and Targeted Cancer Therapeutics. Regul. Toxicol. Pharmacol. 2014, 70, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Constantinidis, T.C.; Vagka, E.; Dallidou, P.; Basta, P.; Drakopoulos, V.; Kakolyris, S.; Chatzaki, E. Occupational Health and Safety of Personnel Handling Chemotherapeutic Agents in Greek Hospitals. Eur. J. Cancer Care 2011, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, P.R. Handling of Cytotoxic Drugs by Healthcare Workers. A Review of the Risks of Exposure. Drug Saf. 1992, 7, 374–380. [Google Scholar] [CrossRef]
- Sessink, P.J.; Kroese, E.D.; van Kranen, H.J.; Bos, R.P. Cancer Risk Assessment for Health Care Workers Occupationally Exposed to Cyclophosphamide. Int. Arch. Occup. Environ. Health 1995, 67, 317–323. [Google Scholar] [CrossRef]
- Sorsa, M.; Hemminki, K.; Vainio, H. Occupational Exposure to Anticancer Drug—Potential and Real Hazards. Mutat. Res. 1985, 154, 135–149. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Falck, K.; Gröhn, P.; Sorsa, M.; Vainio, H.; Heinonen, E.; Holsti, L. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet 1979, 313, 1250–1251. [Google Scholar] [CrossRef]
- The National Institute for Occupational Safety and Health. Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings; The National Institute for Occupational Safety and Health: Washington, DC, USA, 2004.
- van Huizen, P.; Russo, P.L.; Manias, E.; Kuhn, L.; Connell, C.J. Knowledge and Safe Handling Practices Affecting the Occupational Exposure of Nurses and Midwives to Hazardous Drugs: A Mixed Methods Systematic Review. Int. J. Nurs. Stud. 2024, 160, 104907. [Google Scholar] [CrossRef]
- Alehashem, M.; Baniasadi, S. Important Exposure Controls for Protection against Antineoplastic Agents: Highlights for Oncology Health Care Workers. Work 2018, 59, 165–172. [Google Scholar] [CrossRef]
- Topçu, S.; Beşer, A. Oncology Nurses’ Perspectives on Safe Handling Precautions: A Qualitative Study. Contemp. Nurse 2017, 53, 271–283. [Google Scholar] [CrossRef]
- Campbell, K.; Afseth, J.; Dunham, M.; King, M.; Dicksit, D. Global Cancer Nurse’s Experiences and Perceptions of Potential Occupational Exposure to Cytotoxic Drugs: Mixed Method Systematic Review with Framework Synthesis. J. Clin. Nurs. 2024, 33, 4585–4601. [Google Scholar] [CrossRef]
- Simons, A.; Toland, S. Perceived Adverse Effects from Handling Systemic Anti-Cancer Therapy Agents. Br. J. Nurs. 2017, 26, S38–S44. [Google Scholar] [CrossRef]
- Connor, T.H.; Lawson, C.C.; Polovich, M.; McDiarmid, M.A. Reproductive Health Risks Associated with Occupational Exposures to Antineoplastic Drugs in Health Care Settings: A Review of the Evidence. J. Occup. Environ. Med. 2014, 56, 901–910. [Google Scholar] [CrossRef]
- Ratner, P.A.; Spinelli, J.J.; Beking, K.; Lorenzi, M.; Chow, Y.; Teschke, K.; Le, N.D.; Gallagher, R.P.; Dimich-Ward, H. Cancer Incidence and Adverse Pregnancy Outcome in Registered Nurses Potentially Exposed to Antineoplastic Drugs. BMC Nurs. 2010, 9, 15. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Nucci, D.; Villarini, M.; Moretti, M. [Genotoxic risk in nurses handling antiblastic drugs: Systematic review of literature and meta-analysis]. Recenti. Prog. Med. 2017, 108, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Grollino, M.G.; Pavanello, S.; Bonfiglioli, R.; Villarini, M.; Appolloni, M.; Carrieri, M.; Sabatini, L.; Dominici, L.; Stronati, L.; et al. Micronuclei and Chromosome Aberrations in Subjects Occupationally Exposed to Antineoplastic Drugs: A Multicentric Approach. Int. Arch. Occup. Environ. Health 2015, 88, 683–695. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, M.A.; Rogers, B.; Oliver, M.S. Chromosomal Effects of Non-Alkylating Drug Exposure in Oncology Personnel. Environ. Mol. Mutagen. 2014, 55, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Soleyman-Jahi, S.; Zendehdel, K.; Mozdarani, H.; Azimi, C.; Farzanfar, F.; Safari, Z.; Mohagheghi, M.-A.; Khaleghian, M.; Divsalar, K.; et al. Chromosomal Aberrations, Sister Chromatid Exchanges, and Micronuclei in Lymphocytes of Oncology Department Personnel Handling Anti-Neoplastic Drugs. Drug Chem. Toxicol. 2017, 40, 235–240. [Google Scholar] [CrossRef]
- Goodman, J.; Lynch, H. Improving the International Agency for Research on Cancer’s Consideration of Mechanistic Evidence. Toxicol. Appl. Pharmacol. 2017, 319, 39–46. [Google Scholar] [CrossRef]
- Crul, M.; Simons-Sanders, K. Carry-over of Antineoplastic Drug Contamination in Dutch Hospital Pharmacies. J. Oncol. Pharm. Pract. 2018, 24, 483–489. [Google Scholar] [CrossRef]
- Crul, M.; Hilhorst, S.; Breukels, O.; Bouman-d’Onofrio, J.R.C.; Stubbs, P.; van Rooij, J.G. Occupational Exposure of Pharmacy Technicians and Cleaning Staff to Cytotoxic Drugs in Dutch Hospitals. J. Occup. Environ. Hyg. 2020, 17, 343–352. [Google Scholar] [CrossRef]
- Kieffer, C.; Verhaeghe, P.; Lagrassa, S.; Grégoire, R.; Moussaoui, Z.; Casteras-Ducros, C.; Clark, J.E.; Vanelle, P.; Rathelot, P. Preventing the Contamination of Hospital Personnel by Cytotoxic Agents: Evaluation and Training of the Para-Professional Healthcare Workers in Oncology Units. Eur. J. Cancer Care 2015, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, E.; Creta, M.; Vanoirbeek, J.; Zakia, M.; Abdesselam, T.; Lebegge, R.; Poels, K.; Duca, R.-C.; Godderis, L. Environmental Contamination and Occupational Exposure of Algerian Hospital Workers. Front. Public Health 2020, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Pádua, M.; Veiga, A.C.; Carolino, E.; Gomes, M. Antineoplastic Drugs Contamination of Workplace Surfaces in Two Portuguese Hospitals. Environ. Monit. Assess 2014, 186, 7807–7818. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; de Oliveira, A.C.; Carolino, E.; Pádua, M. Occupational Exposure to Cytotoxic Drugs: The Importance of Surface Cleaning to Prevent or Minimise Exposure. Arch. Ind. Hyg. Toxicol. 2018, 69, 238–249. [Google Scholar] [CrossRef]
- ISOPP Standards for the Safe Handling of Cytotoxics. J. Oncol. Pharm. Pract. 2022, 28, S1–S126. [CrossRef]
- Easty, A.C.; Coakley, N.; Cheng, R.; Cividino, M.; Savage, P.; Tozer, R.; White, R.E. Safe Handling of Cytotoxics: Guideline Recommendations. Curr. Oncol. 2015, 22, e27–e37. [Google Scholar] [CrossRef]
- Yoshida, J.; Kosaka, H.; Nishida, S.; Kumagai, S. Actual Conditions of the Mixing of Antineoplastic Drugs for Injection in Hospitals in Osaka Prefecture, Japan. J. Occup. Health 2008, 50, 86–91. [Google Scholar] [CrossRef]
- Working Committee on the Safe Handling of Hazardous Drugs. Prevention Guide: Safe Handling of Hazardous Drugs; IRSST: Montreal, QC, Canada, 2008. [Google Scholar]
- Korczowska, E.; Crul, M.; Tuerk, J.; Meier, K. Environmental Contamination with Cytotoxic Drugs in 15 Hospitals from 11 European Countries—Results of the MASHA Project. Eur. J. Oncol. Pharm. 2020, 3, e24. [Google Scholar] [CrossRef]
- Jun, E.-M.; Kang, S.-W. Effects of Safe Handling Education on Cognition, Compliance and Stress Handling of Antineoplastic Drugs in Clinical Nurses. Nurs. Open 2023, 10, 4144–4152. [Google Scholar] [CrossRef]
- Cotteret, C.; Secretan, P.-H.; Gilles-Afchain, L.; Rousseau, J.; Vidal, F.; Salguero-Hernandez, G.; Batista, J.; Valverde, V.; Guitton, J.; Cisternino, S.; et al. External Contamination of Antineoplastic Drug Vials: An Occupational Risk to Consider. Eur. J. Hosp. Pharm. 2022, 29, 284–286. [Google Scholar] [CrossRef]
- Sessink, P.J.M.; Connor, T.H.; Jorgenson, J.A.; Tyler, T.G. Reduction in Surface Contamination with Antineoplastic Drugs in 22 Hospital Pharmacies in the US Following Implementation of a Closed-System Drug Transfer Device. J. Oncol. Pharm. Pract. 2011, 17, 39–48. [Google Scholar] [CrossRef]
- Ziegler, E.; Mason, H.J.; Baxter, P.J. Occupational Exposure to Cytotoxic Drugs in Two UK Oncology Wards. Occup. Environ. Med. 2002, 59, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Askarian, M.; Azad, H.; Danaei, M. Exposure To Cytotoxic Drugs Threatens The Health Of Staff In Oncology Wards. Russ. Open Med. J. 2021, 10, e0316. [Google Scholar] [CrossRef]
- Simegn, W.; Dagnew, B.; Dagne, H. Knowledge and Associated Factors towards Cytotoxic Drug Handling among University of Gondar Comprehensive Specialized Hospital Health Professionals, Institutional-Based Cross-Sectional Study. Environ. Health Prev. Med. 2020, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration. Controlling Occupational Exposure to Hazardous Drugs; Occupational Safety and Health Administration: Washington DC, USA, 2016.
- European Medicines Agency (EMA). Good Manufacturing Practice (GMP) Guidelines; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2017.
- European Union. Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers From the Risks Related to Exposure to Carcinogens or Mutagens at Work; European Union: Brussels, Belgium, 2004.
- Health and Safety Executive (HSE). Carcinogens and Mutagens: The Control of Substances Hazardous to Health Regulations; Health and Safety Executive (HSE): Bootle, UK, 2015.
- Federal Institute for Occupational Safety and Health (BAuA). Cytotoxic Drugs: Information for Healthcare Professionals; Federal Institute for Occupational Safety and Health (BAuA): Chemnitz, Germany, 2021. [Google Scholar]
- Edwards, C.; Fortingo, N.; Franklin, E. Ergonomics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Waters, T.R.; Dick, R.B. Evidence of Health Risks Associated with Prolonged Standing at Work and Intervention Effectiveness. Rehabil. Nurs. 2015, 40, 148–165. [Google Scholar] [CrossRef]
- Andersen, L.L.; Vinstrup, J.; Sundstrup, E.; Skovlund, S.V.; Villadsen, E.; Thorsen, S.V. Combined Ergonomic Exposures and Development of Musculoskeletal Pain in the General Working Population: A Prospective Cohort Study. Scand. J. Work Environ. Health 2021, 47, 287–295. [Google Scholar] [CrossRef]
- Carayon, P.; Smith, M.J.; Haims, M.C. Work Organization, Job Stress, and Work-Related Musculoskeletal Disorders. Hum. Factors 1999, 41, 644–663. [Google Scholar] [CrossRef]
- Habibi, E.; Pourabdian, S.; Atabaki, A.K.; Hoseini, M. Evaluation of Work-Related Psychosocial and Ergonomics Factors in Relation to Low Back Discomfort in Emergency Unit Nurses. Int. J. Prev. Med. 2012, 3, 564–568. [Google Scholar]
- Lemma, E.; Taye, B.; Hussen, F. Ergonomic Workstations and Work-Related Musculoskeletal Disorders in the Clinical Laboratory. Lab. Med. 2012, 43, e11–e19. [Google Scholar] [CrossRef]
- Erturk Sengel, B.; Tukenmez Tigen, E.; Bilgin, H.; Dogru, A.; Korten, V. Occupation-Related Injuries Among Healthcare Workers: Incidence, Risk Groups, and the Effect of Training. Cureus 2021, 13, e14318. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, H.; Tang, L.; He, Y.; Tian, S. The Factors of Non-Specific Chronic Low Back Pain in Nurses: A Meta-Analysis. J. Back Musculoskelet. Rehabil. 2021, 34, 343–353. [Google Scholar] [CrossRef]
- Barr, A.E.; Barbe, M.F. Pathophysiological Tissue Changes Associated with Repetitive Movement: A Review of the Evidence. Phys. Ther. 2002, 82, 173–187. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M.; Zochowska, A.; Leonard, D.; Crow, M.; Jacklin, A.; Franklin, B.D. Comparing the Upper Limb Disorder Risks Associated with Manual and Automated Cytotoxic Compounding: A Pilot Study. Eur. J. Hosp. Pharm. 2012, 19, 293. [Google Scholar] [CrossRef]
- Moreira, F.; Jesus, Â.; Pinho, C.; Santos, M.; Serdoura, M.; Cruz, A. Ensuring Safety in Cytotoxic Drug Preparation: A Systematic Review of Guidelines Addressing Education for Pharmacy Professionals. J. Am. Pharm. Assoc. (2003) 2025, 65, 102352. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Martínez, M.A.; Ramos Merino, M.; Santos Gago, J.M.; Álvarez Sabucedo, L.M.; Wanden-Berghe, C.; Sanz-Valero, J. Guidelines for Safe Handling of Hazardous Drugs: A Systematic Review. PLoS ONE 2018, 13, e0197172. [Google Scholar] [CrossRef]
- Fazel, S.S.; Keefe, A.; Shareef, A.; Palmer, A.L.; Brenner, D.R.; Nakashima, L.; Koehoorn, M.W.; McLeod, C.B.; Hall, A.L.; Peters, C.E. Barriers and Facilitators for the Safe Handling of Antineoplastic Drugs. J. Oncol. Pharm. Pract. 2022, 28, 1709–1721. [Google Scholar] [CrossRef]
- Favier, B.; Simonin, C.; Tokatian, S.; Guitton, J.; Darnis, S.; Basset, M.; Chabaud, S.; Gilles, L. Cytotoxic Surface Contamination in Hospitals: Current Practices, Challenges and Perspectives. J. Oncol. Pharm. Pract. 2025, 31, 305–314. [Google Scholar] [CrossRef]
- Wallemacq, P.E.; Capron, A.; Vanbinst, R.; Boeckmans, E.; Gillard, J.; Favier, B. Permeability of 13 Different Gloves to 13 Cytotoxic Agents under Controlled Dynamic Conditions. Am. J. Health Syst. Pharm. 2006, 63, 547–556. [Google Scholar] [CrossRef]
- Kosgeroglu, N.; Ayranci, U.; Ozerdogan, N.; Demirustu, C. Turkish Nurses’ Information about, and Administration of, Chemotherapeutic Drugs. J. Clin. Nurs. 2006, 15, 1179–1187. [Google Scholar] [CrossRef]
- Kopp, B.; Schierl, R.; Nowak, D. Evaluation of Working Practices and Surface Contamination with Antineoplastic Drugs in Outpatient Oncology Health Care Settings. Int. Arch. Occup. Environ. Health 2013, 86, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Che Huei, L.; Ya-Wen, L.; Chiu Ming, Y.; Li Chen, H.; Jong Yi, W.; Ming Hung, L. Occupational Health and Safety Hazards Faced by Healthcare Professionals in Taiwan: A Systematic Review of Risk Factors and Control Strategies. SAGE Open Med. 2020, 8, 2050312120918999. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.D.; Gomes, A.N.H.; Zen, P.R.G.; Bica, C.G. Handling of Antineoplastic Drugs: A Health Concern among Health Care Workers. Rev. Bras. Med. Trab. 2021, 18, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; El-Zaemey, S.; Dorji, N.; Rai, B.D.; Fritschi, L. Exposure to Occupational Hazards among Health Care Workers in Low- and Middle-Income Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2603. [Google Scholar] [CrossRef]
- Tang, Y.; Che, X.; Wang, Y.L.; Ye, X.; Cao, W.L.; Wang, Y. Evaluation of Closed System Transfer Devices in Preventing Chemotherapy Agents Contamination During Compounding Process-A Single and Comparative Study in China. Front. Public Health 2022, 10, 827835. [Google Scholar] [CrossRef]
- Gonçalves, A.; de Oliveira, R.A.F.; Fernandes, P.O. Protective Equipment Applicable to a Centralized Cytostatic Preparation Unit. Procedia Comput. Sci. 2022, 196, 663–672. [Google Scholar] [CrossRef]
- Gonçalves, A.; Oliveira, R.; Fernandes, P. The Occupational Risks and Health Effects Resulting from Exposition to Cytotoxic Drugs Preparation. Procedia Comput. Sci. 2023, 219, 1420–1429. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Xu, F. Comparison of Permeabilities of Eight Different Types of Cytotoxic Drugs to Five Gloves with Different Materials by LC-MS/MS Methods to Reduce Occupational Exposure of Medical Personnel. J. Oncol. Pharm. Pract. 2023, 29, 1548–1554. [Google Scholar] [CrossRef]
- Bhirich, N.; Chefchaouni, A.C.; Medkouri, S.E.; Shytry, O.; Belahcen, M.J.; Rahali, Y. Risk Assessment of Personnel Exposure in a Central Cytotoxic Preparation Unit Using the FMECA Method. J. Oncol. Pharm. Pract. 2023, 29, 1884–1892. [Google Scholar] [CrossRef]
- McDiarmid, M.A.; Condon, M. Organizational Safety Culture/Climate and Worker Compliance with Hazardous Drug Guidelines: Lessons from the Blood-Borne Pathogen Experience. J. Occup. Environ. Med. 2005, 47, 740–749. [Google Scholar] [CrossRef]
- Fransman, W.; Vermeulen, R.; Kromhout, H. Dermal Exposure to Cyclophosphamide in Hospitals during Preparation, Nursing and Cleaning Activities. Int. Arch. Occup. Environ. Health 2005, 78, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Villain, A.; Sakji, I.; Bogart, E.; Strobbe, G.; Marliot, G.; Feutry, F. Optimisation of the Preparation of Chemotherapy Based on 5-Fluorouracil by the Use of Peristaltic Pumps. Pharm. Technol. Hosp. Pharm. 2020, 5, 20200003. [Google Scholar] [CrossRef]
- SHPA Committee of Specialty Practice in Oncology. SHPA Standards of Practice for the Safe Handling of Cytotoxic Drugs in Pharmacy Departments. J. Pharm. Pract. Res. 2005, 35, 44–52. [Google Scholar] [CrossRef]
- Yuki, M.; Sekine, S.; Takase, K.; Ishida, T.; Sessink, P.J.M. Exposure of Family Members to Antineoplastic Drugs via Excreta of Treated Cancer Patients. J. Oncol. Pharm. Pract. 2013, 19, 208–217. [Google Scholar] [CrossRef]
- Böhlandt, A.; Sverdel, Y.; Schierl, R. Antineoplastic Drug Residues inside Homes of Chemotherapy Patients. Int. J. Hyg. Environ. Health 2017, 220, 757–765. [Google Scholar] [CrossRef]
- Bláhová, L.; Kuta, J.; Doležalová, L.; Kozáková, Š.; Hojdarová, T.; Bláha, L. Levels and Risks of Antineoplastic Drugs in Households of Oncology Patients, Hospices and Retirement Homes. Environ. Sci. Eur. 2021, 33, 104. [Google Scholar] [CrossRef]
- Çınar, D.; Karadakovan, A. Investigation of Occupational Safety in Oncology Nurses. Int. J. Occup. Saf. Ergon. 2022, 28, 1750–1755. [Google Scholar] [CrossRef]
- Soheili, M.; Taleghani, F.; Jokar, F.; Eghbali-Babadi, M.; Sharifi, M. Oncology Nurses’ Needs Respecting Healthy Work Environment in Iran: A Descriptive Exploratory Study. Asia Pac. J. Oncol. Nurs. 2021, 8, 188–196. [Google Scholar] [CrossRef]
- Abbasi, K.; Hazrati, M.; Mohammadbeigi, A.; Ansari, J.; Sajadi, M.; Hosseinnazzhad, A.; Moshiri, E. Protection Behaviors for Cytotoxic Drugs in Oncology Nurses of Chemotherapy Centers in Shiraz Hospitals, South of Iran. Indian J. Med. Paediatr. Oncol. 2016, 37, 227–231. [Google Scholar] [CrossRef]
- Ramphal, R.; Bains, T.; Goulet, G.; Vaillancourt, R. Occupational Exposure to Chemotherapy of Pharmacy Personnel at a Single Centre. Can. J. Hosp. Pharm. 2015, 68, 104–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).